Abstract
R5 HIV-1 strains resistant to the CCR5 antagonist Maraviroc (MVC) can use drug-bound CCR5. We demonstrate that MVC-resistant HIV-1 exhibits delayed kinetics of coreceptor engagement and fusion during drug-bound versus free CCR5 infection of cell lines. Antibodies directed against the second extracellular loop (ECL2) of CCR5 had greater antiviral activity against MVC-bound compared to MVC-free CCR5 infection. However, in PBMCs, only ECL2 CCR5 antibodies HGS004 and HGS101, but not 2D7, inhibited infection by MVC resistant HIV-1 more potently with MVC-bound than with free CCR5. In addition, HGS004 and HGS101, but not 2D7, restored the antiviral activity of MVC against resistant virus in PBMCs. In flow cytometric studies, CCR5 binding by the HGS mAbs, but not by 2D7, was increased when PBMCs were treated with MVC, suggesting MVC increases exposure of the relevant epitope. Thus, HGS004 and HGS101 have antiviral mechanisms distinct from 2D7 and could help overcome MVC resistance.
Keywords: HIV-1, CCR5, CCR5 antagonists, maraviroc, CCR5 antibodies, antagonist resistance
INTRODUCTION
The identification of CCR5 as an HIV-1 coreceptor (Alkhatib et al., 1996; Choe et al., 1996; Deng et al., 1996; Dragic et al., 1996), prompted by the discovery of CCR5 ligand β-chemokines with antiviral activity (Cocchi et al., 1995), led to the development of antiretroviral CCR5 inhibitors, including small molecules and antibodies. In clinical trials of HIV-1 patients infected with CCR5-tropic HIV-1 only (R5 strains), these agents have achieved potent viral suppression (Currier et al., 2008; Gulick et al., 2008; Gulick et al., 2007; Landovitz et al., 2008; Suleiman et al., 2010; Wilkin et al., 2010; Yeni et al., 2009). At present, the small-molecule CCR5 antagonist Maraviroc (MVC) is the only licensed CCR5 inhibitor (2007; 2009). The CCR5 antibodies PRO 140 and HGS004 have significantly reduced HIV-1 RNA in clinical trials of patients infected with R5 HIV-1 only (Jacobson et al., 2010a; Jacobson et al., 2008; Jacobson et al., 2010b; Lalezari et al., 2008). However, viral resistance to CCR5 inhibitors has been demonstrated (Cooper et al., 2010; Demarest et al., 2009; Gulick et al., 2008; Gulick et al., 2007; Marozsan et al., 2005; Westby et al., 2007).
Resistance to CCR5 inhibitors could arise from a switch to use of the alternative coreceptor CXCR4, either by acquiring mutations in the viral envelope (Env) protein or by selection of preexisting CXCR4-using variants (X4 HIV-1). However, in vitro data indicate that a switch to CXCR4 usage under CCR5 inhibitor pressure is quite rare (McNicholas et al., 2010). In vivo, some patients failing treatment with MVC or other antagonists harbored X4 variants, but DNA sequencing demonstrated that such variants were selected from minor populations already present prior to treatment (Kitrinos et al., 2009; Tsibris et al., 2009; Tsibris et al., 2008; Westby et al., 2006). Resistance could also arise from emergence of mutations that result in increased affinity for CCR5 (Pugach et al., 2007; Trkola et al., 2002; Tsibris et al., 2008; Westby et al., 2007). In genotypic assays, resistance is associated with mutations in Env, generally in the V3 region of gp120 (Kuhmann et al., 2004; Marozsan et al., 2005; Ogert et al., 2008; Tsibris et al., 2008), but no such signature mutations have been identified to date. Resistance is commonly determined using the Phenosense Entry Susceptibility Assay (Monogram Biosciences, San Francisco, CA), a single-cycle, Env-pseudotype assay based on U87 cells expressing high levels of CD4 and CCR5/CXCR4. In this assay, resistance is manifested by decreases in maximum percentage of inhibition (MPI) at saturating concentrations of antagonist (Pugach et al., 2007; Westby et al., 2007). The MPI level reflects the efficiency with which the virus uses the antagonist-free versus antagonist-bound forms of CCR5, with the MPI decreasing as the efficiency with antagonist-bound CCR5 increases. We (Heredia et al., 2008) and others (Pugach et al., 2009) have previously shown that CCR5 density on target cells modulates MPI values.
We now demonstrate that infection of cell lines with an HIV-1 reporter virus bearing the envelope (Env) of a MVC-resistant HIV-1 CC1/85 strain is inhibited by MVC at low CCR5 densities, suggesting a lower viral affinity for MVC-bound than for MVC-free CCR5. We further show that CCR5 mAbs HGS004 and HGS101, but not other CCR5 mAbs, restored MVC inhibition of MVC-resistant HIV-1 infection of PBMCs. We conclude that CCR5 mAbs HGS004 and HGS101 preferentially inhibit MVC-resistant virus infection via antagonist-bound CCR5 and restore sensitivity of resistant virus to MVC, suggesting a potentially effective approach to control resistance to MVC.
METHODS
Cell lines, antibodies and inhibitors
293T cells were cultured in DMEM supplemented with 10% FBS, 100 μg/ml of penicillin and streptomycin, and 0.5 mg/ml of geneticin. JC-6, -10, -20, -57 and -53 cells, derived from HeLa cells and stably expressing CD4 and different CCR5 densities (Platt et al., 1998), were cultured in DMEM supplemented with 10% FBS plus 100 μg/ml penicillin/streptomycin. Maraviroc and T20 were obtained through the NIH AIDS Research and Reference Reagent Program (Germantown, MD). CD4 mAb Q4120 was obtained through the National Institute for Biological Standards and Control (NIBSC, Potters Bar, UK) (Healey et al., 1990). CCR5 antibodies 2D7 and 45523 were purchased from BD Biosciences (San Jose, CA) and R&D Systems (Minneapolis, MN), respectively. CCR5 mAb ROAb14 was a gift from Roche (Palo Alto, CA), and HGS004 and HGS101 were gifts from Human Genome Sciences (Rockville, MD).
Single-cycle HIV-1 entry assay
Replication-defective HIV-1 reporter viruses were produced from 2×106 293T cells transfected with 10 μg of pNL4.3-env –-luc3 and 10 μg of pCI-Env-expressing plasmid (MVCsens or MVCres HIV-1 Env) using calcium phosphate. MVCsens and MVCres HIV-1 Envs, described previously (Westby et al., 2007), correspond to Env genes of primary isolate CC1/85 passaged in PBMCs in the absence and presence of MVC. The MVCsens HIV-1 Env sequence has amino acids 316A, 319A and 323I in V3; whereas MVCres HIV-1 Env contains substitutions 316T, 319A and 323V in V3, which confer resistance to MVC (Westby et al., 2007). Pseudoviruses were collected 48 h after transfection, debris removed by centrifugation and filtration through a 0.45 μm syringe filter, and virus quantified by p24 ELISA. For infection, JC cells were plated in 96 well plates at 8×103 cells/well for 2 days. One hour before infection cells were left untreated or treated with MVC at the indicated concentrations. In experiments evaluating infection by MVC-bound CCR5, cells were pretreated with 10 μM MVC. In some experiments, after MVC pretreatment, cells were incubated for an additional hour with varying dilutions of inhibitor (CCR5 mAbs, CD4 mAb Q4120, or T20). Cells were infected by spinoculation at 1,200×g for 1 h at 4°C using 5 ng p24 (O'Doherty, Swiggard, and Malim, 2000; Platt, Durnin, and Kabat, 2005). Three days later cells were lysed, luciferase activity measured as relative luciferase units (RLU) using the Luciferase Assay System (Promega, Madison, WI), and percentage virus infection calculated as (RLU with inhibitor)/(RLU without inhibitor) × 100. Inhibition data from replicates were plotted using GraphPad Prism software and EC50 values determined using variable slope non-linear regression analysis.
