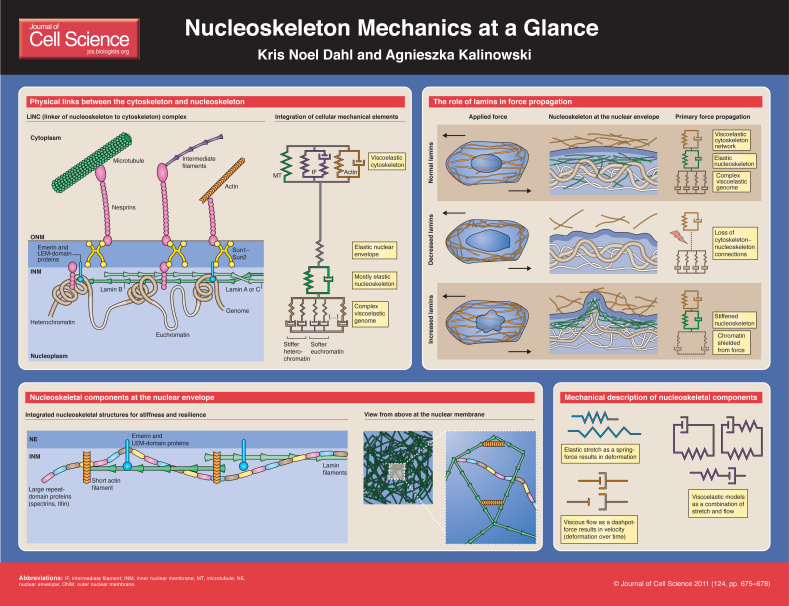
The nucleus contains the genetic information of the cell and all of the regulatory factors that process the genome effectively. The genome is encapsulated by a dense, filamentous meshwork called the nucleoskeleton, which is located at the inner nuclear membrane. The components of the nucleoskeleton are involved in cellular signaling (Wilson and Berk, 2010), but they are also necessary for maintaining nuclear structure, preventing rupture of the nucleus under force and possibly assisting in force transduction (Wang et al., 2009). Here, we present the integrated mechanical structures of the nucleoskeleton, including lamin filaments, multisubunit proteins, short actin filaments and the genome. We also discuss the integration of mechanical elements from the cytoskeleton into the nucleoskeleton. Based on the mechanical contributions of the individual elements, we demonstrate how mutations in nuclear structures might impact force-dependent etiologies of disease. The accompanying poster aims to provide a translational overview between the cell biology of the nucleus and the biophysics of its underlying polymeric structures.
Nucleoskeletal components
The nucleoskeleton is primarily composed of intermediate type V filaments, which consist of lamin proteins. Human cells encode three genes for two types of lamins: the A-type lamins, primarily lamins A and C, which are products of alternative splicing of the LMNA gene; and the B-type lamins, lamin B1 and lamin B2, which are encoded by two separate genes (Gruenbaum et al., 2005). Lamin filaments assemble into homopolymers and mostly separate networks (Delbarre et al., 2006; Furukawa et al., 2009; Moir et al., 2000). B-type lamins are ubiquitously expressed in metazoan cells and are essential to cell survival (Harborth et al., 2001) because of the fundamental roles of lamin B proteins in transcription and other cellular signaling pathways. For example, lamin B1 is essential for RNA synthesis, and activity of RNA polymerases I and II (Tang et al., 2008). However, loss of lamin B1 in mouse embryonic fibroblasts does not result in any of the nuclear softening and cytoskeletal defects that are seen when lamin A is lost (Lammerding et al., 2006). Recently, lamin B2 has been shown to be
required for nuclear migration in neurons (Coffinier et al., 2010).
Lamin A is thought to contribute more significantly to nuclear mechanical functions than B-type lamins (Lammerding et al., 2006; Schape et al., 2009). Nevertheless, cells are able to survive and proliferate without A-type lamins and lmna-knockout mice are viable, but afflicted with muscular dystrophy (Raharjo et al., 2001). In humans, a large number of mutations in LMNA have been described that give rise to at least 13 different diseases, including dominant Emery-Dreifuss muscular dystrophy (and other striated muscle disorders), lipodystrophies and premature aging syndromes (Capell and Collins, 2006; Prokocimer et al., 2009).
