Abstract
Several studies suggested that long-term nitrate therapy may produce negative outcomes in patient mortality and morbidity. A possible mechanism may involve nitrate-mediated activation of various extracellular matrix (ECM) proteases, particularly matrix metalloproteinase-9 (MMP-9), and adhesion molecules in human macrophages, leading to the destabilization of atherosclerotic plaques. We examined the gene and protein regulating effects on THP-1 human macrophages by repeated exposure to therapeutically relevant concentrations of nitroglycerin (NTG) and possible involvement of nuclear factor (NF)-κB signaling mechanism in mediating some of these observed effects. THP-1 human macrophages repeatedly exposed to NTG (at 10 nM, added on days 1, 4 and 7) exhibited extensive alterations in the expression of multiple genes encoding ECM proteases and adhesion molecules. These effects were dissimilar to those produced by a direct nitric oxide donor, diethylenetriamine NONOate. NTG exposure significantly up-regulated NF-κB DNA nuclear binding activity and MMP-9 protein expression, and reduced tissue inhibitor of metalloproteinase-1 (TIMP-1) expression; these effects were abrogated in the presence of the NF-κB inhibitor parthenolide (a chemical inhibitor derived from the feverfew plant). Further, we examined whether our in vitro findings (an elevated MMP-9/TIMP-1 ratio and gelatinase activity) can be translated to in vivo effects, in a rat model. Sprague-Dawley rats exposed continuously to NTG subcutaneously for 8 days via mini-osmotic pumps showed significant induction of plasma MMP-9 dimer concentrations and the expression of a complex of MMP-9 with lipocalin-2 or neutrophil gelatinase associated lipocalin (NGAL). Plasma gelatinase activity was significantly increased by NTG over the entire study period, attaining peak elevation at day 6. Plasma TIMP-1 protein was down-regulated significantly by day 2 and days 4 to 7 in the NTG-treated rats. Pharmacokinetic monitoring of NTG and its dinitrate metabolites indicated that concentrations were well within therapeutic levels observed in humans. Our studies indicate that clinically relevant concentrations of NTG not only altered ECM matrix by changing the expression of multiple genes that govern cellular integrity, affecting cellular MMP-9/TIMP-1 balance in THP-1 human macrophages possibly via NF-κB activation, but also led to systemic changes in MMP-9/TIMP-1 expression and gelatinase activity in rats. These effects may contribute to extracellular matrix degradation and possible atherosclerotic plaque destabilization.
Keywords: Nitroglycerin, Matrix Metalloproteinase-9, Extracellular Matrix, Tissue Inhibitor of Metalloproteinase-1, THP-1, Nuclear Factor-κB
1. INTRODUCTION
While the acute beneficial effects of organic nitrates, including those of nitroglycerin (NTG), are well established in cardiovascular therapy [1], several recent observational studies reported that their chronic use produced insignificant beneficial effects, or even increased cardiovascular mortality and morbidity [2; 3; 4; 5; 6; 7; 8; 9]. This finding is somewhat surprising considering the excellent vasodilator and anti-platelet aggregating effects of these drugs. Although the loss of beneficial activity of organic nitrates upon repeated administration can be explained by the phenomenon of nitrate tolerance [10; 11; 12], yet the mechanism(s) by which nitrates may increase cardiovascular mortality and morbidity are not known at present.
A hypothesis has been advanced [13] that NTG may up-regulate matrix metalloproteinases (MMPs), leading to the destabilization of atherosclerotic plaques. Many MMPs like gelatinase-B (MMP-9), gelatinase-A (MMP-2), stromelysin (MMP-3), interstitial collagenase (MMP-1), and matrilysin (MMP-7) are expressed by plaque macrophages [14; 15]. The activity of MMPs is tightly regulated at their levels of gene and protein expression in concert with those of their endogenous inhibitors tissue inhibitor of matrix metalloproteinases (TIMP). Co-secretion of TIMPs by macrophages has been shown to offer benefits of plaque stabilization [16]. Thus, an imbalance between MMP and TIMP concentrations can lead to atherosclerotic plaque destabilization [17].
Differentiated human monocytic leukemia cells (THP-1) have been shown to be useful for examining the effects of drugs on plaque vulnerability via MMP-9 changes [18]. We have recently shown that acute exposure of these cells to 100 µM NTG over 48 hours led to the up-regulation of MMP-9 and altered mRNA expression of several proteases and adhesion molecules [19]. The activating effect of NTG on MMP-9 was consistent with those observed for S-nitrosocysteine at 200 µM [20], and 500 µM [21] on recombinant MMP-9. However, these effects have been observed at supra-pharmacological doses, and it is not known whether therapeutically relevant concentrations of NTG will affect MMP-9 expression and activity in human macrophages.
NTG can activate the NF-κB signaling mechanism in brain cells where it also activates MMP-9 [22; 23]. However, the applicability of this signaling pathway in human macrophages has not been tested. Here, therefore, we utilized a NF-κB inhibitor, parthenolide (PTN), [24] to examine the possible involvement of NF-κB in mediating the effects of NTG on MMP-9 activity in THP-1 cells. PTN is a sesquiterpene lactone obtained from the anti-inflammatory medicinal herb, Feverfew (Tanacetum parthenum), and is believed to act by inhibiting IκB kinase (IKK) [25].
Substantial evidence exists supporting an in vivo relationship between the systemic activity of matrix metalloproteinases (MMP) and atherosclerotic plaque stability. Through a review of the relevant literature, Konstantino et al. concluded that “MMP-9 can potentially serve as a diagnostic biomarker in acute coronary syndromes (ACS)” and in patients with chronic coronary artery disease [26]. Patients with ACS exhibited elevated peripheral blood levels of MMP-2 and MMP-9 [27]. In vivo macrophage MMP-9 activation has been linked to acute plague rupture in apoE-deficient mice [28]. The ability of NTG to induce systemic changes in MMP concentrations has not been directly tested. Therefore, this study also examined the systemic and time-dependent effects of NTG on MMP-9 in normal Sprague Dawley rats.
2. MATERIALS AND METHODS
2.1. Materials
THP-1 cells were obtained from the American Type Culture Collection (Manassas, VA; ATCC No. TIB-202). HPLC grade methanol, acetonitrile, and water were purchased from Burdick and Jackson (Muskegon, MI). RPMI medium with supplemented glutamine, fetal bovine serum (FBS), 0.05 mM 2-mercaptoethanol, sterile phosphate buffered saline (PBS), 100 U/ml penicillin and 100 µg/ml streptomycin were purchased from Invitrogen Corporation (Carlsbad, CA). NTG solution (5 mg/ml in 30% propylene glycol v/v and 30% ethanol v/v) was obtained from American Reagent Laboratories Inc. (Shirley, NY). DETA-NO was obtained from Cayman Chemicals (Ann Arbor, MI). The sources of other reagents were: RNA extraction kit, SV Total RNA® isolation system, from Promega (Madison, WI); RT2 PCR array system, CAPH-0166, RT2 PCR first strand kit from SABiosciences (Frederick, MD); Recombinant proMMP-9 protein and Quantikine human MMP-9, MMP-2, and TIMP-1 immunoassay kits from R&D system (Minneapolis, MN); 1,2- or 1,3-glyceryl dinitrate (GDN), and 1,2,4-butanetriol-1,4-dinitrate from Cerilliant (Round Rock, TX); Nuclear extraction kit from Chemicon International (Temecula, CA); NF-κB p50/p65 transcription factor assay from Millipore (Billerica, MA); Biotrak MMP-9 activity assay system from GE Healthcare (Piscataway, NJ); Alzet mini-osmotic pumps, model 2ML1, from DURECT Corporation (Cupertino, CA); One-Step Western™ kit for mouse, rabbit primary antibodies, and protein markers from GenScript Corporation (Piscataway, NJ); anti-rat MMP-9 monoclonal antibody from Thermo Fisher Scientific (Fermont, CA); CHEMICON® MMP Gelatinase Activity Assay Kit from Millipore (Billerica, MA); Sirocco protein precipitation plate from Waters (Milford, MA); 1,2- or 1,3-glyceryl dinitrate (GDN), and DualColor™ protein loading buffer from Fermentas (Glen Burnie, MD); recombinant active MMP-9 and MMP-9/ Lipocalin complex from Calbiochem (Gibbstown, NJ); Amersham full-range rainbow molecular weight markers from GE Healthcare (Piscataway, New Jersey), and anti-rat NGAL antibody from Santa Cruz Biotechnology (Santa Cruz, CA). Parthenolide (PTN) and all other chemicals were obtained from Sigma (St. Louis, MA).
2.2. Nitroglycerin and DETA-NO incubation
THP-1 cells were cultured and differentiated into macrophage as previously described [19]. For each treatment (NTG or DETA-NO) or for vehicle control the THP-1 cells were counted using trypan blue staining after centrifugation (2000g for 10 min at 4 °C) and aliquots of 1 ml (1 ×106 cells) cell suspension were placed into six well plates. Cell viability was ≥95%, as determined by trypan blue staining. Cells were differentiated using phorbol 12-myristate 13-acetate (PMA) at a final concentration of 100 ng/ml over 24 h [18]. NTG, DETA-NO, or vehicle control (30% propylene glycol v/v and 30% ethanol v/v) was added at a final NTG or DETA-NO concentration of 10 nM, on days 1, 4 and 7, and the plates were incubated at 37° C for one week. On day 8, the incubation medium was collected, centrifuged (2,000 × g for 5 minutes at 4° C) and then stored at −80° C until measurements for protein activity and expression were taken. The cells were washed twice with 1 ml of cold and sterile PBS and lysed by adding a total of 350 µl of lysis buffer (provided with the SV Total RNA® isolation system) for total RNA extraction for PCR array analysis. In a separate set of experiments, cells were lysed using tris lysis buffer (50 mM tris HCl containing 0.1% triton ×100 v/v) to analyze cellular MMP-2, MMP-9, and TIMP-1 concentrations and MMP-9 activity. Samples from 3 wells were combined as one sample. The pooled sample was centrifuged at 10,000 × g for 10 minutes at 4° C to remove cellular debris before protein and activity determinations.
