Abstract
Introduction
Vitamin D deficiency is common in patients with primary hyperparathyroidism (PHPT). The presence of low levels of vitamin D may affect the skeletal consequences of PHPT.
Methods
In this cross-sectional study, transiliac crest bone biopsies were performed after double tetracycline labeling in patients with mild PHPT and analyzed according to serum levels of 25 hydroxyvitamin D (25OHD).
Results
We studied 30 patients with mild PHPT (age 53±11 years; 67% women; calcium 11.1±1.0 mg/dl; PTH 149±129 pg/ml). Serum 25OHD levels were low in the majority of subjects (mean 21±11 ng/ml) and inversely associated with PTH (r=-0.69; p<0.01). 25OHD levels were directly associated with cortical width (Ct.Wi; r=0.46, p<0.03) and trabecular separation (Tb.Sp; r=0.41; p<0.04), but inversely associated with cancellous bone volume (BV/TV; r=-0.39, p<0.04). Subjects with 25OHD levels <20 ng/ml (n=14) and ≥20 ng/ml (n=16) were compared. Groups did not differ by age, sex, menopausal status, serum calcium, creatinine, or 1,25(OH)2D. PTH was 1.8-fold higher in subjects with 25OHD <20 (265±166 pg/ml vs. 95±50 pg/ml; p <0.01). On histomorphometric analysis, those with low 25OHD had lower Ct.Wi (541±167 μm vs. 712±200 μm; p<0.03). Conversely, measures of trabecular microarchitecture were better in those with lower 25OHD, with higher BV/TV (26.1±6.1 % vs. 20.4±6.4 %; p<0.03), greater trabecular number (Tb.N: 2.0±0.4 mm -1 vs. 1.8±0.4 mm -1; p<0.04) and lower Tb.Sp (371±90 μm vs. 472±137 μm; p<0.04). There were no differences between the groups in bone remodeling indices.
Conclusions
Low levels of 25OHD in patients with PHPT are associated with higher concentrations of PTH, greater catabolic effects in cortical bone and greater anabolic effects in trabecular bone.
Keywords: Primary hyperparathyroidism, secondary hyperparathyroidism, vitamin D deficiency, histomorphometry, cortical bone
1.1 Introduction
Vitamin D insufficiency is common among patients with primary hyperparathyroidism (PHPT), and may be more prevalent among individuals with PHPT than in geographically matched healthy populations.[1, 2] Lower 25-hydroxyvitamin D (25OHD) levels have been reported to be associated with higher circulating parathyroid hormone (PTH), increased parathyroid gland weight, accelerated bone turnover and increased fracture risk.[1, 3-5] The consequences and optimal management of vitamin D insufficiency in these patients are unclear. While it is common for practitioners to avoid repleting low vitamin D levels in patients with PHPT for fear of exacerbating hypercalcemia or hypercalciuria, this strategy has the potential to worsen or unmask underlying bone disease.
Several putative mechanisms have been proposed to account for the increased prevalence of vitamin D deficiency among individuals with PHPT. Chronic vitamin D deficiency may stimulate autonomous parathyroid glands with subsequent development of hyperplasia and transformation to adenoma, or may accelerate the growth of a pre-existing adenoma.[6] Alternatively, the PTH mediated increase in 1,25-dihydroxyvitamin D [1,25(OH)2D] synthesis may reduce 25OHD by inhibiting cutaneous synthesis of vitamin D3, inhibiting hepatic synthesis of 25OHD, and by increasing renal conversion of 25OHD to 1,25(OH)2D.[1, 7, 8]
The skeletal phenotype of patients with PHPT has been characterized by bone densitometry (using DXA technology) and by histomorphometric analysis of bone biopsy specimens.[9-19] Patients with PHPT usually have relatively normal BMD at the lumbar spine, a site rich in cancellous bone, while BMD at the distal 1/3 radius, a site comprised predominantly (95%) of cortical bone, is often low. Consistent with this pattern, quantitative histomorphometry has demonstrated that the cortices are significantly thinner in PHPT patients[20] while trabecular bone is preserved.[10, 18]
Limited data are available regarding the specific histomorphometric changes in individuals with PHPT and vitamin D deficiency. In particular, it is not clear whether the higher PTH levels associated with vitamin D deficiency further augment the effects of parathyroid hormone on bone. In this study, we compared histomorphometry in subjects with mild PHPT with and without vitamin D deficiency, defined as 25OHD <20 ng/ml. We hypothesized that subjects with co-existing mild PHPT and vitamin D deficiency would have more pronounced PTH effect, as evidenced by both greater cortical thinning and more sustained preservation of cancellous bone.
