Abstract
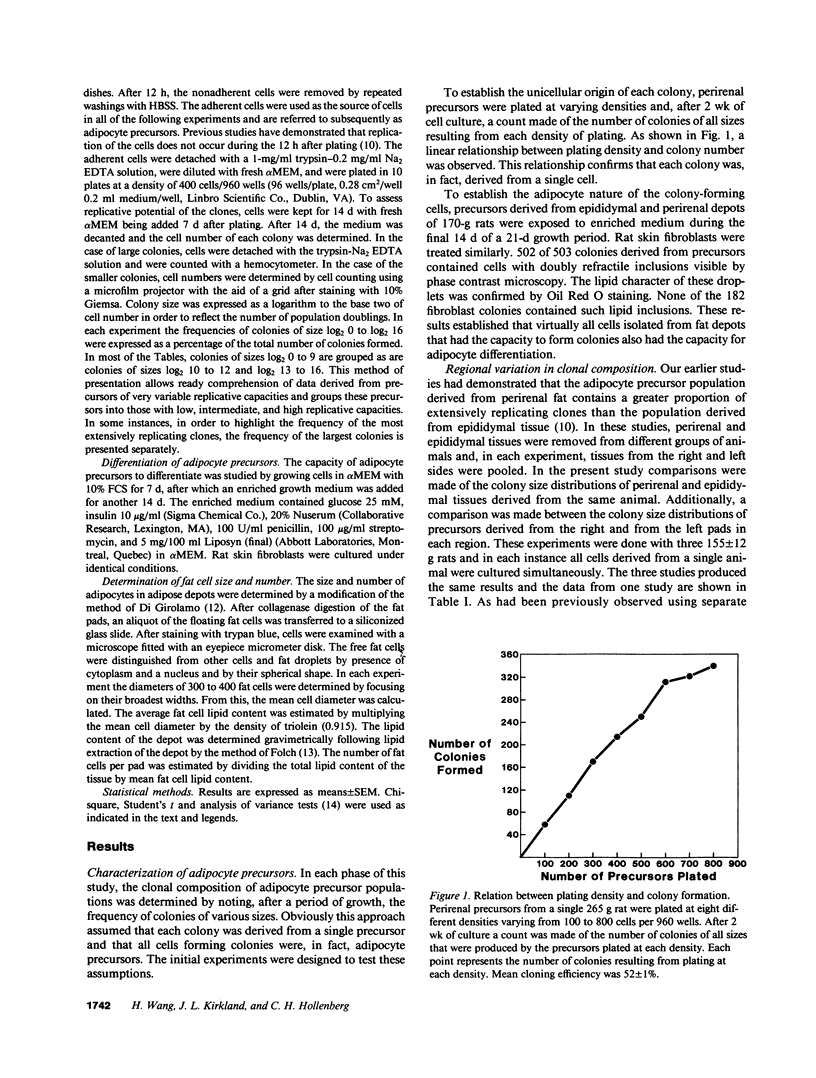
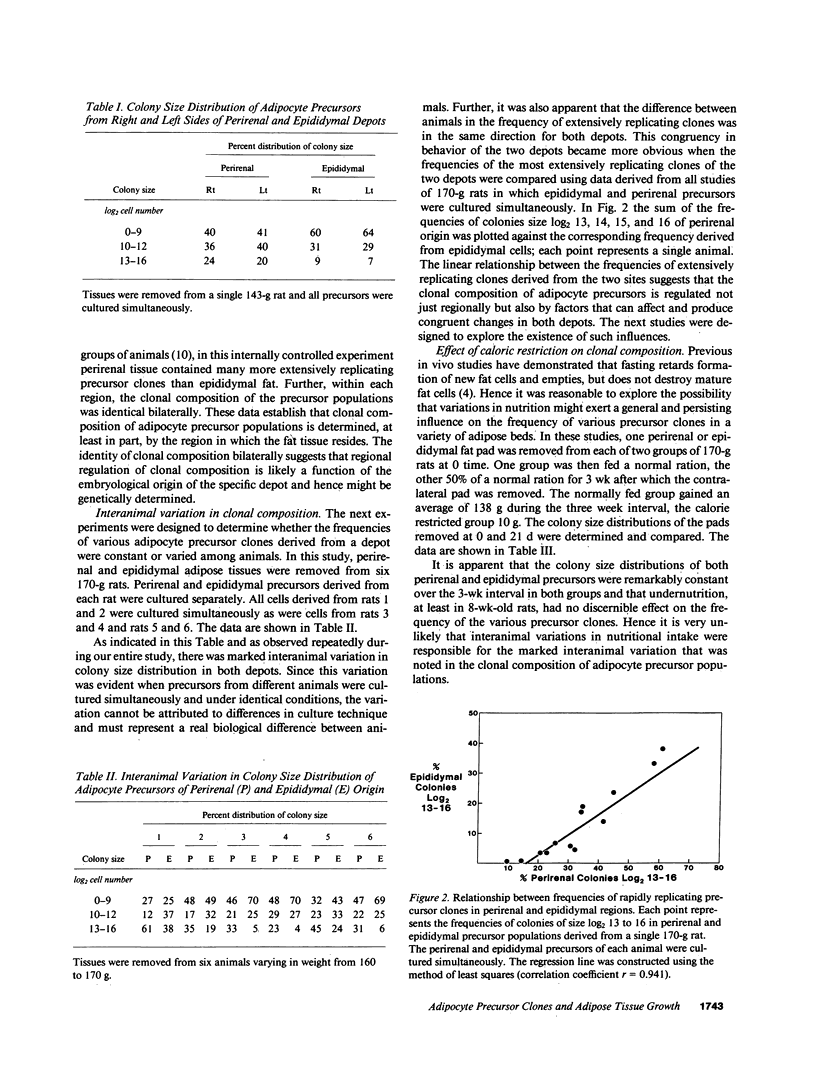
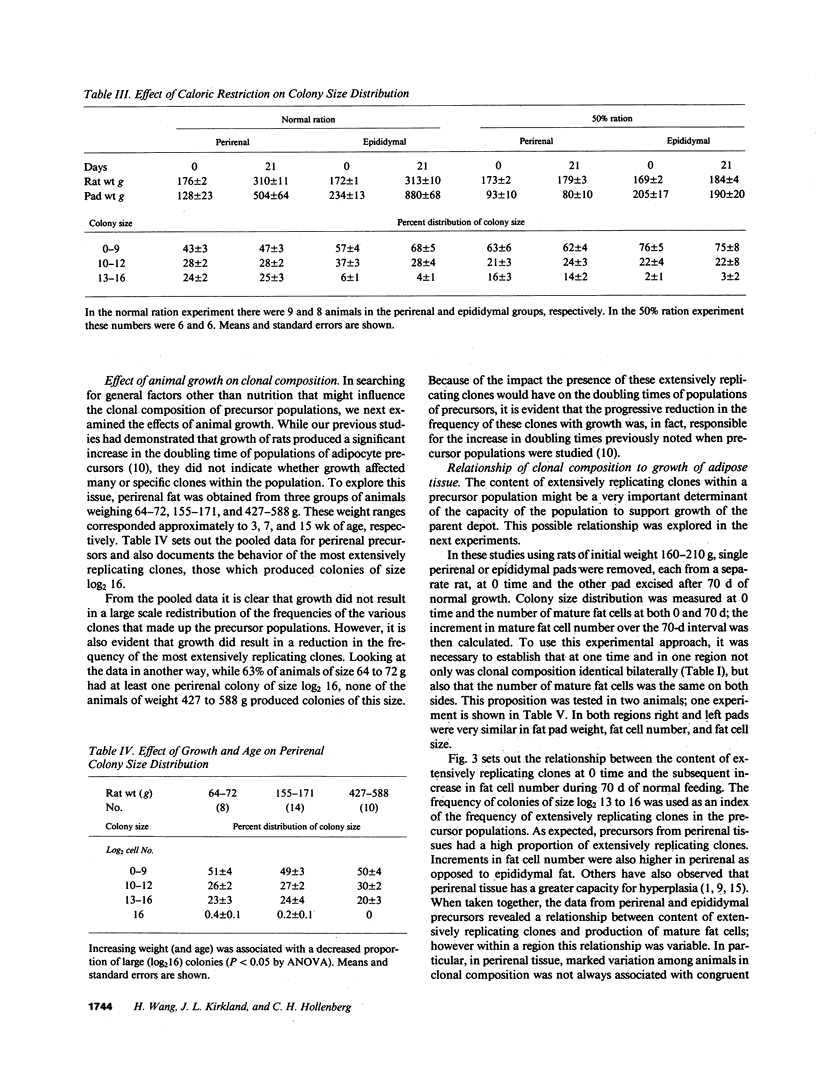
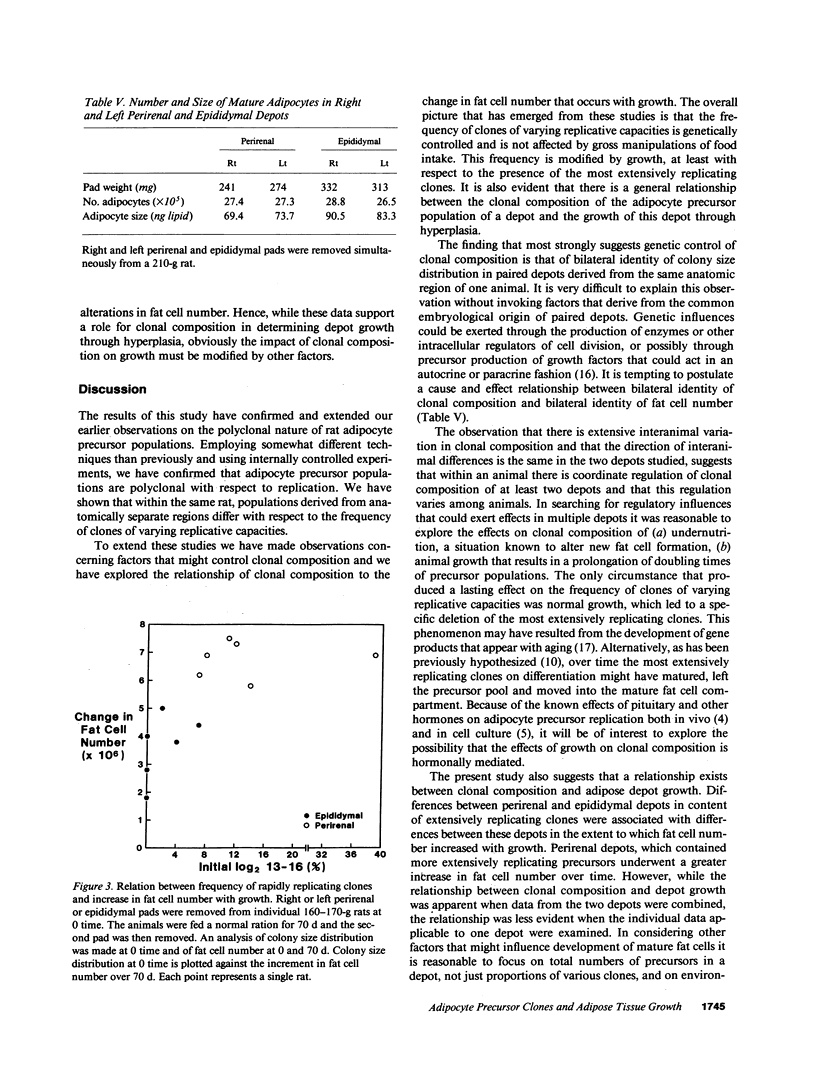
Rat adipocyte precursor populations contain clones varying in capacity for replication. In this study we explored factors controlling the frequency of clones of varying replicative capacities (clonal composition). We also explored the relationship between this frequency and fat depot growth. In perirenal and epididymal depots clonal composition was identical bilaterally; perirenal depots contained more extensively replicating clones. Although there were large interanimal differences in clonal composition, variation between animals was always in the same direction for both depots. Clonal composition was unaffected by undernutrition while with animal growth the frequency of the most extensively replicating clones was reduced. Differentiation of precursors occurred in all clones, while differentiation did not occur in skin fibroblasts cloned under identical conditions. Clonal composition and mature fat cell number were related in that fat cell numbers were identical bilaterally in both depots and increased more extensively with growth in perirenal than epididymal tissue. We conclude (a) that clonal composition of adipocyte precursor populations is regulated genetically and by age, (b) that this composition determines, at least in part, the capacity for adipose depot growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand H. A., Masoro E. J., Yu B. P. Increasing adipocyte number as the basis for perirenal depot growth in adult rats. Science. 