2.3. RT2PCR array analysis
Total RNA was isolated using SV Total RNA® isolation system and quantified spectrophotometrically at 260 nm. RNA integrity was determined by formaldehyde agarose gel electrophoresis. Samples were stored at −80° C until the PCR studies. RT2PCR array was used to assess differential gene expression in treated and control cells. The PCR arrays contained a panel of 96 primer sets with a set of 87 genes, encoding human ECM and adhesion molecules (CAPH-0166, SABiosciences) and five housekeeping genes. RT2 real-time SYBR green PCR master mixes, specific for the Stratagene’s MX 3000P quantitative PCR system, were used for gene amplification. Differential gene expression between treatment (NTG and DETA-NO) and vehicle was determined using both standard unpaired t-tests and the Significance Analysis of Microarrays (SAM) [29] program to control the false discovery rates. Genes reported as significant were significant using either procedure. Fold changes in the gene expression, between treatment and control groups, were calculated using the changes in the normalized Ct values by the ΔΔ Ct method according to the manufacturer’s protocol. A gene stability index [30] was used to determine the expression stability of the housekeeping genes on the array. The stability of the geometric mean of the five housekeeping genes as a normalizing factor was also tested using this procedure. The geometric mean of all of the 5 house keeping genes was chosen to normalize the gene expression data.
2.4. NF-κB p50/p65 transcription factor assay
THP-1 macrophages were repeatedly exposed to NTG (10 nM, added on days 1, 4 and 7), NF-κB inhibitor, PTN (10 µM, added on days 1, 4 and 7), vehicle control (30% propylene glycol v/v and 30% ethanol v/v) or with a combination of NTG and PTN. On day 8, the incubation medium was collected, centrifuged (2,000 × g for 5 minutes at 4° C) and then stored at −80° C until measurements of MMP-9 and TIMP-1 protein expression. The cells were washed twice with 1 ml of cold and sterile PBS. DNA binding activity of NF-κB p50/p65 in the nuclear extracts, obtained after day 8 incubation, using a commercially available nuclear extraction kit, was determined using a non-radioactive chemiluminescent NF-κB p50/p65 transcription factor assay. The detection was carried out using a microplate luminometer (Berthold Technologies, Bath, UK). TNF-α treated HeLA whole cell extract and THP-1 macrophages were used as positive controls. Negative control and a competitor control were also included in the assay.
2.5. In vivo NTG dosing
All rat studies were performed according to the protocols approved by the SUNY Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats, weighing 300–350 g, were purchased from Harlan laboratories. The rats were housed in environmentally controlled room with 12 hour light/dark cycle, with free access to food and water. Animals were anesthetized by intramuscular injection of ketamine (90 mg/kg) and xylazine (9 mg/kg). Two cannulas were implanted, one in the left femoral artery and the other in the right jugular vein, for daily blood withdrawal. Rats were allowed at least 48 hours to recover from surgery before the start of the experiment. Cannulas were flushed daily with heparanised saline (25 U heparin/ml of saline) to avoid any blood clots. Mini-osmotic pumps (model: 2ML1) primed with NTG solution (5 mg/ml) or vehicle control (30% propylene glycol v/v and 30% ethanol v/v) were implanted on the back of the rats slightly posterior to the scapulae to achieve subcutaneous delivery of the drug for eight days (n=5–6 in each group). The rats were allowed to freely move in the cages throughout the study. Blood was collected before implantation of the osmotic pump, and every 24 hours for eight days after implantation of osmotic pump from both jugular vein and femoral artery cannulas. Plasma was separated immediately and stored at −80° C until analysis.
2.6. Determination of plasma MMP-9 immuno-reactive activities
Equal volume of plasma samples collected daily from rat femoral arteries were denatured in DualColor™ protein loading buffer under non-reducing conditions at 90° C for 5 minutes. The proteins were separated using 10% SDS-PAGE gel, and electro-transferred to an ECL nitrocellulose membrane, using a Mini-PROTEAN II Cell System (Bio-RAD, Hercules, CA). The membrane was then treated for One-Step Western blotting according to the prescribed protocol and image acquisition was performed using a Kodak Imager (model 2000MM, Carestream Health Molecular Imaging, New Haven, CT), and analyzed with Kodak ID (ver. 3.6.3) software. Authentic protein samples of recombinant proMMP-9, active MMP-9, and MMP-9/NGAL served as positive controls.
2.7. Determination of MMP-9, MMP-2, TIMP-1 protein expression and MMP-9 / gelatinase activity
MMP-9 (total MMP-9; 92 kDa pro- and 86 kDa active forms), MMP-2 (pro- and active forms), and TIMP-1 protein concentrations were determined in the cell lysate and incubation media samples, using Quantikine human MMP-9 or MMP-2 or TIMP-1 immunoassay, respectively. Plasma TIMP-1 protein concentrations were determined using Quantikine rat TIMP-1 immunoassay. Active MMP-9 concentrations were determined in the cell lysate and incubation media using a Biotrak activity assay system. Endogenous gelatinase activities (active MMP-2 and MMP-9) in rat plasma were determined using CHEMICON® MMP gelatinase activity assay according to the manufacturer’s protocol. In our study, the results are expressed as the percent of the mean gelatinase activity on day zero, i.e., before the implantation of the osmotic pump in each rat. All protein concentrations and MMP-9 / gelatinase activity were normalized by total protein concentration determined by Lowry assay.
2.8. Analysis of concentration of NTG and its metabolites 1,2-GDN and 1,3-GDN
Samples from the incubation media were collected everyday following NTG incubation as described in section 2.2 for NTG, 1,2-GDN, and 1,3-GDN analysis, stored at −80° C until analysis, and analyzed using our published LC-MS/MS method [31]. The samples were diluted with equal volume of solution of 50% methanol and water containing the internal standard, 1,2,4-butanetriol-1,4-dinitrate, centrifuged at 16,000 × g for 20 minutes at 4° C. Samples for constructing standard curves were prepared with the identical proportion of RPMI medium. Concentrations of NTG and its metabolites 1,2-GDN and 1,3-GDN in rat plasma were determined via the LC-MS/MS method [31], with the following minor modifications. Sirocco protein precipitation plate was placed on the collection plate, and 150 µl of acetonitrile was added. Plasma samples (50 µl) were then added forcefully and rapidly into the wells to prevent blocking of the filters. The assembly was sealed using a vented cap mat to prevent leakage and cross-contamination during mixing, which was conducted by vortexing on a mutil-tube vortexer at a medium speed for 1 minute. After mixing, the samples were filtered by centrifuging for 5 minutes at 2000 × g. The filtrate was collected and transferred into 2 ml centrifuge tubes and evaporated under a nitrogen stream. The sample was reconstituted in 100 µl of 50% methanol, vortexed, and centrifuged at 16,000 × g for 30 minutes at 4° C. The supernatant was transferred to an injection vial for LC-MS/MS analysis. Standard curves were prepared similarly by spiking blank plasma and processing it through the protein precipitation plates. A diverter valve was included in the LC-MS/MS assay to inject a sample after 4.5 minutes after the start of a run. The lower limit of detection for NTG and dinitrates was 0.25 ng/ml, and the standard curves were linear from 0.25–20 ng/ml. The method was validated using quality control samples, which were processed similarly as the samples. As described previously [31], the accuracy of the assay for the three nitrates, over the range of 1.5–8 ng/ml, were between 92–117% of the theoretical concentration with a CV<9%.
2.9. Statistical analysis
All data are reported as mean ± standard deviation (SD) unless otherwise stated. Statistical analyses were performed on the untransformed data using Student’s t-test or ANOVA, where appropriate statistical significance was set at p<0.05. Comparisons among different groups were made using ANOVA with post hoc analysis (MINITAB 14.×, Minitab Inc., State College, PA). Repeated measures two-way ANOVA analysis was carried out using GraphPad Prism version 5.02.
3. RESULTS
3.1 NTG alters expression of genes encoding ECM proteases and cell adhesion molecules in human THP-1 macrophages
Repeated exposure of THP-1 human macrophages to NTG significantly altered the expression of 18 out of 87 genes in our gene-array panel (p<0.05, Table 1), including significant down-regulation of TIMP-1, transmembrane molecule (integrin-β-3), collagen (COL6A1), and significant up-regulation of ECM proteases (ADAMTS-8, MMP-13, MMP-8, MMP-26) transmembrane molecules (selectin-E, selectin-P), ECM protease inhibitors (TIMP-3), adhesion molecules (laminin-α-2, thrombospondin-1, tetranectin, tenascin-C), and collagens (COL11A1, COL15A1, COL16A1, COL8A1). In comparison, repeated exposure of THP-1 cells to DETA-NO significantly altered the gene expression of only five genes in our panel (p<0.05, Table 1), including the suppression of the transmembrane molecule integrin-α-L, and significant stimulation of collagens (COL7A1, COL15A1), integrin-α-2, and the adhesion molecule thrombospondin-1. The expression of the housekeeping genes was not altered by repeated exposure to either NTG or DETA-NO. Thus, the geometric mean of the signals exhibited by all housekeeping genes was used for data normalization for all other genes. In addition, two genes exhibited a large but statistical insignificant change (≥5-fold) in average expression after repeated NTG exposure, viz., MMP-16 (4.8 fold increase, p=0.1), and thrombospondin-2 (6.2 fold increase, p=0.2). For DETA-NONOate, genes that exhibited large but insignificant changes were: MMP-16 (5.4 fold increase, p=0.1), selectin-E (4.8 fold increase, p=0.06), TIMP-3 (6.3 fold increase, p=0.1), and MMP-26 (5.5 fold increase, p=0.1).
Table 1.