The majority of lamins are bound to the nuclear envelope through an extensive array of inner nuclear membrane proteins (Schirmer and Foisner, 2007). For example, the LEM-domain proteins emerin, MAN-1 and lamina-associated polypeptide 2 (LAP2), and the newly discovered LEM-domain proteins (Lee and Wilson, 2004) all bind lamins and many are anchored in the inner nuclear membrane (Wagner and Krohne, 2007). These proteins help to stabilize the protein–membrane interactions that lead to a stable nucleoskeleton shell. They also have a number of other binding partners, suggesting that they play possible roles in mechanically sensitive signaling (Wilson and Berk, 2010).
Lamin filaments are primarily responsible for the stiffness of the nucleoskeleton, but other accessory structural proteins found in the nucleoskeleton influence the localized spatial lamin organization and other mechanical properties, such as resilience after stretch. Recently, large repeat-domain proteins, such as titin and αII-spectrin, were shown to have functional roles in organizing lamins and thus are involved in the overall nuclear structure (Zhong et al., 2010b). Loss of titin results in significant nuclear abnormalities, including large blebs and dilations, and heterogeneous labeling of lamins at the nuclear envelope (Zastrow et al., 2006). Loss of αII-spectrin leads to changes in nucleoskeleton organization and a reduced ability to mechanically recover from distension and dilation (Z. Zhong, A. J. Ribeiro, D. Simon et al., unpublished) (Zhong et al., 2010b). These large repeat-domain proteins have multiple or recurring binding sites for LEM-domain proteins, lamins and chromatin-binding proteins. With many binding sites, high concentrations of binding partners can be localized to smaller areas in one dimension, which enhances the stability of large protein complexes.
At the nuclear envelope, small actin structures are also found and can bind emerin (Holaska et al., 2004; Lattanzi et al., 2003). The shortness of these actin structures suggests that they do not provide similar mechanical strength compared with filaments present in the cytoskeleton; their mechanical function in the nuclear envelope, if any, is currently unknown (Pederson and Aebi, 2002). It is possible that these short actin filaments support the overall nucleoskeletal structure by providing mechanical ‘struts’ or binding sites for the stabilization of larger complexes, such as lamin filaments, in the nucleoskeleton.
Connections between the nucleoskeleton and the cytoskeleton
There is a functional connection between structural elements of the cell, the cytoskeleton and the nucleoskeleton. The cytoskeleton is composed of actin microfilaments, microtubules and variable amounts of intermediate filaments. These three elements of the cytoskeleton are interconnected and linked to the nucleoskeleton through proteins of the LINC (linkers of the nucleoskeleton to the cytoskeleton) complex (Crisp et al., 2006; Razafsky and Hodzic, 2009). Only a subset of cytoskeletal filaments are directly attached to the nucleus (Khatau et al., 2009), but the loss of this connectivity through the LINC complex can alter the mechanical function of the cytoskeleton peripheral to the nucleus (Hale et al., 2008; Lee et al., 2007).
LINC complexes consist of a number of proteins, which connect the cytoskeleton with the nuclear interior. Multiple isoforms of the large transmembrane nesprins (also called SYNEs and MYNEs) extend from the outer nuclear membrane and attach to cytoskeletal filaments. There are four known mammalian nesprin proteins, all of which contain spectrin-like repeat subunits and KASH domains (Zhong et al., 2010a). These different nesprin types also have multiple isoforms based on the number of subunits, denoted α, β, δ, γ and so on (Warren et al., 2005). The largest isoforms of nesprin-1, nesprin-2 and nesprin-3 are located in the outer nuclear membrane and bind to either actin (i.e. nesprin-1γ, nesprin-2γ) (Zhang et al., 2002) or plectin (i.e. nesprin-3α and nesprin-3β) (Wilhelmsen et al., 2005), whereas nesprin 4 interacts with microtubules in secretory epithelia (Roux et al., 2009). In the perinuclear space, nesprins bind through their KASH domain to the SUN-domain protein dimer Sun1–Sun2, which extends through the inner nuclear membrane where it binds lamin A and lamin-associated proteins, including emerin (Haque et al., 2010). The smaller nesprin isoforms, such as nesprin-1α and nesprin-2β, are localized to the inner nuclear membrane and directly bind to lamins, emerin and SUN-domain proteins (Haque et al., 2010; Mislow et al., 2002).