Time of inhibitor addition experiments
Pseudoviruses bearing MVCres or MVCsen HIV-1 Env were spinoculated onto JC6 cells (10 ng p24/0.2×106 cells) at 1,600×g for 1 h at 4°C. Plates were placed in a 37°C culture incubator to allow viral entry to proceed. At transfer to 37°C (time 0) and at subsequent time points (10, 20, 30, 40, 60, 90 and 120 min), fully inhibitory concentrations of 2D7 (50 μg/ml) or T20 (10 μM) were added to inhibit CCR5-dependent steps or viral fusion, respectively. Plates were incubated for 3 days, cells lysed and luciferase activity determined. Relative infectivity values were obtained by dividing luciferase activity (RLU) from a given time point by the activity obtained at the last time point (120 min). The infection kinetics data were analyzed by plotting relative infectivity vs. time of addition of inhibitor, and t1/2 values calculated by fitting the data to one-phase exponential association curve using GraphPad Prism.
Infection of PBMCs with replication-competent HIV-1
PBMCs activated for three days with PHA (2.5 μg/ml) were infected with previously described replication-competent chimeric viruses carrying the MVCsens and MVCres Env genes of HIV-1 CC1/85 in a NL4-3 backbone (Westby et al., 2007). In some experiments, CD8 T cells were depleted with Dynal magnetic beads (Invitrogen, Carslbad, CA). Prior to infection, cells were incubated with and without 10 μM MVC for 1 h and, in some experiments, followed by 1 h incubation with CCR5 antibodies. Cells were infected using a multiplicity of infection (MOI) of 0.001, washed and cultured in medium containing IL-2 (100 U/ml) and inhibitors. On day 3, half of the medium was replaced with fresh medium containing inhibitors at the same concentrations as before. Virus replication was evaluated by measuring p24 levels in supernatants on day 7 and EC50 values determined.
Measurement of CCR5 by Flow Cytometry Analysis
PBMCs were cultured for 10 days in complete RPMI culture medium supplemented with 100 U/ml rhIL-2 (Roche, Indianapolis, IN) to upregulate CCR5 expression (Bleul et al., 1997). CCR5 staining was done basically as described (Lalezari et al., 2008; Olson et al., 1999; Wu et al., 1997). Briefly, cells were washed with PBS, incubated with FACS staining buffer (PBS, 10% horse serum, 2% human serum, 0.01% sodium azide), followed by incubation with saturating concentrations of unconjugated 2D7, HGS004 or HGS101 (10 μg/106 cells, as determined in saturation binding optimization experiments). Non-specific binding was detected with 5 μg of mouse IgG2a (Sigma, Saint Louis, MO) for 2D7, and with 5 μg of human IgG4 (Sigma, Saint Louis, MO) for HGS004 or HGS101. 2D7 staining was detected with phycoerythrin (PE)-labeled rat anti-mouse IgG2a (Becton Dickinson, San Jose, CA), while HGS004 and HGS101 were detected with PE-labeled mouse anti-human IgG4 (SouthernBiotech, Birmingham, AL). Lymphocyte subsets were detected using combinations of fluorochrome labeled antibodies directed to CD3, CD4 and CD8 (Becton Dickinson). All incubation steps were done at room temperature for 30 min. Data were collected in a Becton Dickinson FACSCalibur flow cytometeter using CellQuest software and analyzed by FlowJo (TreeStar, Ashland, OR).
RESULTS
MVCres HIV-1 infection in the presence of MVC is inefficient at low CCR5 density
We evaluated MVC inhibition of reporter viruses pseudotyped with Env of MVCsens- and MVCres HIV-1 CC1/85 in a panel of JC clones expressing constant CD4 density (~105 molecules/cell), but differing CCR5 densities (Fig. 1). The panel includes clones expressing levels of CCR5 similar to those in primary CD4+ T cells, typically ranging from ~2×103 to ~15×103 CCR5 molecules/CD4+ T cell (Hladik et al., 2005; Lee et al., 1999b; Reynes et al., 2000). MVCres HIV-1 CC1/85 uses both MVC-bound CCR5 and free CCR5 as coreceptor (Westby et al., 2007). As expected, MVC fully inhibited MVCsens but not MVCres pseudovirus infection, with resistance manifested by plateaus in maximum percentage of inhibition (MPI) of less than 100% (Pugach et al., 2007; Westby et al., 2007). However, MPI levels increased at reduced CCR5 densities. MPI levels in JC53 (130×103 CCR5/cell), JC6 (27×103 CCR5/cell), and JC57 (9×103 CCR5/cell) were 60, 90, and 95%, respectively. In JC10 (2×103 CCR5/cell) and JC20 (0.7×103 CCR5/cell), MVCres HIV-1 Env pseudovirus was fully inhibited in the presence of high MVC concentrations. These results demonstrate a lower efficiency of MVCres HIV-1 Env pseudovirus using MVC-bound CCR5 compared to MVC-free CCR5.
Fig. 1. MVCres HIV-1 infection in the presence of MVC is inefficient at low CCR5 density.
HIV-1 reporter virus pseudotyped with the Env of MVCsens HIV-1 (a) or MVCres HIV-1 (b) were used to infect JC clones with same CD4 but different CCR5 densities, in the presence of varying MVC concentrations. Left panels: Infectivity was determined by measuring luciferase activity in cell lysates on day 3 after infection. In control experiments using uninfected cells or infected cells treated with 10 μM T-20 (a fully inhibitory concentration) luciferase activities ranged between 20 and 40 RLU (not shown). Right panels: Infectivity data in each cell clone were normalized to infectivity values obtained in the absence of drug and percentages of MVC inhibition determined (right panels). Data points are mean ± S.D. of at least 2 replicates and are from one representative experiment of three.
MVCres HIV-1 infection of cell lines via MVC-bound CCR5 is more sensitive to CCR5 mAb 2D7 than infection via free CCR5
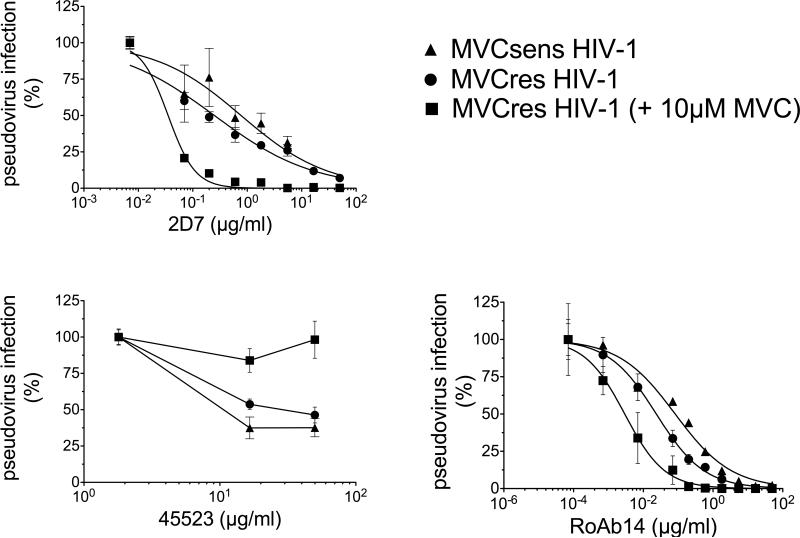
Less efficient use of MVC-bound CCR5 by MVCres HIV-1 could be due to a lower gp120 affinity for bound compared to free CCR5. To test this, we evaluated CCR5 mAb 2D7 inhibition of MVCsens and MVCres HIV-1 infection of JC6 cells in the presence and absence of 10 μM MVC, a saturating drug concentration. 2D7 competes with HIV-1 gp120 for binding to the ECL2 of CCR5 and has similar affinities for MVC-free and MVC-bound CCR5 (Ji et al., 2007b; Maeda et al., 2008; Wu et al., 1997). In the absence of MVC, 2D7 inhibited MVCsens- and MVCres HIV-1 Env pseudotypes with EC50 values of 0.77 and 0.25 μg/ml, respectively (Fig. 2a). In the presence of MVC, MVCsens Env pseudotype was completely inhibited and inhibition was not affected by addition of 2D7, whereas MVCres Env pseudotype became considerably more sensitive to 2D7 (2D7 EC50 = 0.03 μg/ml) when MVC was added. The order of addition of 2D7 and MVC had no effect on antiviral activity (not shown). In a control experiment using mAb 45523, which binds to a multidomain epitope of CCR5 and competes with MVC (Ji et al., 2007b; Maeda et al., 2004), MVCres HIV-1 Env pseudovirus infection was inhibited (albeit modestly) in the absence, but not in the presence, of MVC (Fig. 2b). We confirmed the 2D7 inhibition results using CCR5 mAb ROAb14, which recognizes a region overlapping the 2D7 epitope and, as with 2D7, is unaffected by MVC (Ji et al., 2007b). Similarly to 2D7, ROAb14 inhibited MVCres HIV-1 more potently in the presence of MVC. The ROAb14 EC50 values were 0.08 μg/ml for MVCsens-, 0.02 μg/ml for MVCres-, and only 0.003 μg/ml for MVCres HIV-1 in the presence of MVC (Fig. 2c). Thus, MVCres HIV-1 infection of cell lines through MVC-bound CCR5 is more sensitive to anti-ECL2 CCR5 mAb inhibition than is infection through MVC-free CCR5.