Genes significantly (p<0.05) altered by NTG or DETA-NO treatment versus vehicle control (n=4). The exposure concentration for both agents was 10 nM, added on days 1, 4 and 7).
| Treatment | Gene Expression | Accession # | Gene Title/Symbol | Ratio (Treatment to Control) |
|---|---|---|---|---|
| NTG | Down-regulated | NM_003254 | TIMP metallopeptidase inhibitor(TIMP)-1 | 0.78 |
| NM_000212 | Integrin-β-3(ITGB3) | 0.57 | ||
| NM_001848 | Collagen type-VI-α-1(COL6A1) | 0.75 | ||
| Up-regulated | NM_007037 | ADAM metallopeptidase(ADAMTS8) | 4.21 | |
| NM_002427 | MMP-13 | 7.56 | ||
| NM_002424 | MMP-8 | 1.61 | ||
| NM_021801 | MMP-26 | 18.04 | ||
| NM_000450 | Selectin-E | 8.65 | ||
| NM_003005 | Selectin-P | 9.32 | ||
| NM_000362 | TIMP-3 | 21.05 | ||
| NM_00426 | Laminin, α-2 | 2.59 | ||
| NM_003246 | Thrombospondin-1 | 3.40 | ||
| NM_003278 | Tetranectin | 3.83 | ||
| NM_002160 | Tenascin-C | 2.78 | ||
| NM_080629 | Collagen-type XI-α-1 | 5.51 | ||
| NM_001855 | Collagen-type XV-α-1 | 3.25 | ||
| NM_001856 | Collagen-type-XVI-α-1 | 9.89 | ||
| NM_001850 | Collagen-type-VIII-α-1 | 6.16 | ||
| NM_004994 | MMP-9 | 1.17* | ||
| DETA-NO | Down-regulated | NM_00 2209 | Integrin-α-L (ITGAL) | 0.84 |
| NM_004994 | MMP-9 | 0.88* | ||
| Up-regulated | NM_001855 | Collagen- type-XV-α-1 | 3.38 | |
| NM_000094 | Collagen-type-VII-α-1 | 1.25 | ||
| NM_002203 | Integrin-α-2(ITGA2) | 1.80 | ||
| NM_003246 | Thrombospondin-1( THBS1) | 2.29 |
Change was not statistically significant
3.2. Observed concentrations of NTG and its metabolites in the incubation medium
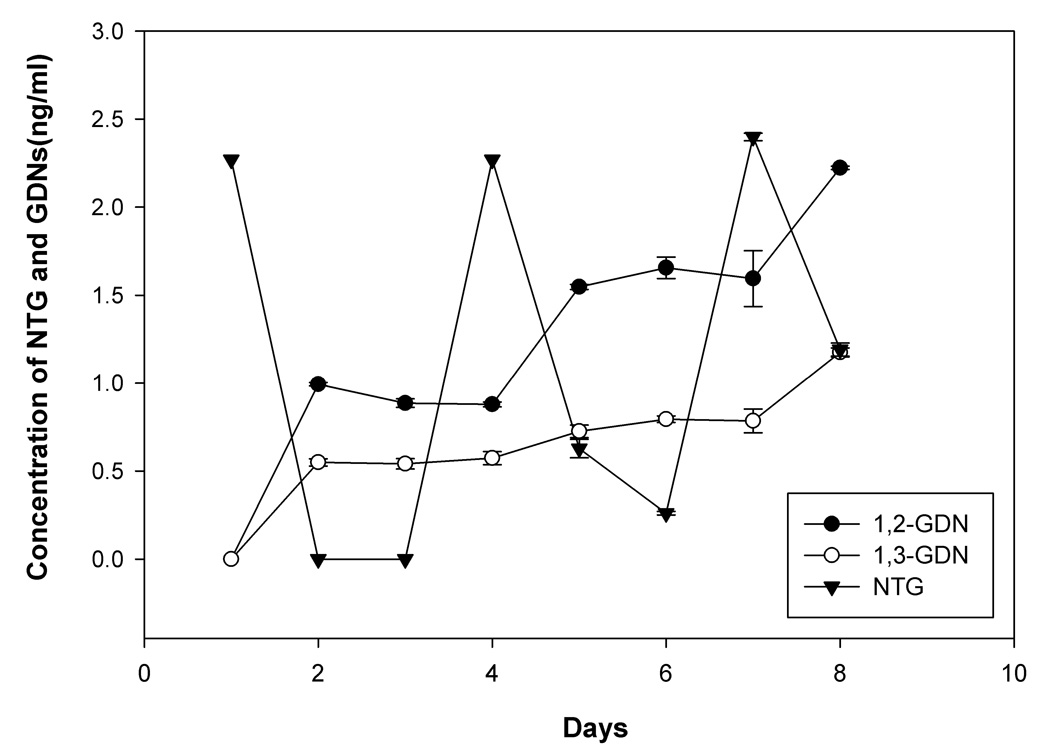
Fig. 1 shows the observed concentrations of NTG, 1,2-GDN, and 1,3-GDN, in the incubation medium over a period of one week under the study conditions. NTG was bioactivated by THP-1 cells to produce the major metabolites 1,2-GDN and 1,3-GDN. The observed concentrations of 1,2-GDN were higher than those of 1,3-GDN.
Fig. 1.
Concentrations of NTG, and dinitrate metabolites 1,2-GDN and 1,3-GDN in the incubation medium after repeated additions of NTG (10 nM, or 2.27 ng/ml, added on days 1, 4 and 7), n=3 each. Error bars indicate SD.
3.3 MMP-9 protein expression and activity
Table 2 shows the total (pro and active) MMP-9 protein concentration and MMP-9 activity after repeated exposure of THP-1 cells to NTG, DETA-NO or vehicle control (30% propylene glycol v/v and 30% ethanol v/v). Upon repeated exposure to the NO donors, cells secreted more MMP-9. The accumulated cellular concentrations of total MMP-9 were increased after exposure to NTG (p<0.05), but not DETA-NO, (p=0.17). Consistent with the increased total MMP-9 concentrations, the activity of MMP-9 in the medium was significantly increased. The corresponding changes in the cell lysates samples were however not significantly different. Overall, these results suggest that NTG can increase the activity of MMP-9 and this effect seemed to be more pronounced than that produced by equimolar concentration of DETA-NO.
Table 2.
Total MMP-9 (in µg/mg protein), active MMP-9, Total MMP-2 and TIMP-1 (all in ng/mg protein) concentrations in cell medium and cell lysates after repeated exposure to NTG, DETA-NO or vehicle control (10 nM added on days 1, 4 and 7, n =6, values represent mean ± SD).
| Control | NTG | DETA-NO | ||||
|---|---|---|---|---|---|---|
| Medium | Cell lysates | Medium | Cell lysates | Medium | Cell lysates | |
| Total MMP-9 | 7.70 ± 1.30 | 0.25 ± 0.08 | 10.2 ± 1.0* # | 0.35 ± 0.09$ | 9.20 ± 0.89* | 0.30 ± 0.11 |
| Active MMP-9 | 0.10 ± 0.13 | 9.38 ± 2.02 | 0.49 ± 0.17* # | 11.5 ± 1.5 | 0.25 ± 0.10* | 11.7 ± 1.4 |
| Total MMP-2 | 33.2 ± 6.3 | 1.53 ± 0.68 | 33.3 ± 5.9 | 1.82 ± 0.38 | 30.4 ± 5.4 | 1.16 ± 0.38 |
| TIMP-1 | 637 ± 90 | 8.40 ± 0.66 | 500 ± 93* | 6.56 ± 0.50$ | 523 ± 90* | 6.02 ± 1.57$ |
p<0.05 versus control medium,
p<0.05 versus control lysate,
p<0.05 versus DETA-NO.
3.4 MMP-2 and TIMP-1 protein expression
No significant change was observed in the concentration of MMP-2 in the cell medium and in cell lysates after either NTG or DETA-NO exposure. In the incubation medium bathing THP-1 macrophages and in cell lysates a significant reduction in TIMP-1 protein concentrations was observed (Table 2).
3.5 Effects of NTG on NF-κB p50/p65 activity in THP-1 cells
Results in Table 3 show that PTN alone significantly decreased NF-κB p50/p65 activity when compared to control samples. Repeated exposure to NTG led to a significant increase in the nuclear NF-κB p50/p65 activity, which was inhibited significantly on co-incubation with PTN. Because the experiments were conducted on different days, the results were expressed as percent of the mean vehicle control value observed for that day. NTG exposure induced MMP-9 protein expression versus control, consistent with our previous observation. PTN not only reduced endogenous MMP-9 concentrations but also significantly inhibited NTG-induced MMP-9 expression in macrophages. Moreover, a significant decrease in TIMP-1 protein concentration was observed when THP-1 cells were exposed to NTG. PTN alone did not significantly affect TIMP-1 concentration versus control, but it reversed the down-regulating effects of NTG (Table 3).
Table 3.
Changes in NF-κB p50/p65 activity, total secreted MMP-9 concentration and TIMP-1 protein expression (the latter two in the cell medium) after repeated exposure to NTG, NF-κB inhibitor parthenolide (PTN) or a combination of NTG and PTN (each added at 10 µM on days 1, 4 and 7, n=6.
| Treatment | NF-κB p50/p65 activity | Total secreted MMP-9 concentration | TIMP-1 protein concentration |
|---|---|---|---|
| Vehicle Control | 100 ± 7% | 100 ± 13 % | 100 ± 8% |
| PTN | 94.0 ± 7.1%$ * | 69.1 ± 14.5%$* | 91.7 ± 8.0%* |
| NTG | 120 ± 5%$ | 126 ± 12%$ | 73.4 ± 9.2%$ |
| PTN + NTG | 104 ± 5%* | 86.5 ± 16.5%$ * | 83.5 ± 10.4%$ * |
p<0.05 versus NTG,
p<0.05 versus vehicle control (by ANOVA and Tukey post hoc test). NF-κB p50/p60 activity was expressed as relative luminescence units (RLUs) in cell nucleus. Total secreted MMP-9 and TIMP-1 protein concentrations were in ng/mg total protein.
3.6 In vivo effects of NTG on the plasma concentrations of MMP-9 immuno-reactive proteins
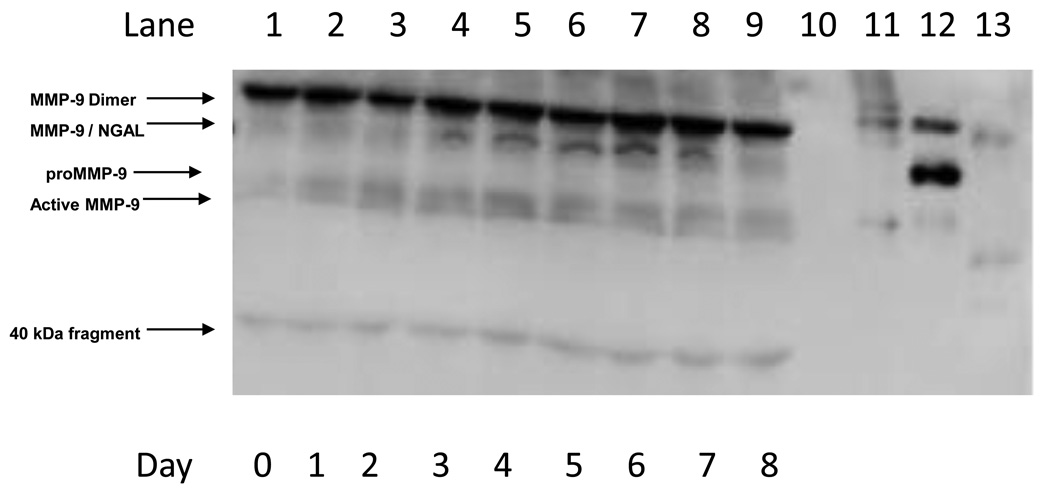
Plasma concentrations of MMP-9 immuno-reactive proteins were monitored daily from 0 to 8 days after implantation of NTG-containing osmotic pumps in vivo. Fig. 2 shows a representative Western blot of MMP-9 reactive bands under non-reducing conditions. In lanes 1–9, which were spotted with plasma samples of a rat from days 0 to 8 after NTG dosing, the presence of several MMP-9 reactive bands was revealed. These included: a band at about 220 kDa representing the MMP-9 dimer, bands of pro and active MMP-9 that co-migrated with the respective recombinant protein standards, a band at approximately 130 kDa, which co-migrated with recombinant MMP-9/NGAL complex, and two lower molecular weight bands around 40 kDa and 20 kDa, which may represent MMP-9 cleavage products [32].