2.1.1 Methods
Patients with PHPT were recruited as part of a longitudinal study designed to evaluate the natural history of PHPT after medical or surgical management.[21, 22] Data from a sequentially enrolled subset of patients who both underwent transiliac crest bone biopsy and in whom 25OHD was measured are presented here. The protocol was approved by the Institutional Review Board of Columbia University Medical Center and all subjects provided written informed consent.
Serum measurements of 25OHD, PTH, 1,25OH2D and creatinine were performed as previously described.[9] PTH levels in the earliest patients enrolled were measured by N-terminal assay. PTH data are included only for those subjects analyzed by IRMA. Percutaneous transiliac crest bone biopsies were performed after double tetracycline labeling. Biopsy specimens were obtained using a Bordier type trephine with an inner diameter of 7.5 mm. Specimens were processed and subjected to histomorphometric analysis as previously described in our laboratory.[11-13] All variables were expressed according to the recommendations of the ASBMR nomenclature committee.[23]
2.1.2 Statistical Methods
Analyses were conducted with STATA version 9.0 (Stata Corp, College Station, Texas). Two-sided p values < 0.05 were considered to indicate statistical significance. Data are presented as mean ± standard deviation (SD). Normality was assessed by Shapiro-Wilk normality test. Those variables that were not normally distributed were logarithmically transformed prior to comparison of between groups differences by Student's t test. Spearman correlation analyses were performed to test associations between variables of interest. Seasonal variation in 25OHD, PTH, and histomorphometry was examined using Kruskal Wallis one way ANOVA.
3.1 Results
We studied 30 patients with mild PHPT. Demographics were typical for patients with PHPT: the mean age was 53 ± 11 years and the majority of patients (67%) were women. Mean serum calcium was 11.1 ± 1.0 mg/dL (nl: 8.4-10.2 mg/dL) and PTH was 149 ± 129 pg/ml (nl: 10-65 pg/ml). Serum 25OHD levels were low in the majority of subjects (mean 21 ± 11 ng/ml, nl: 30-100 ng/ml) while 1,25OH2D levels were within normal limits (mean: 47±17 pg/ml, nl: 15-60). Only four subjects (13%) had 25OHD levels above 30 ng/ml, the threshold currently used to denote sufficiency. Fourteen subjects (47%) had 25OHD levels below the 20 ng/ml cut-point identified by the new international guidelines as the level at which vitamin D should be repleted in PHPT [24]; of these, 10 had levels between 10-< 20 ng/ml and four subjects had levels below 10 ng/ml. Serum 25OHD was inversely associated with PTH (r= -0.69; p<0.01). No association was found between serum 1,25OH2D and either 25OHD or PTH. Vitamin D levels were obtained on most patients in the winter or early spring in New York City (December to May), and no seasonal variability was found.