Within the nucleus, lamins can bind to DNA either directly (Stierle et al., 2003) or indirectly through lamin-binding proteins that are able to interact with DNA and chromatin through histones (Prokocimer et al., 2009). Barrier-to-autointegration factor (BAF), LEM-domain proteins and other lamin-binding proteins, including lamin B receptor, also bind to DNA and to chromatin proteins (Mekhail and Moazed, 2010), thereby generating redundant connections between the nucleoskeleton and the DNA. The functional redundancies of these connections are observed in Caenorhabditis elegans, in which emerin loss is lethal only when MAN1 (also known as LEM-domain-containing protein 3, LEMD3) is also lost (Liu et al., 2003). Lamins are also present in the nuclear interior, where they serve as scaffolds for functional complexes required for gene transcription (Neri et al., 1999). However, these lamin filaments are discontinuous and do not form rigid, percolated (continuous) three-dimensional networks throughout the nucleoplasm. The mechanical consequence of this is that the nuclear interior is primarily governed by the flow of the viscoelastic chromatin, and the functional mechanical networks of lamins, which stretch elastically, are found primarily in the nucleoskeleton (Pajerowski et al., 2007).
Nucleoskeleton mechanics and force transmission
The dense lamin meshwork in the nucleoskeleton acts as an elastic shell that stretches under force; elastic stretch is typically modeled by a spring (Dahl et al., 2004; Pajerowski et al., 2007; Rowat et al., 2005). The nuclear interior deforms as a viscoelastic solid (Pajerowski et al., 2007). Viscoelastic materials deform both by stretching, such as elastic stretch that can be modeled by a spring, and by fluid-like flow, termed viscous flow and is modeled by a ‘dashpot’. Viscoelastic deformation is common for most entangled semi-flexible polymer systems, of both biological and synthetic origin, suggesting that the mechanical characteristics of the nuclear interior are dominated by entangled DNA structures. Furthermore, the time dependence of the deformation viscoelasticity that is observed in nuclei suggests that there are several time and length scales of deformation (Fabry et al., 2001; Stamenovic, 2008), possibly reflecting the higher-order organization of DNA within the nucleus into chromatin and chromosomes, and into chromosome territories. Chromatin can be structurally and functionally divided into heterochromatin and euchromatin (Delcuve et al., 2009; Kanger et al., 2008). Functionally, heterochromatin is considered poor in gene content and is replicated last (Joffe et al., 2010). Heterochromatin itself is a load-bearing mechanical structure within the nucleus. Ablation of heterochromatin-dense regions in the nucleus using lasers results in rapid nuclear shrinkage, which leads to reorganization of the nucleus and the cytoskeleton (Mazumder and Shivashankar, 2010). By contrast, euchromatin has a more open structure with greater fluidity. Many of the heterochromatic regions are localized to the nucleoskeleton at the nuclear envelope. However, pockets of euchromatin exist at the nuclear envelope and throughout the nucleus, and create regions of heterogeneous fluidity within an otherwise stiff nucleus (Fedorova and Zink, 2008). The existing link between viscoelastic cytoskeletal elements (Janmey, 1991) and the nucleus through the LINC complex therefore suggests that forces that are applied to cells can be transmitted to the genome, both globally by the integrated network and locally through individual interconnections (Hale et al., 2008; Lee et al., 2007). This link might be responsible for changes in the conformation of nuclear structures and altered gene expression patterns with applied force.