1978 Sep 29;201(4362):1234–1235. doi: 10.1126/science.151328. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Salans L. B. Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J Lipid Res. 1978 Feb;19(2):269–273. [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S. Role of fat cell size and number in enlargement of epididymal fat pads in three species. Am J Physiol. 1971 Sep;221(3):859–864. doi: 10.1152/ajplegacy.1971.221.3.859. [DOI] [PubMed] [Google Scholar]
- Djian P., Roncari A. K., Hollenberg C. H. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983 Oct;72(4):1200–1208. doi: 10.1172/JCI111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djian P., Roncari D. A., Hollenberg C. H. Adipocyte precursor clones vary in capacity for differentiation. Metabolism. 1985 Sep;34(9):880–883. doi: 10.1016/0026-0495(85)90114-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Hirsch J. Adipose tissue regeneration following lipectomy. Science. 1977 Jul 22;197(4301):391–393. doi: 10.1126/science.877563. [DOI] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976 Jul;5(2):299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Han P. W. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J Lipid Res. 1969 Jan;10(1):77–82. [PubMed] [Google Scholar]
- Hollenberg C. H., Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969 Nov;47(11):2485–2498. doi: 10.1172/JCI105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyde B. J., Hirsch J. Isotopic labeling of DNA in rat adipose tissue: evidence for proliferating cells associated with mature adipocytes. J Lipid Res. 1979 Aug;20(6):691–704. [PubMed] [Google Scholar]
- Knittle J. L., Timmers K., Ginsberg-Fellner F., Brown R. E., Katz D. P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979 Feb;63(2):239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D. C., Roncari D. A., Hollenberg C. H. Release of mitogenic factors by cultured preadipocytes from massively obese human subjects. J Clin Invest. 1987 Feb;79(2):632–636. doi: 10.1172/JCI112859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Pereira-Smith O. M., Smith J. R. Existence of high abundance antiproliferative mRNA's in senescent human diploid fibroblasts. Science. 1986 Apr 18;232(4748):393–395. doi: 10.1126/science.2421407. [DOI] [PubMed] [Google Scholar]
- Roncari D. A. Hormonal influences on the replication and maturation of adipocyte precursors. Int J Obes. 1981;5(6):547–552. [PubMed] [Google Scholar]
- Roncari D. A., Lau D. C., Kindler S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism. 1981 May;30(5):425–427. doi: 10.1016/0026-0495(81)90174-8. [DOI] [PubMed] [Google Scholar]