Fig. 2.
Representative Western blot of MMP-9 under non-reducing conditions. Lane 1: Day 0 plasma sample; Lane 2: Day 1 plasma sample; Lane 3: Day 2 plasma sample; Lane 4: Day 3 plasma sample; Lane 5: Day 4 plasma sample; Lane 6: Day 5 plasma sample; Lane 7: Day 6 plasma sample; Lane 8: Day 7 plasma sample; Lane 9: Day 8 plasma sample; Lane 10: Blank; Lane 11: Recombinant proMMP-9 ; Lane 12; MMP-9/NGAL standard; Lane 13; Recombinant active MMP-9.
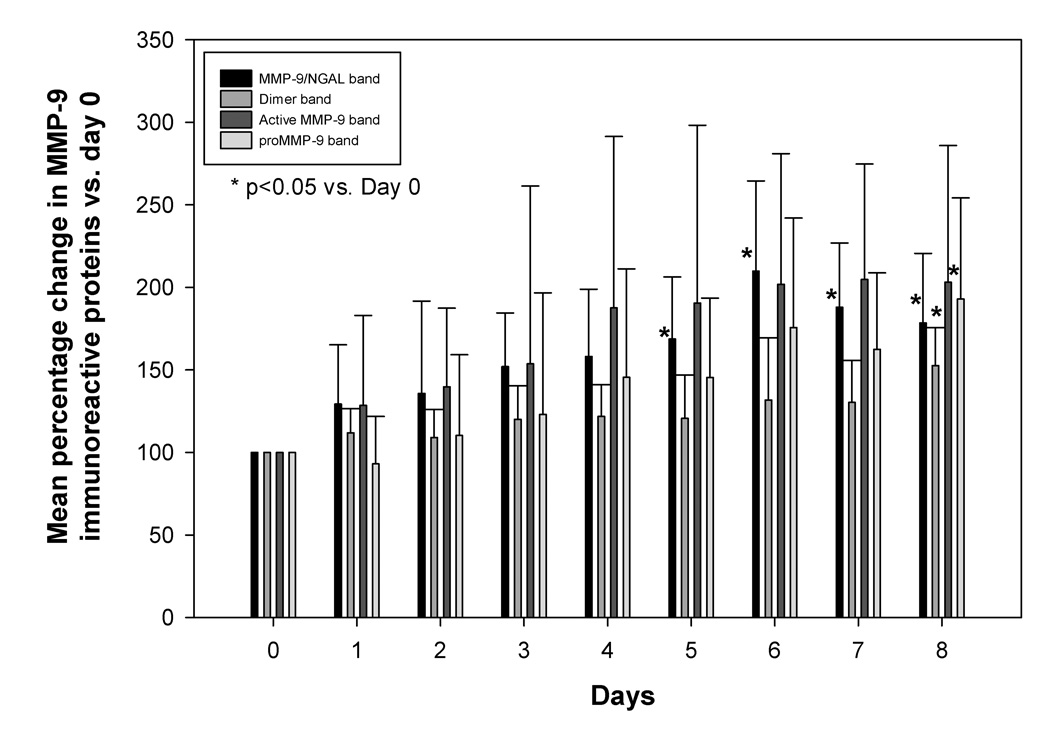
Because each animal provided a different baseline value before dosing (day 0), the data on subsequent days after osmotic pump implantation was normalized as a percentage of the value at day 0 from the same rat. Fig. 3 shows the mean quantified intensity data from all study animals exposed to NTG. Repeated measures ANOVA demonstrated a time-dependent change after NTG treatment (p<0.05), and a post-hoc test showed significant elevation of MMP-9/NGAL complex at days 5–8 versus control animals. Mean percentage change of MMP-9/NGAL band in control samples vs. day 0 ranged from 96.2 ± 2.2 to 101.3 ± 5.6 % for over the period of one week.
Fig. 3.
Comparison of MMP-9/NGAL, dimer protein, active MMP-9, and proMMP-9 protein expression changes in rat plasma samples of the rats implanted with osmotic pumps containing NTG stock solution. The band intensity obtained after Western blot analysis was normalized as percent of band intensity of day 0 for each rat, n=4–5. *p<0.05 (repeated measures ANOVA analysis followed by post-hoc test) versus control (data not plotted).
NTG dosing also increased MMP-9 dimer formation in rat plasma samples (Fig. 3), exhibiting a significant increase on day 8 (1.5-fold, p<0.05). However, in control animals mean percentage change of dimer band ranged from 96.5 ± 1.9 to 102.1 ± 7.1 for over the period of one week and was not significantly different vs day 0 (p>0.05). While there was a trend towards increasing active MMP-9 in plasma after NTG treatment (Fig. 3), statistical significance could not be demonstrated. However, proMMP-9 expression was significantly elevated on day 8 (Fig. 3, p<0.01). In control samples the mean percentage change of active MMP-9 band ranged from 100.1 ± 4.3 to 102.7 ± 4 and proMMP-9 band ranged from 97.6 ± 3.6 to 100.1 ± 7.6 over one week period and were not significantly different from day 0 expression (p>0.05).
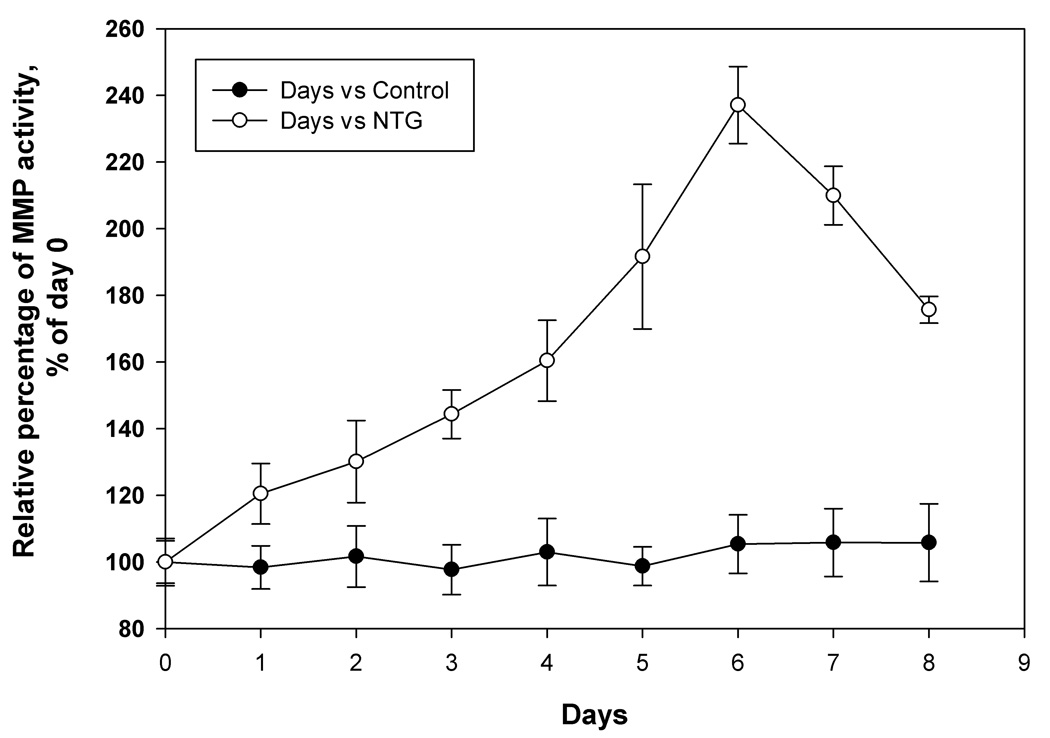
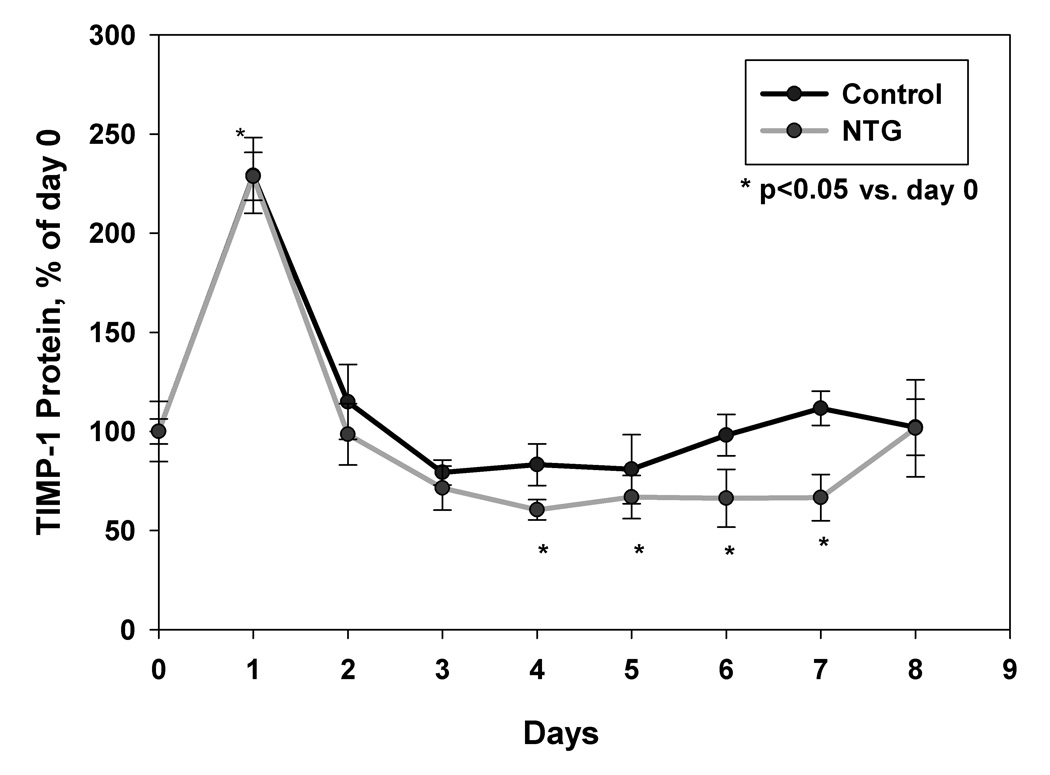
3.7 Effects of NTG dosing on plasma gelatinase activity and TIMP-1 protein levels
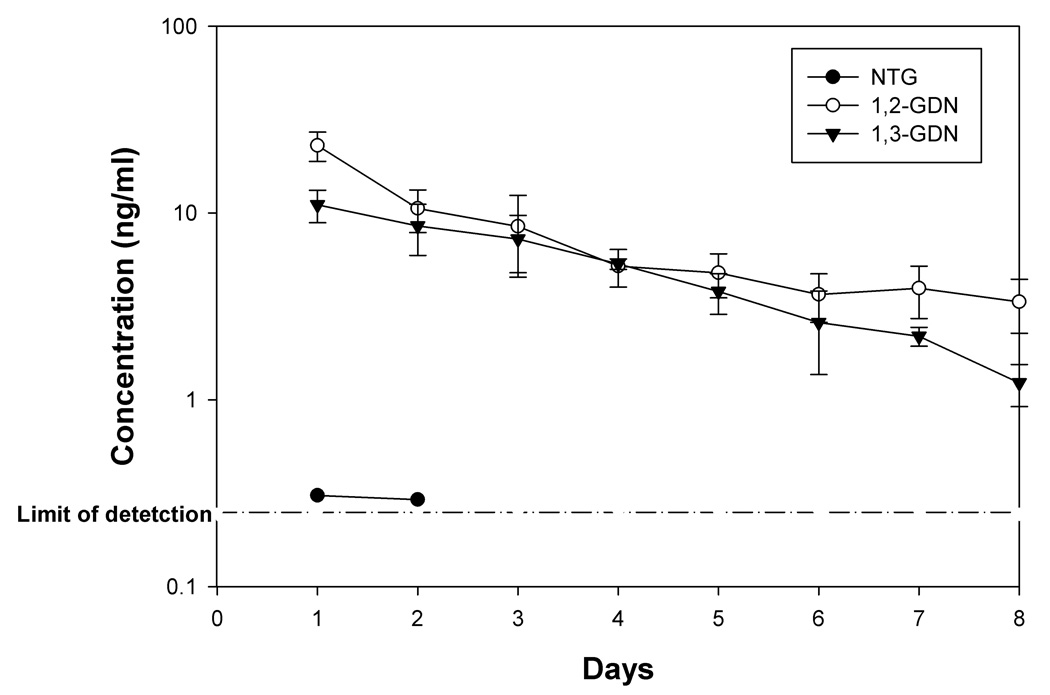
Significant increase in plasma gelatinase activity was observed on all 8 days after NTG treatment, when compared to control animals, as confirmed by two-way repeated measures ANOVA analysis (p<0.05). Peak increase in activity was observed on day 6 followed by a decline on days 7 and 8 (Fig. 4a). In comparison, no significant time-dependent changes in gelatinase activity in the control group was observed (p>0.05, Fig. 4a). A sharp increase in plasma TIMP-1 concentration was seen on day 1 both in treated and control animals, most likely due to the trauma induced by osmotic pump implantation. However, from day 2 onward, plasma TIMP-1 levels returned to near pre-treatment levels. The results showed that NTG dosing induced a significant decline in TIMP-1 protein concentrations on day 2 and days 4 to 7 in the in the NTG-treated animals (Fig. 4b, p<0.05 repeated measures ANOVA analysis followed by post-hoc test). Fig. 5 shows rat plasma NTG and dinitrate metabolite concentrations obtained via the femoral artery cannulas. NTG levels fell below detection limit after the second day. The concentration of the GDN metabolites also decreased over study period. A similar trend was seen in plasma samples obtained from jugular vein (data not shown).
Fig. 4.
(a) MMP activity changes in rat plasma samples of the rats implanted with osmotic pumps containing NTG stock solution versus vehicle control. The MMP activity is expressed as relative percentage of activity as calculated using standard MMP-2 supplied with the assay system and then calculated as percent of activity using mean day 0 activity, n=4. *p<0.05 versus control and (b) TIMP-1 protein expression changes in rat plasma samples of the rats implanted with osmotic pumps containing NTG stock solution versus vehicle control. TIMP-1 protein levels were corrected for total protein content in the samples and then calculated as percent of TIMP-1 using mean day 0 TIMP-1 concentration, n=4. *p<0.05 versus control.
Fig. 5.
Concentration of NTG and dinitrates, 1,2-GDN and 1,3-GDN in rat plasma samples obtained from femoral artery vein determined by LC-MS/MS method, n=4–5.
4. DISCUSSION
Macrophages play a key role in various inflammatory responses and in atherosclerotic plaque rupture. Macrophage cell density is increased in atherosclerosis, where secretion of various proteases by macrophages contributes to the pathology [33]. MMPs are proteolytic enzymes that degrade ECM components and target several regulatory proteins like cytokines and adhesion molecules. The gelatinases, MMP-9 and MMP-2, are mainly responsible for degradation of Type IV collagen, which is the major component of the basement membrane [34]. Hence, MMPs may facilitate tissue repair and growth as well as plaque rupture and tissue invasion [35]. This study extends our previous proof-of-concept study [19] and (a) examined the gene expression and activation of MMPs in human macrophages by NTG at therapeutically relevant concentrations, (b) explored the underlying mechanism behind the gene and protein expression effects mediated by NTG in THP-1 cells, (c) compared the effects of NTG with a direct NO donor, DETA-NO, to further understand the underlying mechanisms, and (d) investigated the in vivo effects of NTG on MMP-9/TIMP-1 balance in rats.