Fig. 2. CCR5 mAb2D7 inhibits MVCres HIV-1 infection of JC6 cells more potently in the presence than in the absence of MVC.
JC6 cells were incubated with and without 10 μM MVC for 1 h, followed by incubation with serial dilutions of CCR5 antibodies 2D7 (a), 45523 (b) or ROAb14 (c) for an additional hour, and then infected with HIV-1 pseudovirus bearing the indicated Env. Infectivity was determined by measuring luciferase activity on day 3, and data normalized to infectivity in the absence of mAb. Data points are mean ± S.D. of at least 2 replicates and are from one representative experiment of four (2D7), two (45523) and three (ROAb14).
MVCres HIV-1 infection via MVC-bound CCR5 exhibits delayed kinetics of coreceptor engagement and fusion
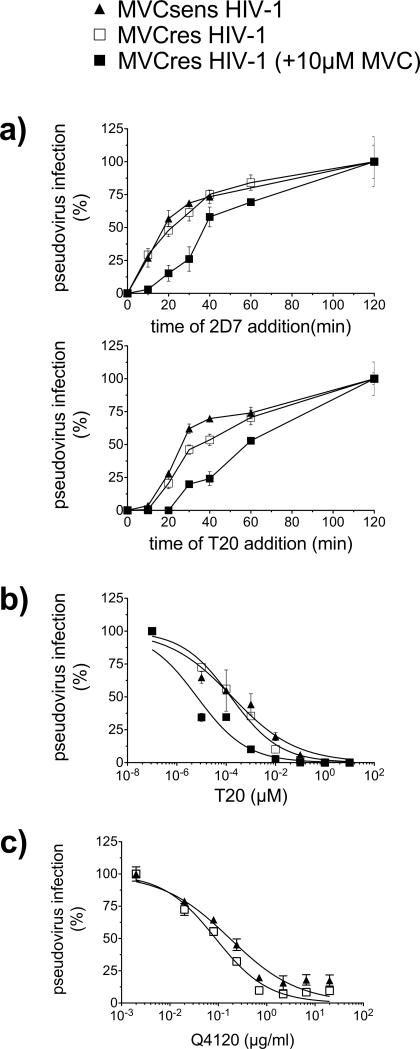
Because the binding efficiency of HIV-1 Env to CCR5 impacts entry kinetics and sensitivity to the fusion inhibitor T20 (Platt, Durnin, and Kabat, 2005; Reeves et al., 2002), we evaluated the kinetics of CCR5 engagement and fusion during pseudovirus infection of JC6 in the presence and absence of 10 μM MVC. This was accomplished using time-of-inhibitor-addition experiments, in which fully inhibitory concentrations of 2D7 or T20 were added at intervals following a synchronized infection (Fig. 3a). The time required for 2D7 half maximal inhibition (t1/2) of MVCsens- and MVCres- HIV-1 entry in the absence of MVC was 19 and 22 min, respectively. In contrast, under MVC-bound CCR5 conditions, MVCres HIV-1 remained sensitive to 2D7 for a longer time, with a 2D7 t½ of 41 min. The T20 t1/2 values of MVCsens and MVCres without MVC were 29 and 38 min, respectively. MVCres HIV-1 had an increased T20 t½ of 59 min for MVC-bound CCR5 infection. Together, the slower kinetics of MVCres HIV-1 engagement of MVC-bound CCR5 compared to that of MVC-free CCR5 are consistent with its higher sensitivity to 2D7 in the presence of MVC (Fig. 2a), suggesting that a slower engagement of MVC-bound CCR5 prolongs the opportunity window for T20 inhibition. We confirmed that MVCres HIV-1 infection via MVC-bound CCR5 is more sensitive to T20 by determining T20 EC50s in the presence and absence of 10 μM MVC (Fig. 3b). In the absence of MVC, T20 EC50s for MVCsens and MVCres HIV-1 were 0.17 and 0.15 nM, respectively. In the presence of MVC, however, T20 inhibition of MVCres HIV-1 was considerably more potent (EC50 = 0.006 nM). To determine whether the kinetics of viral entry were impacted by different efficiencies of CD4 binding, we evaluated sensitivity to a CD4 mAb, Q4120, which recognizes the N-terminal region of CD4 and blocks gp120 binding (Healey et al., 1990). Both MVCsens and MVCres HIV-1 Env pseudovirus were similarly sensitive to Q4120, with EC50s of 0.17 and 0.09 μg/ml, respectively, suggesting similar efficiencies in CD4 binding (Fig. 3c).
Fig. 3. MVCres HIV-1 infection of JC6 cells in the presence of MVC exhibits delayed kinetics of coreceptor engagement and fusion.
(a) Kinetic analysis of CCR5 engagement (upper panel) and fusion (lower panel) of pseudovirus bearing MVCsen and MVCres HIV-1 Env during infection of JC6 cells (with and without 10 μM MVC pretreatment). Pseudovirus particles were spinoculated onto cells at 4°C, cells washed and transferred to 37°C (time 0). At the indicated time points, inhibitors (50 μg/ml 2D7 or 10 μM T20) were added. Luciferase activity was measured on day 3 and data normalized to activity obtained at 120 min. (b,c) Sensitivity of pseudovirus bearing MVCsen and MVCres HIV-1 Env to T20 (b) and CD4 mAb Q4120 (c) during infection of JC6 cells. T20 was added immediately after virus spinoculation, whereas Q4120 was added 1 h before spinoculation. Data (means ± S.D.) are from 3 independent experiments for (a), and from 2 experiments for each (b) and (c).
CCR5 mAbs HGS004 and HGS101, but not 2D7 or ROAb14, preferentially inhibit MVC-bound CCR5 infection of MVCres HIV-1 in PBMCs
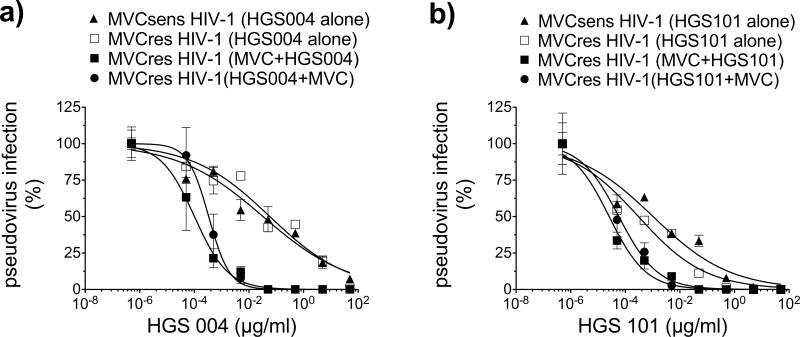
Enhanced inhibition of MVCres HIV-1 infection in the presence of MVC by mAbs targeting CCR5 ECL2, such as 2D7 and ROAb14, suggested a potential approach to control resistance to MVC. We evaluated and compared the antiviral activities of the CCR5 ECL2 mAbs 2D7, ROAb14, HGS004 and HGS101 (a second-generation derivative of HGS004 with improved antiviral activity (Lalezari et al., 2008)) against MVCres HIV-1 in cell lines and PBMCs. In JC6 cells, similarly to 2D7 and ROAb14, HGS004 and HGS101 inhibited MVCres HIV-1 Env pseudovirus infection more potently under MVC-bound CCR5 conditions. Moreover, the antiviral effect was not affected by the order of addition of mAb and MVC, as expected from their independent antiviral mechanisms (Fig. 4). HGS004 EC50s were 0.03 μg/ml for MVCsens, 0.05 μg/ml for MVCres, and 0.0001 μg/ml for MVCres HIV-1 in the presence of MVC. HGS101 gave a similar pattern of inhibition, with EC50s of 0.001 μg/ml for MVCsens, 0.0003 μg/ml for MVCres, and 0.00002 μg/ml for MVCres HIV-1 in the presence of MVC.