Lamin alterations and force transmission
When structures in the nucleus are lost or altered by mutation or dysfunction, they result in significant changes in nuclear mechanical properties, including force transmission through the nucleus. The most severe examples include changes in A-type lamins. Mechanically, lmna−/− fibroblasts show an increased degree of nuclear deformation with mechanical stretching of the cell (Lammerding et al., 2004). Mouse fibroblasts lacking lmna show frequent nuclear rupture under high force (Lammerding et al., 2004). Interestingly, the cytoskeletal response to force is altered in cells lacking lmna, possibly due to the lack of cytoskeletal structures stabilizing the nucleus and an altered cell response caused by the loss of the LINC connection between the cytoskeleton and the nucleus (Hale et al., 2008; Lee et al., 2007). Changes in cytoskeletal mechanics have also been observed in cells lacking LINC complex proteins (Chancellor et al., 2010). Conversely, mutations in LMNA, which cause Hutchinson-Gilford progeria syndrome (HGPS), result in increased accumulation of lamin A at the nuclear envelope (Goldman et al., 2004), which leads to stiffened nuclei in patients suffering from HGPS (Dahl et al., 2006). These nuclei are more resistant to high force (Dahl et al., 2006), but show blebbing (Goldman et al., 2004; Verstraeten et al., 2008) and unique failures such as nuclear lamina cracking under high force (Dahl et al., 2006). In these nuclei, the reorganization of the nuclear interior under force is also reduced, suggesting that an optimal degree of connectivity between cytoskeletal and nucleoskeletal elements exists within a normal cell (Philip and Dahl, 2008). Thus, lamin A in the nucleoskeleton seems to have a direct impact on nuclear stiffness and its mechanical integrity.
The link between nucleoskeleton and gene expression or DNA processing
Forces exerted on the cell alter gene expression through the activation of chemical cues, such as phosphorylation of cellular signaling pathways (Chen, 2008). However, as nucleoskeletal elements are both load-bearing structures and influence gene expression, there might be a more direct role for force in regulating gene expression. Genes in the proximity of the nuclear envelope are mostly repressed by heterochromatization and the presence of large numbers of transcriptional repressors that are associated with lamins and lamin-binding proteins (Ahmed and Brickner, 2007; Towbin et al., 2009). Within the nuclear interior, lamins act in repressing transcription (Lee et al., 2009). Here, lamins A and C participate in cell-cycle regulation of gene expression by forming a scaffold for hyperphosphorylated retinoblastoma (Rb), which represses genes that are required for the G1 to S phase transition of the cell cycle (Boban et al., 2010). Some evidence suggests that lamins A and C can organize into a nucleoplasmic scaffold that is necessary for the elongation phase of replication (Dechat et al., 2008). Lamins A and C also colocalize with proliferation-related proteins, including the activator protein AP-1 (Boban et al., 2010). Short nuclear actins are also involved in many aspects of nuclear function, including transcription and replication (Castano et al., 2010), whereas spectrins located inside the nuclear interior are involved in DNA repair processes (Young and Kothary, 2005). Thus, every important aspect of nuclear function, such as transcription, replication, DNA repair and the control of these processes, is influenced by nucleoskeletal proteins, which possibly serve as scaffolds or regions of localized stiffness. As these proteins are involved in both nuclear mechanics and DNA processing, it is highly likely that these roles are integrated within the nucleus, as manifested by force-induced changes in nuclear function.
Perspectives
Recently, experiments with cells lacking proteins of the LINC complex have shown a reduction in force transmission from the cytoskeleton from one side of the nucleus to other side (Brosig et al., 2010; Chancellor et al., 2010) (Jan Lammerding, personal communication). This trans-nuclear cytoskeletal deformation demonstrates the role of the stiff nucleus in propagating forces from the actin cytoskeleton. It seems that all of the mechanical structures of the cell are connected. The cell might use these mechanical interconnections to maintain its overall mechanical integrity, which is required to preserve tissue mechanics and prevent rupture under high strain. These mechanical interconnections could also allow the exertion of force-induced changes in gene expression through an integrated mechanical network into the nuclear interior. Carefully controlled biophysical and biological experiments must be conducted to determine whether these mechanical interactions are relevant to gene expression. However, decoupling nuclear–cytoskeletal mechanical interactions from mechanically induced chemical signaling within the cell continues to be a major challenge in the field.