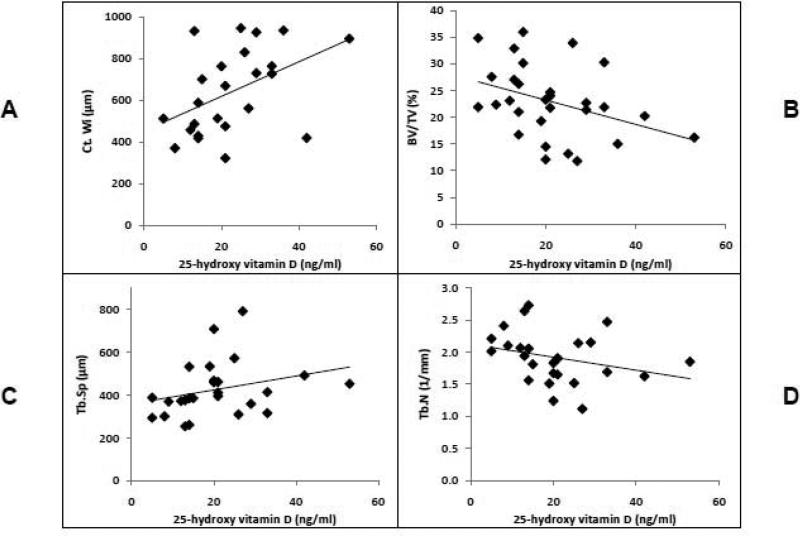
When histomorphometric indices were examined as continuous variables in the entire cohort, lower 25OHD was found to be associated with lower cortical width (r= 0.46, p <0.03; Figure 1A) and with greater cancellous bone volume (r= -0.39, p <0.04; Figure 1B). Lower 25OHD was also associated with lower trabecular separation (r= 0.41; p< 0.04; Figure 1C), and tended to be associated with greater trabecular number (r= -0.37, p= 0.06; Figure 1D).
Figure 1.
Association of 25OHD with cortical width (r= 0.46, p <0.03; panel A), cancellous bone volume (r= -0.39, p <0.04; panel B), trabecular separation (r= 0.41; p< 0.04; panel C), and trabecular number (r= -0.37, p= 0.06; panel D).
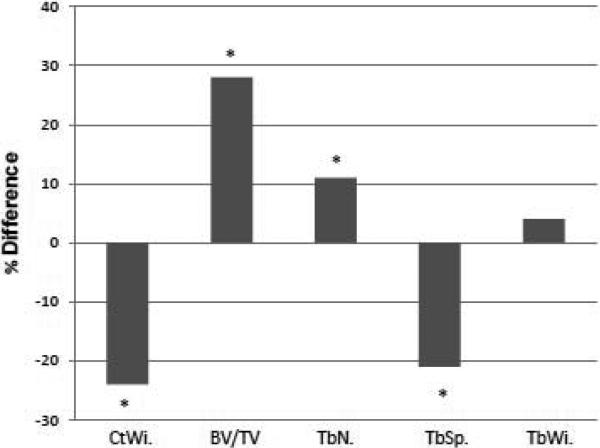
We then compared data from subjects with 25OHD levels <20 (n=14) and ≥ 20 ng/ml (n=16; data are presented in Table 1). These groups did not differ by age, sex, menopausal status, serum calcium, creatinine, or 1,25OH2D. PTH was 1.8 fold higher in subjects with 25OHD < 20 ng/ml (p <0.02). Histomorphometric analysis revealed markedly lower cortical width in subjects with 25OHD < 20 ng/ml (p< 0.04). Cortical porosity did not differ between the groups. In contrast, measures of trabecular microarchitecture were better in these subjects than in those with higher 25OHD. Subjects with 25OHD < 20 ng/ml had higher trabecular bone volume (p < 0.03), higher trabecular number (p< 0.04), and conversely, lower trabecular separation (p< 0.04). Trabecular width did not differ between the two groups, nor did wall width. Differences between histomorphometric indices in those with 25OHD < 20ng/ml and those with 25OHD> 20 ng/ml are presented in Figure 2.
Table 1.
Subject characteristics and histomorphometry by 25OHD level.