Fig. 4. Inhibition of MVCres HIV-1 infection of JC6 cells by CCR5 mAbs HGS004 and HGS101.
JC6 cells were treated with varying concentrations of antibodies HGS004 (a) or HGS101 (b) for 1h under the following conditions: mAb alone, mAb added after 1h preincubation with 10 μM MVC, or mAb added before incubating cells with 10 μM MVC for 1h. Cells were then infected with pseudovirus bearing the indicated Env. Luciferase activity was measured on day 3. For each treatment, data were normalized to luciferase signal in the absence of mAb. Data points are mean ± S.D. of at least 2 replicates and are from one representative experiment of two.
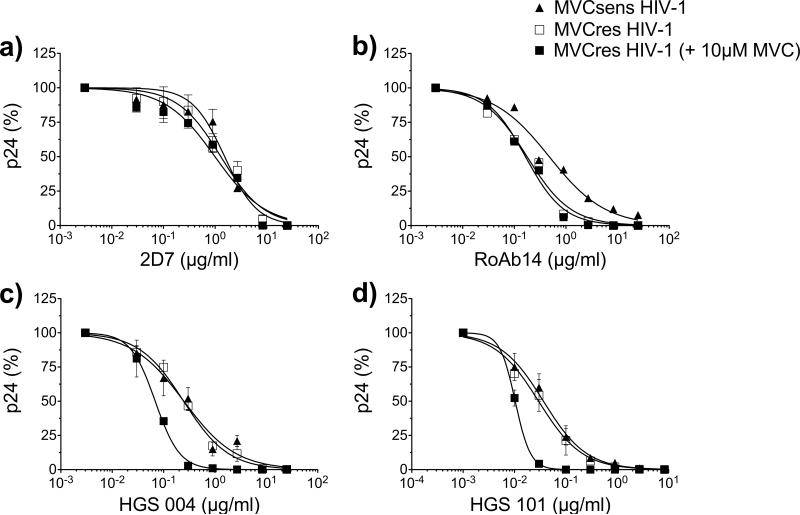
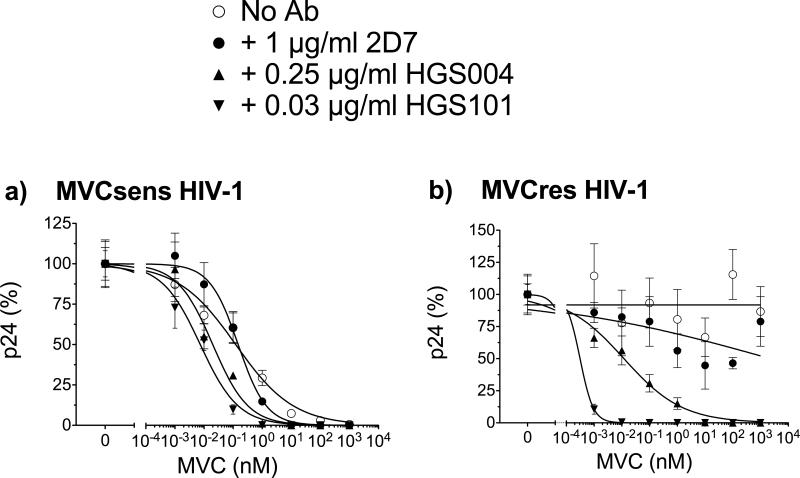
Similar to results with JC6, both 2D7 and ROAb14 inhibited replication-competent MVCsens HIV-1 in PBMCs (EC50s of 1.53 and 0.47 μg/ml for 2D7 and ROAb14, respectively) (Fig. 5). The addition of 10 μM MVC fully inhibited MVCsens HIV-1 with and without mAb (not shown). However, the increased sensitivity of MVCres HIV-1 to 2D7 and ROAb14 antibodies in MVC-treated JC6 cells was not manifested in PBMCs. In PBMCs, 2D7 and ROAb14 inhibited MVCres HV-1 similarly in the absence and presence of 10 μM MVC (2D7 EC50 of 1.29 vs. 1.0 μg/ml in MVC treatment; ROAb14 EC50 of 0.17 vs. 0.16 μg/ml in MVC treatment) (Fig. 5a,b). This was in clear contrast to CCR5 mAbs HGS004 and HGS101. In PBMCs, HGS004 EC50 values were 0.25 μg/ml for MVCsens HIV-1, 0.24 μg/ml for MVCres HIV-1 without MVC and 0.06 μg/ml for MVCres HIV-1 with MVC, respectively (Fig. 5c). Of more significant potential clinical relevance, even greater increases in antiviral potency were observed at EC90 concentrations (HGS004 EC90s of 3 μg/ml for MVCsens, 3 μg/ml for MVCres, and only 0.3 μg/ml for MVCres HIV-1 in the presence of MVC). For HGS101, EC50s were 0.03 for MVCsens, 0.03 μg/ml for MVCres, and 0.01 μg/ml for MVCres HIV-1 in the presence of MVC (Fig. 5d). Again, increased potency was observed at EC90 concentrations, with HGS101 EC90s of 0.4 μg/ml for MVCsens, 0.4 μg/ml for MVCres, and only 0.03 for MVCres HIV-1 in the presence of MVC. These data demonstrate that CCR5 mAbs HGS004 and HGS101, unlike 2D7 and ROAb14, have increased potency against MVCres HIV-1 infection via MVC-bound CCR5 with primary cells.
Fig. 5. CCR5 mAbs HGS004 and HGS101, but not 2D7 or ROAb14, preferentially inhibit MVC-bound CCR5 infection of MVCres HIV-1 in PBMCs.
PHA activated PBMCs were incubated with a without 10 μM MVC for 1h, followed by incubation with varying dilutions of 2D7 (a), ROAb14 (b), HGS004 (c) or HGS101 (d) for an additional hour. Cells were infected with replication-competent, chimeric NL4-3 viruses carrying MVCsens or MVCres HIV-1 Env for 3 h at a MOI of 0.001. Infected cells were cultured in the presence of inhibitors at the same concentrations as before. Data are p24 levels on day 7, normalized to p24 levels in the absence of mAb. In a,c and d, data are means ± S.D. of 2 independent experiments using PBMCs from 2 different donors and are representative of 4 experiments. Data in b are single data points from one experiment representative of 3, each with a different donor.
CCR5 mAbs HGS004 and HGS101, but not 2D7 or ROAb14, restore MVC inhibition of MVCres HIV-1 in PBMCs
In a complementary approach, we tested each mAb at a concentration approximating its EC50 in combination with serial dilutions of MVC (Fig. 6). As expected, MVC fully inhibited MVCsens HIV-1 (MVC EC50 = 0.12 nM; 95% CI: 0.06-0.25). However, the MVC EC50 decreased to 0.02 nM (95% CI: 0.01-0.03) with HGS004 and to 0.007 nM (95% CI: 0.004-0.012) with HGS101, but not with 2D7 (MVC EC50 = 0.14 nM; 95% CI: 0.10-0.20) (Fig. 6a). MVCres HIV-1 was not inhibited by MVC and gave a flat inhibition curve (Fig. 6b), as previously reported for antagonist-resistant HIV-1 infection of PBMCs (Pugach et al., 2007; Westby et al., 2007). 2D7 activity against MVCres HIV-1, normalized to 2D7 inhibition in the absence of MVC, was not affected by MVC. In contrast, MVC regained antiviral activity against MVCres HIV-1 when combined with HGS004 (MVC EC50 = 0.01 nM; 95% CI: 0.005-0.024) or with HGS101 (potent viral inhibition at low MVC concentrations precluded MVC EC50 determinations) (Fig. 6b). Antibody ROAb14 gave similar results to those with 2D7 (supplementary data, Fig. S1). Thus, the combinations of MVC and HGS004/HGS101 differ from those of MVC and 2D7/ROAb14 in that only the former restored MVC activity against MVCres HIV-1. Because the evaluated mAbs can interfere with the binding of β-chemokines to CCR5 (Ji et al., 2007a; Ji et al., 2007b; Lalezari et al., 2008; Olson et al., 1999; Wu et al., 1997), we compared the antiviral activities of 2D7 and MVC versus HGS004 and MVC in CD8-depleted PBMCs (Fig. S2). Results were similar to those with total PBMCs (Fig. 6), suggesting that β-chemokines do not account for the observed antiviral differences between combinations of mAbs and MVC.