Acknowledgments
The project was supported by award number F30AG030905 from the National Institute On Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Aging or the National Institutes of Health. This project was also supported by National Science Foundation CAREER award 0954421. Deposited in PMC for release after 12 months.
References
- Ahmed S., Brickner J. H. (2007). Regulation and epigenetic control of transcription at the nuclear periphery. Trends Genet. 23, 396-402 [DOI] [PubMed] [Google Scholar]
- Boban M., Braun J., Foisner R. (2010). Lamins: ‘structure goes cycling’. Biochem. Soc. Trans. 38, 301-306 [DOI] [PubMed] [Google Scholar]
- Brosig M., Ferralli J., Gelman L., Chiquet M., Chiquet-Ehrismann R. (2010). Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int. J. Biochem. Cell Biol. 42, 1717-1728 [DOI] [PubMed] [Google Scholar]
- Capell B. C., Collins F. S. (2006). Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 7, 940-952 [DOI] [PubMed] [Google Scholar]
- Castano E., Philimonenko V. V., Kahle M., Fukalova J., Kalendova A., Yildirim S., Dzijak R., Dingova-Krasna H., Hozak P. (2010). Actin complexes in the cell nucleus: new stones in an old field. Histochem. Cell Biol. 133, 607-626 [DOI] [PubMed] [Google Scholar]
- Chancellor T. J., Lee J., Thodeti C. K., Lele T. (2010). Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 99, 115-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S. (2008). Mechanotransduction – a field pulling together? J. Cell Sci. 121, 3285-3292 [DOI] [PubMed] [Google Scholar]
- Coffinier C., Chang S. Y., Nobumori C., Tu Y., Farber E. A., Toth J. I., Fong L. G., Young S. G. (2010). Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. USA 107, 5076-5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M., Liu Q., Roux K., Rattner J. B., Shanahan C., Burke B., Stahl P. D., Hodzic D. (2006). Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Sci. 172, 41-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K. N., Kahn S. M., Wilson K. L., Discher D. E. (2004). The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117, 4779-4786 [DOI] [PubMed] [Google Scholar]
- Dahl K. N., Scaffidi P., Islam M. F., Yodh A. G., Wilson K. L., Misteli T. (2006). Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 103, 10271-10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T., Pfleghaar K., Sengupta K., Shimi T., Shumaker D. K., Solimando L., Goldman R. D. (2008). Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 22, 832-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre E., Tramier M., Coppey-Moisan M., Gaillard C., Courvalin J. C., Buendia B. (2006). The truncated prelamin A in Hutchinson-Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum. Mol. Genet. 15, 1113-1122 [DOI] [PubMed] [Google Scholar]
- Delcuve G. P., Rastegar M., Davie J. R. (2009). Epigenetic control. J. Cell. Physiol. 219, 243-250 [DOI] [PubMed] [Google Scholar]
- Fabry B., Maksym G. N., Butler J. P., Glogauer M., Navajas D., Fredberg J. J. (2001). Scaling the microrheology of living cells. Phys. Rev. Lett. 87, 148102 [DOI] [PubMed] [Google Scholar]
- Fedorova E., Zink D. (2008). Nuclear architecture and gene regulation. Biochim. Biophys. Acta 1783, 2174-2184 [DOI] [PubMed] [Google Scholar]
- Furukawa K., Ishida K., Tsunoyama T. A., Toda S., Osoda S., Horigome T., Fisher P. A., Sugiyama S. (2009). A-type and B-type lamins initiate layer assembly at distinct areas of the nuclear envelope in living cells. Exp. Cell Res. 315, 1181-1189 [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Shumaker D. K., Erdos M. R., Eriksson M., Goldman A. E., Gordon L. B., Gruenbaum Y., Khuon S., Mendez M., Varga R. (2004). Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 101, 8963-8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Margalit A., Goldman R. D., Shumaker D. K., Wilson K. L. (2005). The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 6, 21-31 [DOI] [PubMed] [Google Scholar]
