Abstract
Earlier studies indicated that transgenic (tg) mice engineered to express prion protein (PrP) lacking the glycophosphatidylinositol (GPI−/−) membrane anchor formed abnormal proteinase-resistant prion (PrPsc) amyloid deposits in their brains and hearts when infected with the RML strain of murine scrapie. In contrast, RML scrapie infection of normal mice with a GPI-anchored PrP did not deposit amyloid with PrPsc in the brain or the heart. Here we report that scrapie-infected GPI−/− PrP tg mice also deposit PrP and transmissible infectious material in the gut, kidneys, and islets of Langerhans. Similar to previously reported amyloid deposits in the brain and heart, amyloid deposits were found in the gut; however, no amyloid deposited in the islets. By high-resolution electron microscopy, we show PrP is located primarily in α cells and also β cells. Islets contain abundant insulin and there is no abnormality in glucose metabolism in infected GPI−/− PrP tg mice.
Keywords: scrapie, gut, kidney, pancreas, GPI anchor, amyloid, islets of Langerhans, transgenic
INTRODUCTION
Scrapie, transmissible spongiform encephalopathies, or prion diseases are known clinically for causing a progressive fatal neurodegenerative illness involving humans and animals. Biochemically, these disorders are characterized by conversion of the normal host cellular proteinase-sensitive protein (PrPc) to the disease associated form of PrP that is proteinase resistant (PrPsc). Biologically this protein-folding disease is associated with production and transmission of infectivity of tissue containing the PrPsc to normal recipients bearing the same species PrPc.
Previously we generated transgenic mice in which a stop codon was engineered at the COO- end of the PrPc so that the terminal 21 amino acids were not translated creating a PrP lacking its glycophosphatidylinositol (GPI) membrane anchor (Chesebro et al., 2005). This construct was fused to the PrPc promoter and knocked into mice that previously had their PrP gene knocked-out (Bueler et al., 1993; Manson et al., 1994). Such transgenic mice, termed GPI−/− PrP tg mice, lived normal uneventful life spans (600 to 700 days) and reproduced normally (Chesebro et al., 2005). Intracerebral (i.c.) inoculation of GPI−/− PrP tg mice with any of several strains of murine scrapie (RML [Chandler], ME7 or 22L) resulted in the conversion of PrPc to PrPsc and the generation of infectious transmissible material. However, despite abundant deposits of the abnormally folded PrPsc that quantitatively and significantly exceeded PrPsc deposits formed in scrapie-infected mice with a membrane anchored PrP, these scrapie-infected GPI−/− PrP tg mice failed to develop progressive fatal neurodegenerative disease reminiscent of scrapie infection. Instead, they lived normal life spans of 600 to 700 days but displayed abnormalities in CNS electrophysiology and cognitive learning (Trifilo et al., 2008). In contrast, control mice with a membrane-anchored PrPc developed the expected progressively fatal neurodegenerative disease by 150 to 180 days post-scrapie challenge. Two other interesting and novel findings were observed in scrapie infected GPI−/− PrP tg mice. First, the non-amyloid PrPsc deposits accompanying RML infection of GPI membrane-anchored mice converted to PrPsc deposits as amyloid plaques in mice having a non-membrane-anchored PrPc indicating that the GPI anchor plays a role in pathogenesis of prion disease and amyloid deposits (Chesebro et al., 2005; Trifilo et al., 2006). Interestingly, similar amyloid deposits noted in the GPI−/− PrP tg mice have been recently reported for humans with a mutation in their GPI anchor (Jansen et al., 2010). Second, in addition to the CNS PrPsc/amyloid deposits occurring in GPI−/− PrP tg mice infected with scrapie, these mice also displayed extraneural manifestations of PrPsc amyloid deposits in their hearts (Trifilo et al., 2006). As a result, heart function was compromised as hearts from scrapie-infected GPI−/− PrP tg mice but not uninfected GPI−/− PrP tg mice failed to handle an increase in fluid load leading to heart failure (Trifilo et al., 2006).
This paper explores other extraneural manifestations that occur in GPI−/− PrP tg mice infected with RML scrapie, with a focus on the PrP deposits in the gut, kidney, and pancreas. In the gut PrPsc deposits, amyloid and infectious transmissible material was noted. In the kidney PrPsc was found in focal areas of the glomeruli and in the urine. Further, infectivity was transmitted from the urine. For the pancreas, PrP was located in the islets of Langerhans and not the acinar cells. Utilizing high-resolution immuno-electron microscopy, PrP was found primarily in the α-cells and, to a lesser extent, β-cells of the islets but with a clear absence of amyloid deposits. The morphology and numbers of α- and β- cells appear normal. Such scrapie-infected GPI−/− PrP tg mice showed normal blood glucose levels, glucose tolerance tests, and insulin in the islets. Lastly, infectious transmissible material was found in the islets.