Fig. 6. CCR5 mAbs HGS004 and HGS101, but not 2D7, sensitize MVCres HIV-1 to MVC in PBMCs.
PHA-activated PBMCs were incubated with 10-fold serial dilutions of MVC for 1 h, followed by incubation with the indicated concentration of each CCR5 mAb for an additional hour. Cells were infected with replication-competent MVCsens or MVCres HIV-1 for 3 h at a MOI of 0.001. Infected cells were cultured in the presence of inhibitors at the same concentrations as before. Data are p24 levels on day 7, normalized to p24 levels in the absence of MVC. Experimental p24 levels (ng/ml) in the absence of MVC were as follows: 273 ± 38 (no Ab), 142 ± 21 (2D7), 103 ± 11 (HGS004), and 173 ± 14 (HGS101) for MVCsens HIV-1; 180 ± 26 (no Ab), 114 ± 18 (2D7), 113 ± 11 (HGS004), and 103 ± 8 (HGS101) for MVCres HIV-1. Data (means ± S.D.) are from one representative experiment of 2, with a different donor in each experiment.
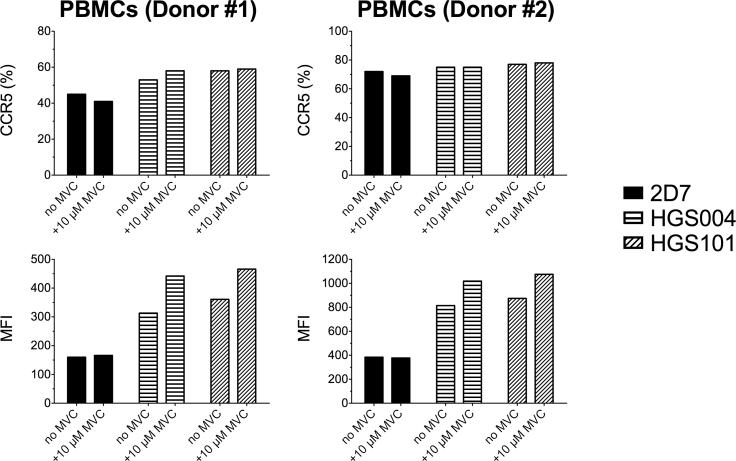
Binding of HGS004 and HGS101, but not 2D7, to CCR5 is higher in the presence than in the absence of MVC in primary cells
The different patterns of viral inhibition between 2D7 (and ROAb14) and the HGS mAbs could reflect differences in binding to free and MVC-bound CCR5. We evaluated mAb binding, with and without MVC, in PBMCs stimulated with IL-2 for 10 days, culture conditions that upregulate CCR5 expression (Bleul et al., 1997). At saturating concentrations (10 μg/106 cells as determined in optimization experiments), both 2D7 and the HGS mAbs stained similar percentages of CCR5+ CD4+ T cells (Fig. 7, upper panels). However, the MFI with 2D7 did not change in the presence of MVC, whereas those with HGS004 and HGS101 were higher in the presence of MVC, suggesting that the latter mAbs recognize a greater proportion of surface CCR5 when CCR5 is bound to MVC (Fig. 7, lower panels).
Fig. 7. Flow cytometry analysis of 2D7, HGS004 and HGS101 binding to MVC-bound and free CCR5 on CD4+ T cells.
10-day IL-2 stimulated PBMCs (1×106) were treated with and without 10 μM MVC, incubated with 10 μg of unconjugated CCR5 antibody or isotype, and stained with the corresponding secondary detection antibody. Lymphocyte subsets were identified using fluorochrome-labeled antibodies for detection of CD3, CD4 and CD8. CCR5 detection in the CD4+ T cell subset was analyzed by comparing percentages of CCR5 positive cells (upper panels) or Mean Fluorescence Intensities (MFI) of the CCR5+ gated populations (lower panels). Representative data from two different donors are shown.
DISCUSSION
MVC resistant variants often emerge during treatment with CCR5 antagonists (Cooper et al.; Gulick et al., 2008; Gulick et al., 2007). Generally, resistant variants remain CCR5 tropic but use both antagonist-free and antagonist-bound CCR5 (Pugach et al., 2007; Westby et al., 2007). In CCR5-expressing cell lines, resistance is manifested by incomplete dose response curves with MPI levels that reflect the relative efficiency with which the virus utilizes antagonist-bound versus antagonist-free CCR5 (Pugach et al., 2007; Westby et al., 2007). In this study, we demonstrate that viruses pseudotyped with the Env of MVCres HIV-1 are inhibited by MVC at low CCR5 densities, suggesting a reduced viral affinity for MVC-bound CCR5. Antibody 2D7, which targets CCR5 ECL2 and inhibits gp120 binding, had higher activity against infection of cell lines by MVCres HIV-1 via MVC-bound CCR5 compared to that using free CCR5. Consistent with these results, the kinetics of CCR5 engagement and viral fusion were clearly slower for infection mediated by MVC-bound CCR5. We confirmed the 2D7 results with CCR5 mAb ROAb14, whose epitope overlaps that of 2D7 and which also blocks gp120 binding (Ji et al., 2007b). Similar patterns of inhibition were also observed with mAb HGS004 and its HGS101 derivative.
In contrast, in primary PBMCs, HGS004 and HGS101 but not 2D7 or ROAb14, inhibited MVCres HIV-1 with greater potency (~10-fold reduction in EC90s) in MVC-bound than in free CCR5 infection (Fig. 5). In combination with HGS004 or HGS101, MVC became active against resistant HIV-1 and inhibited sensitive HIV-1 more potently (Fig. 6). These dissimilarities in antiviral activity between 2D7 and ROAb14 versus HGS004 and HGS101 in diverse cell types support the notion of CCR5 having multiple forms (e.g., conformation, covalent modifications, sulfation differences), varying among cell types and with different affinity for antagonist and potential to support viral entry in the free and bound forms (Anastassopoulou et al., 2009; Hill et al., 1998; Lee et al., 1999a; Olson et al., 1999). Our data suggest that HGS004 and HGS101 interfere with the ability of CCR5 in either free or antagonist-bound conformations to serve as coreceptors for MVC resistant HIV-1 in both primary cells and cell lines.
The binding sites of all four mAbs map to the ECL2 of CCR5. 2D7 and ROAb14 have overlapping epitopes (Zhang et al., 2007), which differ from those of HGS004 and HGS101 (Lalezari et al., 2008) (Thi Migone, Personal communication). That both 2D7 and ROAb14 inhibit resistant HIV-1 with similar potencies in the presence and absence of MVC in PBMCs probably reflects comparable affinities of MVCres HIV-1 Env for free and antagonist bound CCR5 in these cells, as manifested by flat inhibition curves (Fig. 6b) (Pugach et al., 2007; Westby et al., 2007). A greater inhibition of MVC-bound than of free CCR5 infection in PBMCs by HGS004/HGS101 suggests that their epitopes remain somewhat exposed on MVC-bound CCR5. Consistent with this, both HGS mAbs, but not 2D7, gave higher mean fluorescence intensities (MFI) with MVC-bound CCR5 versus free CCR5 on CD4+ T cells. Although we have not yet determined antibody affinities or off-rates, the data suggest that the HGS mAbs recognize a greater proportion of CCR5 molecules when these are occupied by MVC than does 2D7. As the formation of a fusion pore by R5 HIV-1 likely requires the engagement of several CCR5 molecules (about 4 to 6) (Kuhmann et al., 2000; Sougrat et al., 2007), we hypothesize that a greater proportion of occupied CCR5 molecules may prevent effective engagement of sufficient CCR5s by sensitive or MVC-resistant viruses. Because MVC-resistant viruses require higher coreceptor densities when using the MVC-bound form (Fig. 1)(Heredia et al., 2008; Pugach et al., 2009), an increase in antibody-bound CCR5 molecules could further prevent fusion pore formation.