- Hale C. M., Shrestha A. L., Khatau S. B., Stewart-Hutchinson P. J., Hernandez L., Stewart C. L., Hodzic D., Wirtz D. (2008). Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 95, 5462-5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F., Mazzeo D., Patel J. T., Smallwood D. T., Ellis J. A., Shanahan C. M., Shackleton S. (2010). Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J. Biol. Chem. 285, 3487-3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborth J., Elbashir S. M., Bechert K., Tuschl T., Weber K. (2001). Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 114, 4557-4565 [DOI] [PubMed] [Google Scholar]
- Holaska J. M., Kowalski A. K., Wilson K. L. (2004). Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2, E231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A. (1991). Mechanical properties of cytoskeletal polymers. Curr. Opin. Cell Biol. 3, 4-11 [DOI] [PubMed] [Google Scholar]
- Joffe B., Leonhardt H., Solovei I. (2010). Differentiation and large scale spatial organization of the genome. Curr. Opin. Genet. Dev. 20, 562-569 [DOI] [PubMed] [Google Scholar]
- Kanger J. S., Subramaniam V., van Driel R. (2008). Intracellular manipulation of chromatin using magnetic nanoparticles. Chromosome Res. 16, 511-522 [DOI] [PubMed] [Google Scholar]
- Khatau S. B., Hale C. M., Stewart-Hutchinson P. J., Patel M. S., Stewart C. L., Searson P. C., Hodzic D., Wirtz D. (2009). A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA 106, 19017-19022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Schulze P. C., Takahashi T., Kozlov S., Sullivan T., Kamm R. D., Stewart C. L., Lee R. T. (2004). Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Fong L. G., Ji J. Y., Reue K., Stewart C. L., Young S. G., Lee R. T. (2006). Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 281, 25768-25780 [DOI] [PubMed] [Google Scholar]
- Lattanzi G., Cenni V., Marmiroli S., Capanni C., Mattioli E., Merlini L., Squarzoni S., Maraldi N. M. (2003). Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts. Biochem. Biophys. Res. Commun. 303, 764-770 [DOI] [PubMed] [Google Scholar]
- Lee D. C., Welton K. L., Smith E. D., Kennedy B. K. (2009). A-type nuclear lamins act as transcriptional repressors when targeted to promoters. Exp. Cell Res. 315, 996-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Hale C. M., Panorchan P., Khatau S. B., George J. P., Tseng Y., Stewart C. L., Hodzic D., Wirtz D. (2007). Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 93, 2542-2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Wilson K. L. (2004). All in the family: evidence for four new LEM-domain proteins Lem2 (NET-25), Lem3, Lem4 and Lem5 in the human genome. Symp. Soc. Exp. Biol. 329-339 [PubMed] [Google Scholar]
- Liu J., Lee K. K., Segura-Totten M., Neufeld E., Wilson K. L., Gruenbaum Y. (2003). MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 100, 4598-4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Shivashankar G. V. (2010). Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. J. R. Soc. Interface 7 Suppl. 3, S321-S330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail K., Moazed D. (2010). The nuclear envelope in genome organization, expression and stability. Nat. Rev. Mol. Cell Biol. 11, 317-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mislow J. M., Holaska J. M., Kim M. S., Lee K. K., Segura-Totten M., Wilson K. L., McNally E. M. (2002). Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 525, 135-140 [DOI] [PubMed] [Google Scholar]
- Moir R. D., Yoon M., Khuon S., Goldman R. D. (2000). Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151, 1155-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri L. M., Raymond Y., Giordano A., Capitani S., Martelli A. M. (1999). Lamin A is part of the internal nucleoskeleton of human erythroleukemia cells. J. Cell. Physiol. 178, 284-295 [DOI] [PubMed] [Google Scholar]
- Pajerowski J. D., Dahl K. N., Zhong F. L., Sammak P. J., Discher D. E. (2007). Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA 104, 15619-15624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Aebi U. (2002). Actin in the nucleus: what form and what for? J. Struct. Biol. 140, 3-9 [DOI] [PubMed] [Google Scholar]
- Philip J. T., Dahl K. N. (2008). Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J. Biomech. 41, 3164-3170 [DOI] [PubMed] [Google Scholar]