After transdermal NTG administration in humans, plasma concentrations ranged from 0.44–40.7 nM or 0.1–9.24 ng/ml [36; 37; 38; 39; 40; 41; 42]. The concentrations of 1,2-GDN and 1,3-GDN observed clinically were 0.3–119.5 ng/ml and 0.5–42.2 ng/ml, respectively [38; 41; 43; 44; 45]. In the present study, we added NTG at 10 nM or 2.27 ng/ml in the incubation medium on days 1, 4 and 7. NTG can be bioactivated to its major metabolites 1,2-GDN or 1,3-GDN by enzymatic or non-enzymatic pathways with the production of 1,2-GDN considered as an index of “mechanism-based” metabolism that parallels vasodilatation and the production of 1,3-GDN associated with the nonenzymatic activation as its appearance is unrelated to relaxation and tolerance induction [10; 12]. Cellular metabolism of NTG was observed forming the major dinitrate metabolites of NTG (Fig. 1). The nitrate concentrations were therefore well within their therapeutic ranges.
The RT2 profiler PCR arrays systems used here offers a wide dynamic range, reproducibility, specificity, and greater sensitivity for studying differential gene expression. We added 3 more genes, viz., testican-1, MMP-26, and cathepsin-L, to the commercially available panel, based on our previous findings that significant altered regulation of testican-1 and MMP-26 was observed after acute NTG exposure at 100 µM [19]. Since testican-1 has been shown to have inhibitory effects on another important protease cathepsin-L, which contributes to atherosclerosis progression [46; 47], the gene regulation of cathepsin-L was also examined in this study.
Our results confirmed previous observations that exposure to NTG may cause extensive changes in cellular and tissue gene regulation, as were shown for THP-1 macrophages [19], and in vascular tissues [48; 49]. However, the specific genes that were affected in the present experiment differed substantially from those observed after acute high-concentration exposure [19]. The only gene commonly altered was MMP-26, but in this study, we observed an up-regulation while the opposite was seen under acute, high-concentration conditions. VCAM-1 showed a 1.5 fold increase here but, unlike that seen under acute conditions, the change was not statistically significant. The reason for these observed differences in gene expression effects are most likely due to differences in exposure conditions at which different cellular transcriptional controls might be activated. Additionally, the samples were analyzed at only one time-point and time-dependent changes, which could be quite complex, were not examined. Also, different bioactivation mechanisms can be involved at these different exposure conditions leading to different fractions of GDN metabolites and these gene expression differences. Upon exposure at 10 nM for 8 days the concentration of 1,2-GDN metabolite was higher than 1,3-GDN in the incubation medium (Fig. 1). In contrast at higher dose exposure as in our previous publication [19] (100 µM for 48 hours) the concentration of 1,3-GDN was higher than 1,2-GDN in the incubation medium (NTG: 12.8 ± 1.0; 1,3-GDN: 5.75 ± 1.09, and 1,2-GDN: 3.01 ± 1.08 ng/ml). Nevertheless, the results suggest extensive cellular effects of NTG on ECM remodeling genes.
Under the conditions of repeated exposure studied here, NTG significantly up-regulated several ECM degrading proteases like MMP-8, MMP-13, MMP-26, and ADAMTS-8. The two collagenases, MMP-8 (collagenase-2) and MMP-13 (collagenase-3), like others in this enzyme family, degrade triple helical fibrillar collagen. Increased MMP-8 activity has been linked to vulnerable plaques [50; 51], and may serve as a predictor of plaque instability and cardiovascular mortality [52; 53]. Using ApoE and MMP-13 double knockout mice, a recent study showed the importance of MMP-13 in collagen regulation in plaques [54]. Gene expression of MMP-26 (matrilysin-2), a newly identified member of the MMP family, increased after NTG exposure vs. control. Unlike other MMPs, it lacks the hemopexin domain [55], and it has been shown to activate proMMP-9 [56].
Like MMPs, ADAMTS is another family of proteinases capable of degrading the ECM. ADMATS-8 is the member of this family that can degrade proteoglycans like aggrecan and versican, which are expressed in blood vessels. In our study, ADMATS-8 was significantly up-regulated by NTG treatment. A recent study reported ADAMTS-8 expression in macrophage-rich areas of atherosclerotic plaques [57], but its role in plaque progression is still unclear.
The gene expression of several adhesion molecules (selectin-P, selectin-E, laminin-α-2, thrombospondin-1, tetranectin, tenascin-C, and integrin-β-3) were altered after repeated NTG exposure. Cell adhesion molecules like selectin-E, selectin-P, and integrins play an important role in leukocyte extravasation and may serve as biomarkers for plaque destabilization [58]. In our study, we found an up-regulation of thrombospondin-1 after repeated exposure to NTG. We observed an up-regulation of tetranectin, a protein found in human plasma, which is suspected to participate in tissue degeneration and proteolysis via plasminogen binding [59], and to play a significant role in cancer metastasis [60]. Tenascin-C, an ECM glycoprotein was also up-regulated, which has been shown to induce the expression of MMP-9 [61] that may play a role in the remodeling of atherosclerotic plaque matrix composition [62]. In addition, repeated exposure to NTG altered various genes encoding collagens, down-regulating COL6A1 but inducing the gene expression of COL11A1, COL15A1, COL16A1, and COL8A1. Collagens constitute 60% of the plaque protein, and can stimulate smooth muscle cell migration and proliferation by up-regulating some ECM molecules like tenascin-C.