Another possible interpretation is that the HGS mAbs disrupt conformational changes in CCR5 required for viral entry, especially by resistant virus and antagonist-bound CCR5. Viruses resistant to CCR5 antagonists often become more dependent on interactions with the N-terminal region (Berro et al., 2009; Laakso et al., 2007; Lin et al., 2007; Nolan et al., 2009; Pfaff et al., 2010), with the ECL2 region (Sterjovski et al., 2010), or with both the N-terminal and ECL2 regions of CCR5 (Agrawal-Gamse et al., 2009; Ogert et al., 2010; Tilton et al., 2010). We are currently investigating the dependence of MVC resistant viruses upon the N-terminal and ECL2 regions.
In summary, HGS004 and HGS101, but not the other mAbs tested, were more potent against MVCres HIV-1 in the presence than in the absence of MVC and restored MVC inhibition of resistant HIV-1 in PBMCs. In addition, both HGS004 and HGS101 enhanced MVC inhibition of MVCsens HIV-1. In a monotherapy clinical trial of HGS004, treatment was safe but HIV-1 RNA was reduced by > 1 log10 in only 54% of patients (Lalezari et al., 2008). Retrospectively, it was found that sensitivity of the patients’ viruses to HGS004 predicted antiviral responses (Lalezari et al., 2008). Although HGS004 is currently in clinical development for treating autoimmune disorders, our results suggest that combinations of MVC and HGS004 could potentially enhance antiviral responses and control MVC-resistant viruses in patients with R5 HIV-1. Even better antiviral responses might be achieved by combining MVC and HGS101, a more potent, second-generation derivative of HGS004.
Supplementary Material
Fig. S1. Inhibition of MVCsens and MVCres HIV-1 by the combination of MVC and ROAb14 in PBMCs. PHA-activated PBMCs were incubated with serial dilutions of MVC for 1 h, followed by incubation with the indicated concentration of CCR5 mAb ROAb14 for an additional hour. Cells were infected with replication-competent MVCsens or MVCres HIV-1 for 3 h at a MOI of 0.001. Infected cells were cultured in the presence of inhibitors at the same concentrations as before. Data are p24 levels on day 7, normalized to p24 levels in the absence of MVC. Experimental p24 levels (ng/ml) in the absence of MVC were as follows: 470 ± 64 (no Ab), 200 ± 38 (ROAb14) for MVCsens HIV-1; and 376 ± 59 (no Ab) and 229 ± 38 (ROAb14) for MVCres HIV-1. Data (means ± S.D.) are from one representative experiment of 2, with a different donor in each experiment.
Fig. S2. CCR5 mAb HGS004, but not 2D7, sensitizes MVCres HIV-1 to MVC in CD8-depleted PBMCs. CD8-depleted PBMCs were activated with PHA for 3 days, incubated with 10-fold serial dilutions of MVC for 1 h, followed by incubation with the indicated concentration of each CCR5 mAb for an additional hour. Cells were infected with replication-competent MVCsens or MVCres HIV-1 for 3 h at a MOI of 0.001. Infected cells were cultured in the presence of inhibitors at the same concentrations as before. Data are p24 levels on day 7, normalized to p24 levels in the absence of MVC. Experimental p24 levels (ng/ml) in the absence of MVC were as follows: 554 ± 74 (no Ab), 395 ± 49 (2D7) and 406 ± 62 (HGS004) for MVCsens HIV-1; 479 ± 60 (no Ab), 294 ± 42 (2D7) and 221 ± 36 (HGS004) for MVCres HIV-1. Data (means ± S.D. of duplicates) are from one single experiment.
Acknowledgements
We thank David Kabat (Oregon Health and Science University, Portland, OR) for providing JC cells, Nathaniel Landau (University of New York School of Medicine, New York, NY) for plasmid constructs, Changhua Ji (Roche, Palo Alto, CA) for mAb ROAb14, Thi Migone (Human Genome Sciences, Rockville, MD) for HGS004/101, and Becky Jubb and Hernan Valdez (Pfizer, Sandwich, United Kingdom) for viruses. We also thank Becky Jubb and Robert C. Gallo for critical review of the manuscript.
This project was funded by NIH NIAID grant AI084417.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- FDA notifications. Maraviroc approved as a CCR5 co-receptor antagonist. AIDS Alert. 2007;22(9):103. [PubMed] [Google Scholar]
- FDA Approves Expanded Use of Selzentry® for Appropriate Patients Starting HIV Antiretroviral Therapy for the First Time. 2009 Availalble at: http://www.viivhealthcare.com/media-room/press-releases/2009-11-20.aspx.
- Agrawal-Gamse C, Lee FH, Haggarty B, Jordan AP, Yi Y, Lee B, Collman RG, Hoxie JA, Doms RW, Laakso MM. Adaptive mutations in a human immunodeficiency virus type 1 envelope protein with a truncated V3 loop restore function by improving interactions with CD4. J Virol. 2009;83(21):11005–15. doi: 10.1128/JVI.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Anastassopoulou CG, Ketas TJ, Klasse PJ, Moore JP. Resistance to CCR5 inhibitors caused by sequence changes in the fusion peptide of HIV-1 gp41. Proc Natl Acad Sci U S A. 2009;106(13):5318–23. doi: 10.1073/pnas.0811713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro R, Sanders RW, Lu M, Klasse PJ, Moore JP. Two HIV-1 variants resistant to small molecule CCR5 inhibitors differ in how they use CCR5 for entry. PLoS Pathog. 2009;5(8):e1000548. doi: 10.1371/journal.ppat.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94(5):1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85(7):1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Cooper DA, Heera J, Goodrich J, Tawadrous M, Saag M, Dejesus E, Clumeck N, Walmsley S, Ting N, Coakley E, Reeves JD, Reyes-Teran G, Westby M, Van Der Ryst E, Ive P, Mohapi L, Mingrone H, Horban A, Hackman F, Sullivan J, Mayer H. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis. 2010;201(6):803–13. doi: 10.1086/650697. [DOI] [PubMed] [Google Scholar]
- Currier J, Lazzarin A, Sloan L, Clumeck N, Slims J, McCarty D, Steel H, Kleim JP, Bonny T, Millard J. Antiviral activity and safety of aplaviroc with lamivudine/zidovudine in HIV-infected, therapy-naive patients: the ASCENT (CCR102881) study. Antivir Ther. 2008;13(2):297–306. [PubMed] [Google Scholar]
- Demarest JF, Amrine-Madsen H, Irlbeck DM, Kitrinos KM. Virologic failure in first-line human immunodeficiency virus therapy with a CCR5 entry inhibitor, aplaviroc, plus a fixed-dose combination of lamivudine-zidovudine: nucleoside reverse transcriptase inhibitor resistance regardless of envelope tropism. Antimicrob Agents Chemother. 2009;53(3):1116–23. doi: 10.1128/AAC.01055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381(6584):661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, Nadler J, Clotet B, Karlsson A, Wohlfeiler M, Montana JB, McHale M, Sullivan J, Ridgway C, Felstead S, Dunne MW, van der Ryst E, Mayer H. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359(14):1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick RM, Su Z, Flexner C, Hughes MD, Skolnik PR, Wilkin TJ, Gross R, Krambrink A, Coakley E, Greaves WL, Zolopa A, Reichman R, Godfrey C, Hirsch M, Kuritzkes DR. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196(2):304–12. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- Healey D, Dianda L, Moore JP, McDougal JS, Moore MJ, Estess P, Buck D, Kwong PD, Beverley PC, Sattentau QJ. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990;172(4):1233–42. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia A, Latinovic O, Gallo RC, Melikyan GB, Reitz M, N L, Redfield R. Reduction of CCR5 with low-dose Rapamycin enhances the antiviral activity of Vicriviroc against both sensitive and drug-resistant HIV-1. Proc Natl Acad Sci U S A. 2008;105(51):20476–20481. doi: 10.1073/pnas.0810843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Kwon D, Jones M, Davis CB, Marmon S, Daugherty BL, DeMartino JA, Springer MS, Unutmaz D, Littman DR. The amino terminus of human CCR5 is required for its function as a receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology. 1998;248(2):357–71. doi: 10.1006/viro.1998.9283. [DOI] [PubMed] [Google Scholar]