- Prokocimer M., Davidovich M., Nissim-Rafinia M., Wiesel-Motiuk N., Bar D., Barkan R., Meshorer E., Gruenbaum Y. (2009). Nuclear lamins: key regulators of nuclear structure and activities. J. Cell. Mol. Med. 13, 1059-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raharjo W. H., Enarson P., Sullivan T., Stewart C. L., Burke B. (2001). Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci. 114, 4447-4457 [DOI] [PubMed] [Google Scholar]
- Razafsky D., Hodzic D. (2009). Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J. Cell Biol. 186, 461-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux K. J., Crisp M. L., Liu Q., Kim D., Kozlov S., Stewart C. L., Burke B. (2009). Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc. Natl. Acad. Sci. USA 106, 2194-2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowat A. C., Foster L. J., Nielsen M. M., Weiss M., Ipsen J. H. (2005). Characterization of the elastic properties of the nuclear envelope. J. R. Soc. Interface 2, 63-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schape J., Prausse S., Radmacher M., Stick R. (2009). Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy. Biophys. J. 96, 4319-4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer E. C., Foisner R. (2007). Proteins that associate with lamins: many faces, many functions. Exp. Cell Res. 313, 2167-2179 [DOI] [PubMed] [Google Scholar]
- Stamenovic D. (2008). Rheological behavior of mammalian cells. Cell. Mol. Life Sci. 65, 3592-3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierle V., Couprie J., Ostlund C., Krimm I., Zinn-Justin S., Hossenlopp P., Worman H. J., Courvalin J. C., Duband-Goulet I. (2003). The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochem. 42, 4819-4828 [DOI] [PubMed] [Google Scholar]
- Tang C. W., Maya-Mendoza A., Martin C., Zeng K., Chen S., Feret D., Wilson S. A., Jackson D. A. (2008). The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J. Cell Sci. 121, 1014-1024 [DOI] [PubMed] [Google Scholar]
- Towbin B. D., Meister P., Gasser S. M. (2009). The nuclear envelope-a scaffold for silencing? Curr. Opin. Genet. Dev. 19, 180-186 [DOI] [PubMed] [Google Scholar]
- Verstraeten V. L., Ji J. Y., Cummings K. S., Lee R. T., Lammerding J. (2008). Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell 7, 383-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N., Krohne G. (2007). LEM-domain proteins: new insights into lamin-interacting proteins. Int. Rev. Cytol. 261, 1-46 [DOI] [PubMed] [Google Scholar]
- Wang N., Tytell J. D., Ingber D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75-82 [DOI] [PubMed] [Google Scholar]
- Warren D. T., Zhang Q., Weissberg P. L., Shanahan C. M. (2005). Nesprins: intracellular scaffolds that maintain cell architecture and coordinate cell function? Expert Rev. Mol. Med. 7, 1-15 [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K., Litjens S. H., Kuikman I., Tshimbalanga N., Janssen H., van den Bout I., Raymond K., Sonnenberg A. (2005). Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 171, 799-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. L., Berk J. M. (2010). The nuclear envelope at a glance. J. Cell Sci. 123, 1973-1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. G., Kothary R. (2005). Spectrin repeat proteins in the nucleus. BioEssays 27, 144-152 [DOI] [PubMed] [Google Scholar]
- Zastrow M. S., Flaherty D. B., Benian G. M., Wilson K. L. (2006). Nuclear titin interacts with A- and B-type lamins in vitro and in vivo. J. Cell Sci. 119, 239-249 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Ragnauth C., Greener M. J., Shanahan C. M., Roberts R. G. (2002). The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics 80, 473-481 [PubMed] [Google Scholar]
- Zhong Z., Chang S. A., Kalinowski A., Wilson K. L., Dahl K. N. (2010a). Stabilization of the spectrin-like domains of nesprin-1alpha by the evolutionarily conserved “adaptive” domain. Cell. Mol. Bioeng. 3, 139-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wilson K. L., Dahl K. N. (2010b). Beyond lamins: other structural components of the nucleoskeleton. Methods Cell Biol. 98, 97-119 [DOI] [PMC free article] [PubMed] [Google Scholar]