| 25OHD<20 N=14 | 25OHD ≥20 N=16 | P-value | |
|---|---|---|---|
| Age | 55 ± 12 | 52 ± 11 | NS |
| Sex (% female) | 71% | 63% | NS |
| Calcium (8.8-10.4 mg/dl) | 11.0 ±0.8 | 11.2 ±1.2 | NS |
| Creatinine (0.6-1.5 mg/dl) | 1.0 ± 0.2 | 1.1 ± 0.2 | NS |
| PTH (10-65 pg/ml) | 265 ± 166 | 95 ±50 | <0.02 |
| 1,25(OH)2D (15-60 pg/ml) | 54.6 ± 33.0 | 46.9 ±17.9 | NS |
| 25OHD (30-100 ng/ml) | 12.5 ± 4.4 | 28.5 ± 9.3 | |
| STRUCTURAL INDICES: | |||
| Cortical Width (μm; Ct.Wi) | 541 ± 167 | 712 ± 200 | <0.04 |
| Cancellous bone volume (%; BV/TV) | 26.1 ± 6.1 | 20.4± 6.4 | <0.03 |
| Trabecular number (1/mm; Tb.N) | 2.0 ± 0.4 | 1.8 ± 0.4 | <0.04 |
| Trabecular separation (μm; Tb.Sp) | 371.1± 90.4 | 472.2± 137.4 | <0.04 |
| Trabecular width (μm; Tb.Wi) | 122.5 ± 21.4 | 117.3± 30.0 | NS |
| REMODELING INDICES: | |||
| Osteoid surface (%) | 31.4±14.6 | 26.3±10.4 | NS |
| Osteoid width (No. lamellae) | 12.7±3.0 | 14.4±2.3 | NS |
| Mineralization lag time (days) | 46.6±40.2 | 37.3±7.6 | NS |
| Mineralizing surface (%) | 20.6±10.8 | 18.1±10.0 | NS |
| Mineral apposition rate (μm/day) | 0.64±01.3 | 0.63±0.10 | NS |
| Bone formation rate (μm3/μm2/day) | 0.13±0.09 | 0.11±0.05 | NS |
| Activation frequency (cycles/year) | 1.08±0.75 | 0.83±0.23 | NS |
Figure 2.
Comparison of structural histomorphometric parameters in subjects with 25OHD <20 and ≥20. Data presented as % difference of low vitamin D vs. higher vitamin D groups. *p<0.05.
Bone remodeling indices, including osteoid surface, osteoid width, activation frequency, mineralization lag time, mineralizing surface, mineral apposition rate or bone formation rate did not differ by vitamin D status (Table 1). Although there were no linear associations between these variables and 25OHD observed in the group as a whole, in the subset of subjects with 25OHD < 20 ng/ml, low 25OHD tended to be associated with increased OS (r= -0.53, p= 0.09), increased OWi (r= -0.63, p= 0.09) and increased ActF (r= -0.68, p= 0.06).
Associations between PTH and histomorphometric measurements were also examined. Higher PTH was associated with greater cortical porosity (r=0.61; p<0.02) and tended to be associated with lower trabecular separation (r=-0.50; p<0.10). There was no other association between PTH and structural parameters. Subjects with higher PTH also tended to have greater osteoid surface (r=0.47; p=0.09), mineralizing surface (r=0.43; p=0.12) and eroded surface (r=0.44; p=0.12).
4.1 Discussion
Patients with PHPT who had low vitamin D levels had higher PTH levels and significant differences in structural indices of bone histomorphometry, with more marked cortical thinning and improved trabecular indices. These findings are consistent with an enhanced effect of PTH, which is anabolic in trabecular bone and catabolic in cortical bone.
In PHPT, bone densitometry is consistent with relative preservation of cancellous bone and cortical bone loss.[9, 15] Parisien et al. found that cancellous bone volume and trabecular number were increased and cortical thickness reduced in 20 men and women with PHPT compared to autopsy controls.[12] Further, age related changes that were observed in controls did not occur in PHPT subjects, suggesting that elevated PTH may modulate the deleterious effects of age on both cancellous and cortical bone. Other age related changes (increase in marrow space star volume or decrease in mean plate density seen in controls) also have been absent in PHPT.[18] Newer analysis of 3-dimensional structure of cancellous bone biopsy specimens confirm these observations.[14] While cancellous bone and indeed trabecular connectivity are preserved in postmenopausal women with PHPT [11] an increase in cortical porosity, and decreased cortical width in this group has also been reported.[16, 17] In the present study, we observed that the previously reported structural changes in subjects with PHPT, namely preservation of cancellous bone and cortical thinning, were more pronounced in those subjects with PHPT and coexisting vitamin D deficiency. These differences may reflect the higher concentration of PTH in the more D deficient group, or they may be due to a PTH independent effect of low 25OHD.