Finally, consistent with a previous report in which NTG concentration was not defined [13], repeated exposure to NTG significantly down-regulated the endogenous MMP inhibitor TIMP-1 (NTG treatment/control vehicle treatment=0.78, p<0.05). Higher MMP-9/TIMP-1 ratios were observed in acute coronary syndrome patients and this ratio may be an independent predictor of plaque stability [63]. TIMP-1 concentration, by itself, can also serve as a predictor of cardiovascular death [64]. Our study also detected a significant up-regulation of TIMP-3 (NTG treatment/control vehicle treatment= 21.05, p<0.05).
The gene expression effects after repeated exposure to DETA-NO were substantially different from those observed for NTG. The gene encoding thrombospondin-1 was the only gene that was up-regulated by both treatments. Nunez et al. [65] showed that, unlike DETA-NO, NTG did not inhibit O2 consumption by vascular mitochondria, nor was NO fluorescence detected from NTG. These investigators therefore concluded that NTG does not release free NO within their experimental concentration range (up to 1022126 M). In the present study, metabolism studies clearly showed that NTG was bioactivated in the cell system, producing its dinitrate metabolites (Fig. 1). The half-life of DETA-NO release of NO has been reported to be 20 hrs at 37°C. Fig. 1 shows that in the incubation medium, NTG disappearance was near complete 24 hrs after addition, followed by formation of its dinitrate metabolites, which could also release NO upon further metabolism. The observed differences in gene expression effects of NTG and DETA-NO therefore could be due to differences in the release rates of NO from these compounds or the distribution of the various NO species released and the subsequent chemical interaction with ECM proteases.
Our results (Table 2) indicated that NTG and DETA-NO induced the protein expression of total MMP-9 in THP-1 human macrophages. The effects of NTG appeared to be higher than those from DETA-NO, at the same molar concentration. NTG and DETA-NO exposure led to a 5-fold and 2.5-fold increase, respectively, in active MMP-9 concentrations in the incubation medium bathing human THP-1 macrophages. These observations suggest both NO donors not only increased the expression of total MMP-9, but also increased its activity. The biochemical mechanism of interaction of NTG with the cysteine switch of MMP-9 is not known. Recently, we have demonstrated pro-oxidant effects of NTG [66] on cellular thiols, which may present one of the possible activating mechanisms of the cysteine switch of MMP-9. Unlike the results from Death et al. [13], no significant change in MMP-2 protein expression was observed here. The reason for this discordant finding is not known, but might relate to different exposure conditions. In contrast, we found that after repeated exposure to NTG, TIMP-1 protein expression was significantly down-regulated, consistent with the suppressed gene expression results seen in the microarray study (Table 1).
NF-κB inhibition by PTN has been shown to retard atherosclerotic lesions in apoE knockout mice [67; 68], an effect that is possibly mediated via inhibition of IκB phosphorylation and degradation [24; 25]. Several PTN-containing products are available over the counter, reportedly producing beneficial effects on blood vessel tone, in migraine relief, cancer, blood aggregation, and arthritis [69; 70; 71; 72; 73]. Here, we showed that NTG significantly enhanced NF-κB p50/p65 DNA binding activity in THP- 1 macrophages, which was negated in the presence of the NF-κB inhibitor PTN (Table 3). Our results also indicated that NTG-induced changes in MMP-9 and TIMP-1 expression can be linked to NF-κB activation, based on the abrogating effects of PTN. Other investigators have already shown that PTN reduced MMP-9 expression [74; 75]; here we demonstrated that it also significantly inhibited NTG induced MMP-9 protein secretion (Table 3).
The promoter region of MMP-9 gene contains a NF-κB element that is responsible for induction by various cytokine inducers [76; 77]. Several studies have reported NF-κB activation by NO or by NO related species like peroxynitrite [78; 79]. NTG has been shown to stimulate NF-κB binding activity via IKK-κ activation in TNF-κ activated endothelial cells and in vivo in rat brain nuclei [22; 23]. Pandey et al. [80] have shown that IKK activity can be modulated through its cysteine residue 179. We have shown that NTG can oxidize protein thiols, either in small molecules [81], or when they are present in cysteine residues of proteins [66; 82], forming disulfide and S-glutathionylated products. Moreover, NO has been shown to activate p21ras, a G-protein family member and cellular signaling mediator [83]. We have shown recently that NTG can activate p21ras by S-glutathionylation in human endothelial cells and porcine epithelial cells at a concentration of 100 µM [66], similar to that reported for peroxynitrite in bovine aortic endothelial cells at a concentration of 100 µM [84]. Activation of p21ras increases the activity of kinases Raf-1 and phosphatidylinositol 3-kinase, which in turn can activate IKK and increase IκB degradation, leading to enhanced activity of NF-κB [85]. Thus, the observed NTG effects may be mediated by the upstream activation of p21ras, which in turn lead to NF-κB activation.
Our in vitro studies suggested that NTG may alter the MMP-9/TIMP-1 balance in vitro. Literature review indicates a strong link between elevated MMP-9 activity to atherosclerotic plaque destabilization [26; 27; 28]. A recent study by Kim et al. demonstrated activation of MMP-9 by a continuous short-term infusion (10 µg/kg/minute for 30 minutes) of supra-pharmacological doses of NTG in rat meningeal blood vessels [23]. However, the overall in vivo effects of NTG on MMP-9 / TIMP-1 expression, and MMP activity were not reported. Earlier, Uemura et al. [86] showed that increased plasma gelatinase activity can be demonstrated in Sprague-Dawley rats after induction of diabetes. These findings indicate that this simple animal model may potentially allow for the detection of changes in plasma gelatinase activities after in vivo NTG dosing. We therefore examined the systemic and time-dependent in vivo dynamic effects, on continuous NTG exposure, at therapeutically relevant concentrations, on MMP-9/TIMP-1 expression and MMP activity in Sprague-Dawley rats.
Western blot analysis was carried out to examine changes in MMP-9 immuno-reactive activities in plasma over 8 days in rats, which were exposed continuously to NTG. Plasma samples, rather than serum samples, were utilized because literature reports indicated an artificial increase in MMP or TIMP values in the latter samples, due to release of MMP/TIMP by platelets or polymorphonuclear neutrophils during clot formation [87]. Our results showed that active MMP-9 protein expression trended upwards in NTG-treated animals, but statistical difference could not be demonstrated, because of the high data variability (Fig. 3). However, significant induction of MMP-9/NGAL complex (Fig. 3) and proMMP-9 (Fig. 3) in NTG-treated animals were observed while no such changes were observed in the control group. NGAL or Lipocalin-2 is a 25 kDa glycoprotein [88] that is induced in several inflammatory conditions like atherosclerosis, and it may serve as a predictor of cardiovascular mortality after ischemia [89]. It is secreted as a monomer or as a complex with MMP-9. Increased levels of NGAL have also been linked to NF-κB activation by IKK activation [90]. NGAL can have positive effects on MMP-9 activity via several processes: (i) direct activation of proMMP-9, (ii) prevention of autolysis of MMP-9, and (iii) synergistic effects on proMMP-9 activation with other MMP activating agents [32; 91; 92]. Thus, NGAL may play an important role in vascular remodeling and plaque instability [89; 93; 94]. The intensity of the MMP-9 dimer trended upward in NTG-treated animals versus control, and statistical significance could be demonstrated at day 8 (Fig. 3). Taken together, these results showed that continuous NTG dosing in rats produced significant in vivo elevation of MMP-9 immuno-reactivity in plasma.
Gelatinase activity assay using a biotinylated gelatinase substrate was carried out to examine MMP activity changes in rat plasma samples of NTG-treated versus control animals. The sensitivity of the assay used is similar to that obtained from traditional zymography method, but the procedure is more expedient. Significant increase in gelatinase activity was observed from day 1 with a peak at day 6, with a peak increase of 2.4 fold observed on day 6 (Fig. 4a) as compared to control animals.
The overall MMP activity also involves contribution from its natural inhibitor TIMP-1, which binds to both proMMP-9 and active MMP-9 to inhibit their activity [95]. In the present study, TIMP-1 protein expression was significantly down-regulated by NTG on day 2, and days 4 to 7 in the NTG-treated rats. A maximum decline in TIMP-1 concentrations, around 40%, in NTG-treated animals was observed on day 4, when compared to a 20% decrease in control animals (Fig. 4b). A characteristic sharp increase in TIMP-1 expression was observed in both control and treated animals on day 1 (Fig. 4b), which might be due to stress associated with surgery. A decline in plasma TIMP-1 level favors plaque instability due to an elevated MMP-9/TIMP-1 balance.
The pharmacodynamic effects observed over the period of 8 days in NTG-treated rats therefore included: an induction of plasma MMP-9 dimer and MMP-9/NGAL complex, as well as increased gelatinase activity, and decreased TIMP-1 protein concentration. To determine if the NTG dosing in our study provided therapeutic relevant levels, we measured arterial and venous plasma concentrations of NTG and its two major dinitrate metabolites (Fig. 5). NTG concentration was undetectable (< 0.25 ng/ml) after the second day in arterial plasma samples. GDN concentrations ranged between 5–10 ng/ml and showed a gradual decline over the study period. Follow-up studies (data not shown) indicated that this decline was due to reduced release of NTG from the osmotic pump with time, possibly due to drug adsorption onto the plastic components of the infusion device. In follow-up studies examining the delivery of NTG from osmotic pumps, we verified that there was continuous zero order release of NTG solution over the study duration (data not shown). GDN metabolites were not detected in the solutions within the pump or pump washings, so it was clear that NTG degradation was not the cause for the declining plasma metabolite concentrations. The possibility of metabolic induction after continuous exposure can be ruled out, because chronic intravenous infusion in humans has been shown to be associated with a slight increase (not decrease) in plasma concentrations, contrary to metabolic induction [96].
The plasma concentrations of NTG, 1,2-GDN, and 1,3-GDN, observed in patients ranged widely, depending on the route of administration and the NTG doses administered. For example, in patients on long-term intravenous NTG infusions at doses ranging from 1.3 to 12.0 mg/hour for 1 to 16 days, Torfgard et al. [43] observed plasma concentrations of 0.8 to 894.5 ng/ml for NTG, undetected to 119.5 ng/ml (for 1,2-GDN), and 0.8–42.2 ng/ml (for 1,3-GDN), respectively. The composite plasma concentrations observed after transdermal NTG administration, at doses of 5 to 105 mg per day, ranged from 0.1 to 9.24 ng/ml (NTG), 0.3–3.5 ng/ml (1,2-GDN), and 0.5–0.8 ng/ml (1,3-GDN), respectively [36; 38; 39; 40; 41; 42; 44; 45]. The plasma concentrations observed in the present study, i.e., NTG: 0.3 to 0.7 ng/ml, 1,2-GDN: 2 to 23 ng/ml, and 1,3-GDN: 1.2 –11 ng/ml, were well within these clinically observed values. Thus, the observed pharmacodynamic effects on MMP-9/TIMP-1 imbalance and enhanced MMP-9 activity might well be translatable in patients.