- Hladik F, Liu H, Speelmon E, Livingston-Rosanoff D, Wilson S, Sakchalathorn P, Hwangbo Y, Greene B, Zhu T, McElrath MJ. Combined effect of CCR5-Delta32 heterozygosity and the CCR5 promoter polymorphism -2459 A/G on CCR5 expression and resistance to human immunodeficiency virus type 1 transmission. J Virol. 2005;79(18):11677–84. doi: 10.1128/JVI.79.18.11677-11684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JM, Lalezari JP, Thompson MA, Fichtenbaum CJ, Saag MS, Zingman BS, D'Ambrosio P, Stambler N, Rotshteyn Y, Marozsan AJ, Maddon PJ, Morris SA, Olson WC. Phase 2a Study of the CCR5 Monoclonal Antibody PRO 140 Administered Intravenously to HIV-infected Adults. Antimicrob Agents Chemother. 2010a doi: 10.1128/AAC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JM, Saag MS, Thompson MA, Fischl MA, Liporace R, Reichman RC, Redfield RR, Fichtenbaum CJ, Zingman BS, Patel MC, Murga JD, Pemrick SM, D'Ambrosio P, Michael M, Kroger H, Ly H, Rotshteyn Y, Buice R, Morris SA, Stavola JJ, Maddon PJ, Kremer AB, Olson WC. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis. 2008;198(9):1345–52. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- Jacobson JM, Thompson MA, Lalezari JP, Saag MS, Zingman BS, D'Ambrosio P, Stambler N, Rotshteyn Y, Marozsan AJ, Maddon PJ, Morris SA, Olson WC. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J Infect Dis. 2010b;201(10):1481–7. doi: 10.1086/652190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Brandt M, Dioszegi M, Jekle A, Schwoerer S, Challand S, Zhang J, Chen Y, Zautke L, Achhammer G, Baehner M, Kroetz S, Heilek-Snyder G, Schumacher R, Cammack N, Sankuratri S. Novel CCR5 monoclonal antibodies with potent and broad-spectrum anti-HIV activities. Antiviral Res. 2007a;74(2):125–37. doi: 10.1016/j.antiviral.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ji C, Zhang J, Dioszegi M, Chiu S, Rao E, Derosier A, Cammack N, Brandt M, Sankuratri S. CCR5 small-molecule antagonists and monoclonal antibodies exert potent synergistic antiviral effects by cobinding to the receptor. Mol Pharmacol. 2007b;72(1):18–28. doi: 10.1124/mol.107.035055. [DOI] [PubMed] [Google Scholar]
- Kitrinos KM, Amrine-Madsen H, Irlbeck DM, Word JM, Demarest JF. Virologic failure in therapy-naive subjects on aplaviroc plus lopinavir-ritonavir: detection of aplaviroc resistance requires clonal analysis of envelope. Antimicrob Agents Chemother. 2009;53(3):1124–31. doi: 10.1128/AAC.01057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhmann SE, Platt EJ, Kozak SL, Kabat D. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J Virol. 2000;74(15):7005–15. doi: 10.1128/jvi.74.15.7005-7015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhmann SE, Pugach P, Kunstman KJ, Taylor J, Stanfield RL, Snyder A, Strizki JM, Riley J, Baroudy BM, Wilson IA, Korber BT, Wolinsky SM, Moore JP. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J Virol. 2004;78(6):2790–807. doi: 10.1128/JVI.78.6.2790-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MM, Lee FH, Haggarty B, Agrawal C, Nolan KM, Biscone M, Romano J, Jordan AP, Leslie GJ, Meissner EG, Su L, Hoxie JA, Doms RW. V3 loop truncations in HIV-1 envelope impart resistance to coreceptor inhibitors and enhanced sensitivity to neutralizing antibodies. PLoS Pathog. 2007;3(8):e117. doi: 10.1371/journal.ppat.0030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari J, Yadavalli GK, Para M, Richmond G, Dejesus E, Brown SJ, Cai W, Chen C, Zhong J, Novello LA, Lederman MM, Subramanian GM. Safety, pharmacokinetics, and antiviral activity of HGS004, a novel fully human IgG4 monoclonal antibody against CCR5, in HIV-1-infected patients. J Infect Dis. 2008;197(5):721–7. doi: 10.1086/527327. [DOI] [PubMed] [Google Scholar]
- Landovitz RJ, Angel JB, Hoffmann C, Horst H, Opravil M, Long J, Greaves W, Fatkenheuer G. Phase II study of vicriviroc versus efavirenz (both with zidovudine/lamivudine) in treatment-naive subjects with HIV-1 infection. J Infect Dis. 2008;198(8):1113–22. doi: 10.1086/592052. [DOI] [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, Doranz BJ, Vakili J, Setoh P, Berg E, Liu G, Guy HR, Durell SR, Parmentier M, Chang CN, Price K, Tsang M, Doms RW. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999a;274(14):9617–26. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999b;96(9):5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Bertolotti-Ciarlet A, Haggarty B, Romano J, Nolan KM, Leslie GJ, Jordan AP, Huang CC, Kwong PD, Doms RW, Hoxie JA. Replication-competent variants of human immunodeficiency virus type 2 lacking the V3 loop exhibit resistance to chemokine receptor antagonists. J Virol. 2007;81(18):9956–66. doi: 10.1128/JVI.00385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Das D, Yin PD, Tsuchiya K, Ogata-Aoki H, Nakata H, Norman RB, Hackney LA, Takaoka Y, Mitsuya H. Involvement of the second extracellular loop and transmembrane residues of CCR5 in inhibitor binding and HIV-1 fusion: insights into the mechanism of allosteric inhibition. J Mol Biol. 2008;381(4):956–74. doi: 10.1016/j.jmb.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Nakata H, Koh Y, Miyakawa T, Ogata H, Takaoka Y, Shibayama S, Sagawa K, Fukushima D, Moravek J, Koyanagi Y, Mitsuya H. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J Virol. 2004;78(16):8654–62. doi: 10.1128/JVI.78.16.8654-8662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marozsan AJ, Kuhmann SE, Morgan T, Herrera C, Rivera-Troche E, Xu S, Baroudy BM, Strizki J, Moore JP. Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D). Virology. 2005;338(1):182–99. doi: 10.1016/j.virol.2005.04.035. [DOI] [PubMed] [Google Scholar]
- McNicholas P, Wei Y, Whitcomb J, Greaves W, Black TA, Tremblay CL, Strizki JM. Characterization of emergent HIV resistance in treatment-naive subjects enrolled in a vicriviroc phase 2 trial. J Infect Dis. 2010;201(10):1470–80. doi: 10.1086/652189. [DOI] [PubMed] [Google Scholar]
- Nolan KM, Del Prete GQ, Jordan AP, Haggarty B, Romano J, Leslie GJ, Hoxie JA. Characterization of a human immunodeficiency virus type 1 V3 deletion mutation that confers resistance to CCR5 inhibitors and the ability to use aplaviroc-bound receptor. J Virol. 2009;83(8):3798–809. doi: 10.1128/JVI.01751-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74(21):10074–80. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogert RA, Hou Y, Ba L, Wojcik L, Qiu P, Murgolo N, Duca J, Dunkle LM, Ralston R, Howe JA. Clinical resistance to vicriviroc through adaptive V3 loop mutations in HIV-1 subtype D gp120 that alter interactions with the N-terminus and ECL2 of CCR5. Virology. 2010;400(1):145–55. doi: 10.1016/j.virol.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Ogert RA, Wojcik L, Buontempo C, Ba L, Buontempo P, Ralston R, Strizki J, Howe JA. Mapping resistance to the CCR5 co-receptor antagonist vicriviroc using heterologous chimeric HIV-1 envelope genes reveals key determinants in the C2-V5 domain of gp120. Virology. 2008;373(2):387–99. doi: 10.1016/j.virol.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Olson WC, Rabut GE, Nagashima KA, Tran DN, Anselma DJ, Monard SP, Segal JP, Thompson DA, Kajumo F, Guo Y, Moore JP, Maddon PJ, Dragic T. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73(5):4145–55. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff JM, Wilen CB, Harrison JE, Demarest JF, Lee B, Doms RW, Tilton JC. HIV-1 resistance to CCR5 antagonists associated with highly efficient use of CCR5 and altered tropism on primary CD4+ T cells. J Virol. 2010;84(13):6505–14. doi: 10.1128/JVI.00374-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt EJ, Durnin JP, Kabat D. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J Virol. 2005;79(7):4347–56. doi: 10.1128/JVI.79.7.4347-4356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugach P, Marozsan AJ, Ketas TJ, Landes EL, Moore JP, Kuhmann SE. HIV-1 clones resistant to a small molecule CCR5 inhibitor use the inhibitor-bound form of CCR5 for entry. Virology. 2007;361(1):212–28. doi: 10.1016/j.virol.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugach P, Ray N, Klasse PJ, Ketas TJ, Michael E, Doms RW, Lee B, Moore JP. Inefficient entry of vicriviroc-resistant HIV-1 via the inhibitor-CCR5 complex at low cell surface CCR5 densities. Virology. 2009;387(2):296–302. doi: 10.1016/j.virol.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JD, Gallo SA, Ahmad N, Miamidian JL, Harvey PE, Sharron M, Pohlmann S, Sfakianos JN, Derdeyn CA, Blumenthal R, Hunter E, Doms RW. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A. 2002;99(25):16249–54. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynes J, Portales P, Segondy M, Baillat V, Andre P, Reant B, Avinens O, Couderc G, Benkirane M, Clot J, Eliaou JF, Corbeau P. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181(3):927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- Sougrat R, Bartesaghi A, Lifson JD, Bennett AE, Bess JW, Zabransky DJ, Subramaniam S. Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog. 2007;3(5):e63. doi: 10.1371/journal.ppat.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterjovski J, Roche M, Churchill MJ, Ellett A, Farrugia W, Gray LR, Cowley D, Poumbourios P, Lee B, Wesselingh SL, Cunningham AL, Ramsland PA, Gorry PR. An altered and more efficient mechanism of CCR5 engagement contributes to macrophage tropism of CCR5-using HIV-1 envelopes. Virology. 2010;404(2):269–78. doi: 10.1016/j.virol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman J, Zingman BS, Diaz RS, Madruga JV, DeJesus E, Slim J, Mak C, Lee E, McCarthy MC, Dunkle LM, Walmsley S. Vicriviroc in combination therapy with an optimized regimen for treatment-experienced subjects: 48-week results of the VICTOR-E1 phase 2 trial. J Infect Dis. 2010;201(4):590–9. doi: 10.1086/650342. [DOI] [PubMed] [Google Scholar]
- Tilton JC, Wilen CB, Didigu CA, Sinha R, Harrison JE, Agrawal-Gamse C, Henning EA, Bushman FD, Martin JN, Deeks SG, Doms RW. A maraviroc-resistant HIV-1 with narrow cross-resistance to other CCR5 antagonists depends on both N-terminal and extracellular loop domains of drug-bound CCR5. J Virol. 2010;84(20):10863–76. doi: 10.1128/JVI.01109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A, Kuhmann SE, Strizki JM, Maxwell E, Ketas T, Morgan T, Pugach P, Xu S, Wojcik L, Tagat J, Palani A, Shapiro S, Clader JW, McCombie S, Reyes GR, Baroudy BM, Moore JP. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc Natl Acad Sci U S A. 2002;99(1):395–400. doi: 10.1073/pnas.012519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibris AM, Korber B, Arnaout R, Russ C, Lo CC, Leitner T, Gaschen B, Theiler J, Paredes R, Su Z, Hughes MD, Gulick RM, Greaves W, Coakley E, Flexner C, Nusbaum C, Kuritzkes DR. Quantitative deep sequencing reveals dynamic HIV-1 escape and large population shifts during CCR5 antagonist therapy in vivo. PLoS One. 2009;4(5):e5683. doi: 10.1371/journal.pone.0005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibris AM, Sagar M, Gulick RM, Su Z, Hughes M, Greaves W, Subramanian M, Flexner C, Giguel F, Leopold KE, Coakley E, Kuritzkes DR. In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject. J Virol. 2008;82(16):8210–4. doi: 10.1128/JVI.00444-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby M, Lewis M, Whitcomb J, Youle M, Pozniak AL, James IT, Jenkins TM, Perros M, van der Ryst E. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol. 2006;80(10):4909–20. doi: 10.1128/JVI.80.10.4909-4920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby M, Smith-Burchnell C, Mori J, Lewis M, Mosley M, Stockdale M, Dorr P, Ciaramella G, Perros M. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J Virol. 2007;81(5):2359–71. doi: 10.1128/JVI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin TJ, Su Z, Krambrink A, Long J, Greaves W, Gross R, Hughes MD, Flexner C, Skolnik PR, Coakley E, Godfrey C, Hirsch M, Kuritzkes DR, Gulick RM. Three-year safety and efficacy of vicriviroc, a CCR5 antagonist, in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr. 2010;54(5):470–6. doi: 10.1097/qai.0b013e3181e2cba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore JP, Mackay CR. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186(8):1373–81. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeni P, Lamarca A, Berger D, Cimoch P, Lazzarin A, Salvato P, Smaill FM, Teofilo E, Madison SJ, Nichols WG, Adkison KK, Bonny T, Millard J, McCarty D. Antiviral activity and safety of aplaviroc, a CCR5 antagonist, in combination with lopinavir/ritonavir in HIV-infected, therapy-naive patients: results of the EPIC study (CCR100136). HIV Med. 2009;10(2):116–24. doi: 10.1111/j.1468-1293.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rao E, Dioszegi M, Kondru R, DeRosier A, Chan E, Schwoerer S, Cammack N, Brandt M, Sankuratri S, Ji C. The second extracellular loop of CCR5 contains the dominant epitopes for highly potent anti-human immunodeficiency virus monoclonal antibodies. Antimicrob Agents Chemother. 2007;51(4):1386–97. doi: 10.1128/AAC.01302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Inhibition of MVCsens and MVCres HIV-1 by the combination of MVC and ROAb14 in PBMCs. PHA-activated PBMCs were incubated with serial dilutions of MVC for 1 h, followed by incubation with the indicated concentration of CCR5 mAb ROAb14 for an additional hour. Cells were infected with replication-competent MVCsens or MVCres HIV-1 for 3 h at a MOI of 0.001. Infected cells were cultured in the presence of inhibitors at the same concentrations as before. Data are p24 levels on day 7, normalized to p24 levels in the absence of MVC. Experimental p24 levels (ng/ml) in the absence of MVC were as follows: 470 ± 64 (no Ab), 200 ± 38 (ROAb14) for MVCsens HIV-1; and 376 ± 59 (no Ab) and 229 ± 38 (ROAb14) for MVCres HIV-1. Data (means ± S.D.) are from one representative experiment of 2, with a different donor in each experiment.
Fig. S2. CCR5 mAb HGS004, but not 2D7, sensitizes MVCres HIV-1 to MVC in CD8-depleted PBMCs. CD8-depleted PBMCs were activated with PHA for 3 days, incubated with 10-fold serial dilutions of MVC for 1 h, followed by incubation with the indicated concentration of each CCR5 mAb for an additional hour. Cells were infected with replication-competent MVCsens or MVCres HIV-1 for 3 h at a MOI of 0.001. Infected cells were cultured in the presence of inhibitors at the same concentrations as before. Data are p24 levels on day 7, normalized to p24 levels in the absence of MVC. Experimental p24 levels (ng/ml) in the absence of MVC were as follows: 554 ± 74 (no Ab), 395 ± 49 (2D7) and 406 ± 62 (HGS004) for MVCsens HIV-1; 479 ± 60 (no Ab), 294 ± 42 (2D7) and 221 ± 36 (HGS004) for MVCres HIV-1. Data (means ± S.D. of duplicates) are from one single experiment.