Static and dynamic indices of bone remodeling, osteoid surface, osteoid volume, mineralizing surface, eroded surface, and bone formation rate, are increased in patients with PHPT compared to normal controls [11-13, 17, 18]; osteoid surface and eroded surface positively correlate with PTH levels.[12] Activation frequency was also elevated in PHPT subjects, and at the level of the basic structural unit, an increase in wall width, adjusted apposition rate and active formation period has been observed.[13, 16, 18] We previously reported that subjects with vitamin D deficiency and PHPT had an increase in osteoid surface and mineralizing surface in subjects in the lowest tertile of 25OHD compared to those in the highest tertile.[1] Structural parameters were not examined at that time. These findings were consistent with an enhancement of the remodeling changes seen in PHPT in general. In the present study, we did not compare our subjects to normal controls, and therefore cannot speak to whether remodeling in our subjects was greater than normal. However, we did not observe pronounced differences in remodeling parameters among PHPT subjects with and without vitamin D deficiency nor did we find an association between remodeling and 25OHD in the cohort as a whole. Similar to previous reports, we observed trends between higher PTH with eroded surface, osteoid surface and mineralizing surface; our findings did not reach significance, likely due to the small sample size. It is conceivable that we did not observe associations between remodeling parameters and 25OHD in the group as a whole because the effects of PTH on remodeling parameters were dominant except in those subjects with very low levels of 25OHD. This hypothesis is supported by the trend toward associations of 25OHD levels with greater remodeling in subjects who were vitamin D deficient. We may not have observed differences in remodeling because structural parameters reflected the effects of elevated PTH and low 25OHD over time, whereas the remodeling parameters solely reflected conditions at the time of the biopsy. Again, lack of statistical power and few subjects with adequate vitamin D stores in the cohort may also have precluded our ability to detect remodeling differences.
Histomorphometric studies in subjects with vitamin D deficiency have almost exclusively focused on patients deficient in the active moiety (1,25(OH)2D) secondary to end stage kidney disease. In these subjects, osteomalacia, or profound 1,25(OH)2D deficiency is reflected in increased osteoid surface, thickened osteoid seams, and prolonged mineralization lag time.[25, 26] Orwoll et al. found that, mineralization lag time was increased and improved similarly after treatment with calcium or calcium and vitamin D in osteoporotic subjects, whose mean 25OHD levels were 14-18 ng/ml.[27] These authors suggest that the mineralization defect was more likely a result of insufficient calcium availability as opposed to lack of vitamin D. Two recent studies have evaluated bone biopsies in patients with normal kidney function and 25OHD deficiency. Need at al.[28] found seasonal changes in histomorphometry in 121 osteoporotic subjects, with a median age 66, in South Australia. Although no subject had osteomalacia by biopsy, osteoid thickness and osteoid maturation time were greatest in the winter, when 25OHD levels were lowest, and were inversely correlated with 25OHD. No association between 1,25(OH)2D and these parameters was found. BV/TV did not change throughout the year and other structural parameters (TbN, TbSp) are not reported. Armas et al. also reported that osteoid thickness, mineralization lag time and osteoid maturation time were greater during winter months in postmenopausal but not premenopausal women.[29] While we observed a trend toward increased osteoid width and osteoid surface with 25OHD level, perhaps with a greater number of subjects our findings would have been more similar to those of the above studies. It is also conceivable that differences in 25OHD levels have a greater impact on the postmenopausal skeleton [29], and therefore were less pronounced in our cohort which included a range of ages, both genders and variable menopausal status. Further, as suggested by the results of Orwoll et al., the mild elevation in serum calcium among our subjects may have masked some of the increase in remodeling that would be expected with vitamin D deficiency alone. Finally, the lack of seasonal variability in our patients may have been due to the relative homogeneity of season of analysis in this small group.