5. CONCLUSIONS
Our results indicate that NTG, at therapeutically relevant concentrations, significantly altered the expression of multiple genes encoding a number of ECM proteases and adhesion molecules, as well as the protein expression and activity of MMP-9, and TIMP-1 protein expression. Further studies show that repeated exposure of NTG increased intranuclear NF-κB binding, elevated MMP-9 secretion, and down-regulated TIMP-1 in human macrophages. All these effects are negated in the presence of the NF-κB inhibitor PTN.
Our in vivo results in rats indicated that therapeutic concentrations of NTG induced time-dependent changes in plasma MMP immuno-reactivity, activation of plasma gelatinases, and inhibition of TIMP-1 protein expression. In addition, we observed that NTG exposure induced the formation of a complex of MMP-9 with NGAL, a NF-κB inducible protein. Several literature reports have pointed out that up-regulation of gelatinases can cause atherosclerotic plaque destabilization [26; 27; 28]. To the best of our knowledge, the present study demonstrated for the first time that NTG at therapeutically relevant concentrations can produce adverse effects on MMP-9/TIMP-1 ratio in an experimental animal model. Further studies to reverse these adverse effects, possibly via the use of an NF-κB inhibitor, as shown in our in vitro studies, may aid in the future design of better therapeutic strategies when using this life-saving cardiovascular drug.
ACKNOWLEDGMENTS
Research supported in part by NIH [grant HL081580] and by a graduate fellowship awarded from the John Kapoor Foundation to ASK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tfelt-Hansen PC, Tfelt-Hansen J. Nitroglycerin headache and nitroglycerin-induced primary headaches from 1846 and onwards: a historical overview and an update. Headache. 2009;49:445–456. doi: 10.1111/j.1526-4610.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi T, Hagiwara N, Kasanuki H, Koyanagi R, Ogawa H. Long-term nitrate use in acute myocardial infarction (the Heart Institute of Japan, Department of Cardiology nitrate evaluation program) Cardiovasc Drugs Ther. 2008;22:177–184. doi: 10.1007/s10557-008-6089-8. [DOI] [PubMed] [Google Scholar]
- 3.Anon GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 4.Anon ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 5.Ishikawa K, Kanamasa K, Ogawa I, Takenaka T, Naito T, Kamata N, Yamamoto T, Nakai S, Hama J, Oyaizu M, Kimura A, Yamamoto K, Aso N, Arai M, Yabushita H, Katori Y. Long-term nitrate treatment increases cardiac events in patients with healed myocardial infarction. Secondary Prevention Group. Jpn Circ J. 1996;60:779–788. doi: 10.1253/jcj.60.779. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa K, Yamamoto T, Kanamasa K, Hayashi T, Takenaka T, Kimura A, Miyataka M, Yabushita H, Kitayama K. Intermittent nitrate therapy for prior myocardial infaraction does not induce rebound angina nor reduce cardiac events. Intern Med. 2000;39:1020–1026. doi: 10.2169/internalmedicine.39.1020. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Multicenter Myocardial Ischemia Research Group. Am Heart J. 1999;138:577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- 8.Nagao K, Kanmatsuse K, Ooiwa K, Satou K, Watanabe I, Yamasita M, Kikushima K. The effects of long-acting nitrates on 5-year cardiac events of patients with coronary thrombolytic therapy for acute myocardial infarction. Intern Med. 2000;39:877–884. doi: 10.2169/internalmedicine.39.877. [DOI] [PubMed] [Google Scholar]
- 9.Kanamasa K, Hayashi T, Kimura A, Ikeda A, Ishikawa K. Long-term, continuous treatment with both oral and transdermal nitrates increases cardiac events in healed myocardial infarction patients. Angiology. 2002;53:399–408. doi: 10.1177/000331970205300405. [DOI] [PubMed] [Google Scholar]
- 10.Fung HL. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol. 2004;44:67–85. doi: 10.1146/annurev.pharmtox.44.101802.121646. [DOI] [PubMed] [Google Scholar]
- 11.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 12.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles. Br J Pharmacol. 2008;155:170–184. doi: 10.1038/bjp.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Death AK, Nakhla S, McGrath KC, Martell S, Yue DK, Jessup W, Celermajer DS. Nitroglycerin upregulates matrix metalloproteinase expression by human macrophages. J Am Coll Cardiol. 2002;39:1943–1950. doi: 10.1016/s0735-1097(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 14.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allaire E, Forough R, Clowes M, Starcher B, Clowes AW. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–1420. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 18.Rival Y, Beneteau N, Chapuis V, Taillandier T, Lestienne F, Dupont-Passelaigue E, Patoiseau JF, Colpaert FC, Junquero D. Cardiovascular drugs inhibit MMP-9 activity from human THP-1 macrophages. DNA Cell Biol. 2004;23:283–292. doi: 10.1089/104454904323090912. [DOI] [PubMed] [Google Scholar]
- 19.Krishnatry AS, Brazeau DA, Fung HL. Broad regulation of matrix and adhesion molecules in THP-1 human macrophages by nitroglycerin. Nitric Oxide. 2010;22:11–17. doi: 10.1016/j.niox.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy SM, Bove PF, Matthews DE, Akaike T, van der Vliet A. Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry. 2008;47:5832–5840. doi: 10.1021/bi702496v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greco R, Tassorelli C, Cappelletti D, Sandrini G, Nappi G. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology. 2005;26:795–800. doi: 10.1016/j.neuro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Kim GM, Jin KS, Chung CS. Differential effects of corticosteroids on the expression of cyclooxygenase-2, tumour necrosis factor-alpha and matrix metalloproteinase-9 in an animal model of migraine. Cephalalgia. 2008;28:1179–1187. doi: 10.1111/j.1468-2982.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 24.Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- 25.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 26.Konstantino Y, Nguyen TT, Wolk R, Aiello RJ, Terra SG, Fryburg DA. Potential implications of matrix metalloproteinase-9 in assessment and treatment of coronary artery disease. Biomarkers. 2009;14:118–129. doi: 10.1080/13547500902765140. [DOI] [PubMed] [Google Scholar]
- 27.Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 28.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyayama T, Tsou PS, Fung SM, Fung HL. Simultaneous determination of nitroglycerin and dinitrate metabolites in metabolism studies using liquid chromatography-mass spectrometry with electrospray ionization. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;835:21–26. doi: 10.1016/j.jchromb.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 32.Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem. 2001;268:1918–1928. doi: 10.1046/j.1432-1327.2001.02066.x. [DOI] [PubMed] [Google Scholar]
- 33.Daugherty A, Webb NR, Rateri DL, King VL. Thematic review series: The immune system and atherogenesis. Cytokine regulation of macrophage functions in atherogenesis. J Lipid Res. 2005;46:1812–1822. doi: 10.1194/jlr.R500009-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 35.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 36.Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med. 2006;16:259–265. doi: 10.1016/j.tcm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Parker JO, Fung HL. Transdermal nitroglycerin in angina pectoris. Am J Cardiol. 1984;54:471–476. doi: 10.1016/0002-9149(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 38.Sun JX, Piraino AJ, Morgan JM, Joshi JC, Cipriano A, Chan K, Redalieu E. Comparative pharmacokinetics and bioavailability of nitroglycerin and its metabolites from Transderm-Nitro, Nitrodisc, and Nitro-Dur II systems using a stable-isotope technique. J Clin Pharmacol. 1995;35:390–397. doi: 10.1002/j.1552-4604.1995.tb04079.x. [DOI] [PubMed] [Google Scholar]
- 39.Bustard MA, Ryan G, Seaward G, Saleniak ME, Smith GN. Human maternal and fetal plasma glyceryl trinitrate concentrations. Am J Obstet Gynecol. 2003;189:1777–1778. doi: 10.1016/s0002-9378(03)00866-4. [DOI] [PubMed] [Google Scholar]
- 40.Gerardin A, Gaudry D, Moppert J, Theobald W, Fankhauser P. Glycerol trinitrate (nitroglycerin) plasma concentrations achieved after application of transdermal therapeutic systems to healthy volunteers. Arzneimittelforschung. 1985;35:530–532. [PubMed] [Google Scholar]
- 41.Auclair B, Sirois G, Ngoc AH, Ducharme MP. Population pharmacokinetics of nitroglycerin and of its two metabolites after a single 24-hour application of a nitroglycerin transdermal matrix delivery system. Ther Drug Monit. 1998;20:607–611. doi: 10.1097/00007691-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto S, Kobayashi A. Clinical pharmacokinetics and pharmacodynamics of glyceryl trinitrate and its metabolites. Clin Pharmacokinet. 2003;42:205–221. doi: 10.2165/00003088-200342030-00001. [DOI] [PubMed] [Google Scholar]
- 43.Torfgard KE, Svedjeholm R, Hakansson E, Ahlner J. Tolerance development and arterial and venous tissue-/plasma levels of glyceryl trinitrate and glyceryl dinitrate in patients treated with nitroglycerin infusion. Arzneimittelforschung. 1995;45:963–966. [PubMed] [Google Scholar]
- 44.Santoro A, Rovati LC, Follet M, Setnikar I, Caplain H, Gualano V. Plasma levels of glyceryl trinitrate and dinitrates during application of three strengths of a new glyceryl trinitrate transdermal patch. Arzneimittelforschung. 2000;50:786–794. doi: 10.1055/s-0031-1300290. [DOI] [PubMed] [Google Scholar]
- 45.Williams RL, Thakker KM, John V, Lin ET, Liang-Gee W, Benet LZ. Nitroglycerin absorption from transdermal systems: formulation effects and metabolite concentrations. Pharm Res. 1991;8:744–749. doi: 10.1023/a:1015802101272. [DOI] [PubMed] [Google Scholar]
- 46.Jormsjo S, Wuttge DM, Sirsjo A, Whatling C, Hamsten A, Stemme S, Eriksson P. Differential expression of cysteine and aspartic proteases during progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2002;161:939–945. doi: 10.1016/S0002-9440(10)64254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bocock JP, Edgell CJ, Marr HS, Erickson AH. Human proteoglycan testican-1 inhibits the lysosomal cysteine protease cathepsin L. Eur J Biochem. 2003;270:4008–4015. doi: 10.1046/j.1432-1033.2003.03789.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang EQ, Lee WI, Brazeau D, Fung HL. cDNA microarray analysis of vascular gene expression after nitric oxide donor infusions in rats: implications for nitrate tolerance mechanisms. AAPS PharmSci. 2002;4:E10. doi: 10.1208/ps040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pi X, Yan C, Kim D, Chen J, Berk BC. Differential expression of genes from nitrate-tolerant rat aorta. J Vasc Res. 2002;39:304–310. doi: 10.1159/000065542. [DOI] [PubMed] [Google Scholar]