Limitations of this study include the retrospective, cross-sectional design as well as the, and lack of a normal control group. The sample size was moderate, and there were missing PTH data due to changes in assay methodology that may have further reduced our ability to detect all of the expected effects of PTH on histomorphometry. The distribution of 25OHD levels in our cohort, with very few subjects who had levels above 30 ng/ml, precluded our ability to evaluate histomorphometry in individuals with primary hyperparathyroidism who were vitamin D sufficient. Further as very few subjects had 25OHD levels in the severely deficient range (below10 ng/ml), we were not able to determine whether there is a threshold below which the effects of low 25OHD itself would predominate over those of elevated PTH.
Based upon these data, it is not possible to isolate the specific effects of high PTH compared to those of low 25OHD on bone structure. Nonetheless, the data offer a window into the consequences of these coexisting conditions on the skeleton. A prospective study that included PHPT subjects across the full range from severely deficient to those who are vitamin D replete might provide greater insight in this area. Ultimately, we would like to be able to extrapolate these data to clinical expectations. However, at this point we do not know whether the observed cortical thinning leads to an increase in fracture incidence in this population, or whether the improvement seen in cancellous indices might offset this effect.
5.1 Conclusions
In summary, individuals with concurrent PHPT and vitamin D deficiency have higher levels of parathyroid hormone, and structural changes by histomorphometry, in both cortical (lower cortical width), and cancellous (higher cancellous bone volume, higher trabecular number, and lower trabecular separation) bone. The skeleton demonstrates greater catabolic consequences in cortical bone and greater anabolic effects in trabecular bone. While these effects are consistent with an enhanced PTH effect, larger studies are needed to delineate the specific contributions of high PTH and low 25OHD to this skeletal phenotype.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med. 1999;107:561–7. doi: 10.1016/s0002-9343(99)00294-6. [DOI] [PubMed] [Google Scholar]
- 2.Boudou P, Ibrahim F, Cormier C, Sarfati E, Souberbielle JC. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J Endocrinol Invest. 2006;29:511–5. doi: 10.1007/BF03344140. [DOI] [PubMed] [Google Scholar]
- 3.Rao DS, Honasoge M, Divine GW, Phillips ER, Lee MW, Ansari MR, Talpos GB, Parfitt AM. Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J Clin Endocrinol Metab. 2000;85:1054–8. doi: 10.1210/jcem.85.3.6440. [DOI] [PubMed] [Google Scholar]
- 4.Nordenstrom E, Westerdahl J, Lindergard B, Lindblom P, Bergenfelz A. Multifactorial risk profile for bone fractures in primary hyperparathyroidism. World J Surg. 2002;26:1463–7. doi: 10.1007/s00268-002-6433-2. [DOI] [PubMed] [Google Scholar]
- 5.Rao DS, Agarwal G, Talpos GB, Phillips ER, Bandeira F, Mishra SK, Mithal A. Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: a global perspective. J Bone Miner Res. 2002;17(Suppl 2):N75–80. [PubMed] [Google Scholar]
- 6.Parfitt AM. Parathyroid growth: Normal and abnormal. In: Bilezikian JP, Levine MA, Marcus R, editors. The Parathyroids: Basic and Clinical Concepts. Academic Press; New York: 2001. pp. 293–330. [Google Scholar]
- 7.Mosekilde L, Charles P, Lindegreen P. Determinants for serum 1,25-dihydroxycholecalciferol in primary hyperparathyroidism. Bone Miner. 1989;5:279–90. doi: 10.1016/0169-6009(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 8.Clements MR, Davies M, Fraser DR, Lumb GA, Mawer EB, Adams PH. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci (Lond) 1987;73:659–64. doi: 10.1042/cs0730659. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–91. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 10.Parisien M, Mellish RW, Silverberg SJ, Shane E, Lindsay R, Bilezikian JP, Dempster DW. Maintenance of cancellous bone connectivity in primary hyperparathyroidism: trabecular strut analysis. J Bone Miner Res. 1992;7:913–9. doi: 10.1002/jbmr.5650070808. [DOI] [PubMed] [Google Scholar]
- 11.Parisien M, Cosman F, Mellish RW, Schnitzer M, Nieves J, Silverberg SJ, Shane E, Kimmel D, Recker RR, Bilezikian JP, et al. Bone structure in postmenopausal hyperparathyroid, osteoporotic, and normal women. J Bone Miner Res. 1995;10:1393–9. doi: 10.1002/jbmr.5650100917. [DOI] [PubMed] [Google Scholar]
- 12.Parisien M, Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, Dempster DW. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab. 1990;70:930–8. doi: 10.1210/jcem-70-4-930. [DOI] [PubMed] [Google Scholar]
- 13.Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 1999;84:1562–6. doi: 10.1210/jcem.84.5.5652. [DOI] [PubMed] [Google Scholar]
- 14.Dempster DW, Muller R, Zhou H, Kohler T, Shane E, Parisien M, Silverberg SJ, Bilezikian JP. Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone. 2007;41:19–24. doi: 10.1016/j.bone.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen P, Steiniche T, Brixen K, Hessov I, Melsen F, Charles P, Mosekilde L. Primary hyperparathyroidism: biochemical markers and bone mineral density at multiple skeletal sites in Danish patients. Bone. 1997;21:93–9. doi: 10.1016/s8756-3282(97)00078-1. [DOI] [PubMed] [Google Scholar]
- 16.Brockstedt H, Christiansen P, Mosekilde L, Melsen F. Reconstruction of cortical bone remodeling in untreated primary hyperparathyroidism and following surgery. Bone. 1995;16:109–17. doi: 10.1016/s8756-3282(94)00017-4. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen P, Steiniche T, Brockstedt H, Mosekilde L, Hessov I, Melsen F. Primary hyperparathyroidism: iliac crest cortical thickness, structure and remodeling evaluated by histomorphometric methods. Aarhus Bone and Mineral Research Group. Bone. 1993;14:403–8. doi: 10.1016/8756-3282(93)90171-6. [DOI] [PubMed] [Google Scholar]
- 18.Christiansen P, Steiniche T, Vesterby A, Mosekilde L, Hessov I, Melsen F. Primary hyperparathyroidism: iliac crest trabecular bone volume, structure, remodeling, and balance evaluated by histomorphometric methods. Bone. 1992;13:41–9. doi: 10.1016/8756-3282(92)90360-9. [DOI] [PubMed] [Google Scholar]
- 19.Steiniche T, Christiansen P, Vesterby A, Ullerup R, Hessov I, Mosekilde LE, Melsen F. Primary hyperparathyroidism: bone structure, balance, and remodeling before and 3 years after surgical treatment. Bone. 2000;26:535–43. doi: 10.1016/S8756-3282(00)00260-X. [DOI] [PubMed] [Google Scholar]
- 20.Parisien M, Silverberg SJ, Shane E, de la CL, Lindsay R, Bilezikian JP, Dempster DW. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin.Endocrinol.Metab. 1990;70:930–938. doi: 10.1210/jcem-70-4-930. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 22.Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93:3462–70. doi: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 24.Bilezikian JP, Khan AA, Potts JT., Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parfitt AM, Qiu S, Rao DS. The mineralization index--a new approach to the histomorphometric appraisal of osteomalacia. Bone. 2004;35:320–5. doi: 10.1016/j.bone.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Compston J. Vitamin D. In: Feldman D, Glorieux FH, Pike JW, editors. Bone Histomorphometry. Academic Press; New York: 1997. pp. 573–86. [Google Scholar]
- 27.Orwoll ES, McClung MR, Oviatt SK, Recker RR, Weigel RM. Histomorphometric effects of calcium or calcium plus 25-hydroxyvitamin D3 therapy in senile osteoporosis. J Bone Miner Res. 1989;4:81–8. doi: 10.1002/jbmr.5650040112. [DOI] [PubMed] [Google Scholar]
- 28.Need AG, Horowitz M, Morris HA, Moore R, Nordin C. Seasonal change in osteoid thickness and mineralization lag time in ambulant patients. J Bone Miner Res. 2007;22:757–61. doi: 10.1359/jbmr.070203. [DOI] [PubMed] [Google Scholar]
- 29.Armas L, Heaney RP, Recker RR. Seasonal variation in bone histomorphometry. J Bone Miner Res. 2008;23:301. doi: 10.1359/jbmr.071026. author reply 301. [DOI] [PubMed] [Google Scholar]