- 50.Molloy KJ, Thompson MM, Jones JL, Schwalbe EC, Bell PR, Naylor AR, Loftus IM. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation. 2004;110:337–343. doi: 10.1161/01.CIR.0000135588.65188.14. [DOI] [PubMed] [Google Scholar]
- 51.Sluijter JP, Pulskens WP, Schoneveld AH, Velema E, Strijder CF, Moll F, de Vries JP, Verheijen J, Hanemaaijer R, de Kleijn DP, Pasterkamp G. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37:235–239. doi: 10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- 52.Tuomainen AM, Nyyssonen K, Laukkanen JA, Tervahartiala T, Tuomainen TP, Salonen JT, Sorsa T, Pussinen PJ. Serum matrix metalloproteinase-8 concentrations are associated with cardiovascular outcome in men. Arterioscler Thromb Vasc Biol. 2007;27:2722–2728. doi: 10.1161/ATVBAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 53.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 54.Deguchi JO, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 55.de Coignac AB, Elson G, Delneste Y, Magistrelli G, Jeannin P, Aubry JP, Berthier O, Schmitt D, Bonnefoy JY, Gauchat JF. Cloning of MMP-26. A novel matrilysin-like proteinase. Eur J Biochem. 2000;267:3323–3329. doi: 10.1046/j.1432-1327.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- 56.Uria JA, Lopez-Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60:4745–4751. [PubMed] [Google Scholar]
- 57.Wagsater D, Bjork H, Zhu C, Bjorkegren J, Valen G, Hamsten A, Eriksson P. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514–522. doi: 10.1016/j.atherosclerosis.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Haverslag R, Pasterkamp G, Hoefer IE. Targeting adhesion molecules in cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets. 2008;8:252–260. doi: 10.2174/187152908786786188. [DOI] [PubMed] [Google Scholar]
- 59.Christensen L. The distribution of fibronectin, laminin and tetranectin in human breast cancer with special attention to the extracellular matrix. APMIS Suppl. 1992;26:1–39. [PubMed] [Google Scholar]
- 60.Hogdall CK, Christensen L, Clemmensen I. Tetranectin, a plasma and tissue protein--a prognostic marker of breast and ovarian cancer. Ugeskr Laeger. 1994;156:6190–6195. [PubMed] [Google Scholar]
- 61.Kenji K, Hironori U, Hideya Y, Michinori I, Yasuhiko H, Nobuoki K. Tenascin-C is associated with coronary plaque instability in patients with acute coronary syndromes. Circ J. 2004;68:198–203. doi: 10.1253/circj.68.198. [DOI] [PubMed] [Google Scholar]
- 62.Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, Sharifi BG. Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation. 1999;99:1284–1289. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]
- 63.Cheng M, Hashmi S, Mao X, Zeng QT. Relationships of adiponectin and matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio with coronary plaque morphology in patients with acute coronary syndrome. Can J Cardiol. 2008;24:385–390. doi: 10.1016/s0828-282x(08)70602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, Cambien F, Tiret L, Munzel T, Blankenberg S. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study. Eur Heart J. 2006;27:150–156. doi: 10.1093/eurheartj/ehi582. [DOI] [PubMed] [Google Scholar]
- 65.Nunez C, Victor VM, Tur R, Alvarez-Barrientos A, Moncada S, Esplugues JV, D'Ocon P. Discrepancies between nitroglycerin and NO-releasing drugs on mitochondrial oxygen consumption, vasoactivity, and the release of NO. Circ Res. 2005;97:1063–1069. doi: 10.1161/01.RES.0000190588.84680.34. [DOI] [PubMed] [Google Scholar]
- 66.Tsou PS, Addanki V, Haas JA, Page NA, Fung HL. Role of Glutaredoxin-Mediated Protein S-Glutathionylation in Cellular Nitroglycerin Tolerance. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.149997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez-Franco O, Hernandez-Vargas P, Ortiz-Munoz G, Sanjuan G, Suzuki Y, Ortega L, Blanco J, Egido J, Gomez-Guerrero C. Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1864–1870. doi: 10.1161/01.ATV.0000229659.94020.53. [DOI] [PubMed] [Google Scholar]
- 68.Hishikawa K, Nakaki T, Fujita T. Oral flavonoid supplementation attenuates atherosclerosis development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:442–446. doi: 10.1161/01.ATV.0000148404.24271.fc. [DOI] [PubMed] [Google Scholar]
- 69.Tassorelli C, Greco R, Morazzoni P, Riva A, Sandrini G, Nappi G. Parthenolide is the component of tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: studies in an animal model of migraine. Cephalalgia. 2005;25:612–621. doi: 10.1111/j.1468-2982.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- 70.Parada-Turska J, Paduch R, Majdan M, Kandefer-Szerszen M, Rzeski W. Antiproliferative activity of parthenolide against three human cancer cell lines and human umbilical vein endothelial cells. Pharmacol Rep. 2007;59:233–237. [PubMed] [Google Scholar]
- 71.Tomita T, Kunugiza Y, Nomura K, Morimoto D, Kuroda S, Yoshikawa H. Application of NFkappaB inhibitor for arthritis. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:71–76. doi: 10.2177/jsci.32.71. [DOI] [PubMed] [Google Scholar]
- 72.Wang W, Adachi M, Zhang R, Zhou J, Zhu D. A novel combination therapy with arsenic trioxide and parthenolide against pancreatic cancer cells. Pancreas. 2009;38:e114–e123. doi: 10.1097/MPA.0b013e3181a0b6f2. [DOI] [PubMed] [Google Scholar]
- 73.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15:143–152. [PubMed] [Google Scholar]
- 74.Dell'agli M, Galli GV, Bosisio E, D'Ambrosio M. Inhibition of NF-kB and metalloproteinase-9 expression and secretion by parthenolide derivatives. Bioorg Med Chem Lett. 2009 doi: 10.1016/j.bmcl.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 75.Gomez-Hernandez A, Martin-Ventura JL, Sanchez-Galan E, Vidal C, Ortego M, Blanco-Colio LM, Ortega L, Tunon J, Egido J. Overexpression of COX-2, Prostaglandin E synthase-1 and prostaglandin E receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: regulation by nuclear factor-kappaB. Atherosclerosis. 2006;187:139–149. doi: 10.1016/j.atherosclerosis.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 76.Borden P, Heller RA. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997;7:159–178. doi: 10.1615/critreveukargeneexpr.v7.i1-2.90. [DOI] [PubMed] [Google Scholar]
- 77.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 78.Kang JL, Lee K, Castranova V. Nitric oxide up-regulates DNA-binding activity of nuclear factor-kappaB in macrophages stimulated with silica and inflammatory stimulants. Mol Cell Biochem. 2000;215:1–9. doi: 10.1023/a:1026581301366. [DOI] [PubMed] [Google Scholar]
- 79.Lander HM, Sehajpal P, Levine DM, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993;150:1509–1516. [PubMed] [Google Scholar]
- 80.Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J Biol Chem. 2007;282:17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 81.Chong S, Fung HL. Biochemical and pharmacological interactions between nitroglycerin and thiols. Effects of thiol structure on nitric oxide generation and tolerance reversal. Biochem Pharmacol. 1991;42:1433–1439. doi: 10.1016/0006-2952(91)90456-f. [DOI] [PubMed] [Google Scholar]
- 82.Lee WI, Fung HL. Mechanism-based partial inactivation of glutathione S-transferases by nitroglycerin: tyrosine nitration vs sulfhydryl oxidation. Nitric Oxide. 2003;8:103–110. doi: 10.1016/s1089-8603(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 83.Brennan LA, Wedgwood S, Bekker JM, Black SM. Nitric oxide activates p21ras and leads to the inhibition of endothelial NO synthase by protein nitration. DNA Cell Biol. 2003;22:317–328. doi: 10.1089/104454903322216662. [DOI] [PubMed] [Google Scholar]
- 84.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. Faseb J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 85.Arsura M, Mercurio F, Oliver AL, Thorgeirsson SS, Sonenshein GE. Role of the IkappaB kinase complex in oncogenic Ras- and Raf-mediated transformation of rat liver epithelial cells. Mol Cell Biol. 2000;20:5381–5391. doi: 10.1128/mcb.20.15.5381-5391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001;88:1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 87.Rossignol P, Cambillau M, Bissery A, Mouradian D, Benetos A, Michel JB, Plouin PF, Chatellier G, Jacob MP. Influence of blood sampling procedure on plasma concentrations of matrix metalloproteinases and their tissue inhibitors. Clin Exp Pharmacol Physiol. 2008;35:464–469. doi: 10.1111/j.1440-1681.2008.04897.x. [DOI] [PubMed] [Google Scholar]
- 88.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 89.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 90.Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, Yan ZQ. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB. Am J Pathol. 2006;169:2245–2253. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CB, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 92.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 93.Diamanti-Kandarakis E, Livadas S, Kandarakis SA, Margeli A, Papassotiriou I. Serum concentrations of atherogenic proteins neutrophil gelatinase-associated lipocalin and its complex with matrix metalloproteinase-9 are significantly lower in women with polycystic ovary syndrome: hint of a protective mechanism? Eur J Endocrinol. 2008;158:525–531. doi: 10.1530/EJE-07-0822. [DOI] [PubMed] [Google Scholar]
- 94.Leclercq A, Houard X, Philippe M, Ollivier V, Sebbag U, Meilhac O, Michel JB. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007;82:1420–1429. doi: 10.1189/jlb.1106671. [DOI] [PubMed] [Google Scholar]
- 95.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr Med Chem. 2001;8:425–474. doi: 10.2174/0929867013373417. [DOI] [PubMed] [Google Scholar]
- 96.Zimrin D, Reichek N, Bogin KT, Aurigemma G, Douglas P, Berko B, Fung HL. Antianginal effects of intravenous nitroglycerin over 24 hours. Circulation. 1988;77:1376–1384. doi: 10.1161/01.cir.77.6.1376. [DOI] [PubMed] [Google Scholar]