RESULTS
Extraneural manifestations of scrapie infection
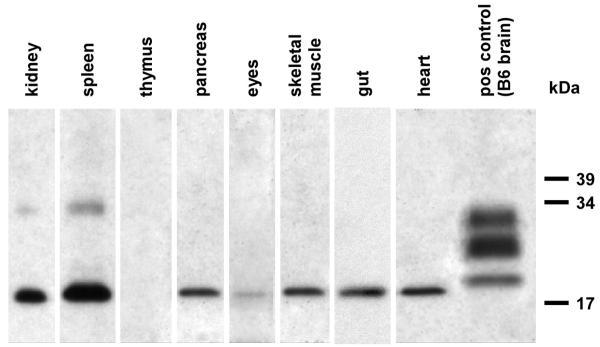
Twenty 8 to 10 week old GPI−/− PrP tg mice were infected i.c. with RML scrapie and followed for 400 days to determine whether there was conversion of PrP found at multiple extraneural sites. Similarly assayed were ten age- and sex-matched GPI−/− PrP tg mice not receiving scrapie. As shown in Figure 1 by Western blot, scrapie infected GPI−/− PrP tg mice converted PrPc to PrPsc in their kidneys, spleen, pancreas, eye, skeletal muscle, heart, gut, and brain. Conversion to PrPsc occurred at this time in 18 of 20 (90%) of the scrapie-infected GPI−/− PrP tg mice in the kidney, pancreas, eye, skeletal muscle and heart, and in 20 of 20 (100%) in the gut and brain (data not shown). PrPsc conversion from PrPc was not detected in the thymus of these mice. As Figure 1 illustrates, the PrPsc was mono-glycosylated (by Western blot) which corresponded to our early observations with brain and heart (Chesebro et al., 2005; Trifilo et al., 2008; Trifilo et al., 2006). None of the tissues obtained from uninfected GPI−/− PrP tg mice displayed PrPsc by either Western blot or immunohistochemistry (data not shown).
Fig. 1.
PrPsc deposition in extraneural tissues. Western blots showing PrPsc in various tissue homogenates from the infected GPI−/− PrP tg mouse. Tissues were taken at 400 days post-infection, and homogenized to a 2% final concentration. All samples were treated with 25 μg/mL proteinase K prior to SDS-PAGE, and were blotted for PrP using the D18 monoclonal anti-PrP antibody at 1:300. Brain homogenate from an infected B6 mouse is shown as a control.
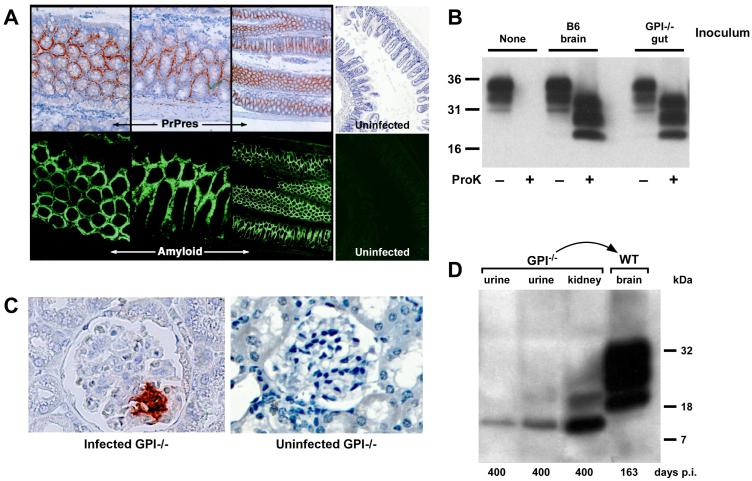
Next, we limited our focus to studies of the gut, kidney, and pancreas. Since GPI−/− PrP tg mice infected with RML scrapie expressed PrPsc with amyloid deposits in their hearts and brains, we analyzed the gut from all twenty infected mice for PrPsc-associated amyloid deposits. As seen in Figure 2A, PrPsc (PrPres) occurred as amyloid deposits. This was observed in all twenty PrPsc-infected GPI−/− PrP tg mice, but PrPsc and amyloid were absent in all ten GPI−/− PrP tg non-scrapie infected control mice studied. Infectious material was found in the gut tissue and feces by i.c. transmission to sentinel B6 mice resulting in their developing clinical scrapie 160 to 170 days post-inoculation (data not shown). Additionally, gut tissue was transmitted i.c. to tga20 mice which developed clinical scrapie 60 to 70 days post-infection, confirmed by finding PrPsc by Western blot analysis (Figure 2B). Glomeruli and tubules in the kidney showed PrPsc by immunohistochemistry in GPI−/− PrP tg mice infected with scrapie (Figure 2C). A 10% cleared suspension of either kidney or urine (Figure 2D) transmitted infectivity to brains of B6 mice within 170 days following i.c. inoculation. Western blots confirmed the presence of fully glycosylated PrPsc in the recipient brain tissue (Figure 2D). For the glomeruli, by immunohistochemistry only focal areas of PrPsc were noted, usually encompassing 10% to 20% of the glomerular tuffs but on occasion up to 30%. There was no associated protein in the urine in any of the scrapie-infected mice supporting the histopathological picture of essentially normal anatomical morphology of the kidney in scrapie-infected mice whose PrP was not anchored.
Fig. 2.
(A) PrPsc (PrPres) staining in the gut by immunohistochemistry. Sections of gut tissue from GPI−/− PrP tg mice were stained for PrPsc and amyloid by D18 and Thioflavin S staining, respectively (50x magnification). Note that PrPsc appears to deposit exclusively as amyloid in the gut. Uninfected control stains are shown on the right (50x magnification). (B) Western blot of brain homogenates of tga20 mice infected with either infected B6 brain homogenate or infected GPI−/− PrP tg mouse gut homogenate. Proteinase K digestion confirms that the PrP in the tga20 brain is PrPsc. (C) Immunohistochemical staining of the kidney reveals PrPsc deposition in the glomerulus after 400 days of infection (400x magnification). An uninfected GPI−/− PrP tg mouse kidney is shown as a control (400x magnification). (D) Urine and kidney tissue were taken 400 days post-infection and was transferred into B6 mice by intracranial inoculation. Both the urine and kidney samples contained PrPsc, as shown by Western blot, and were able to pass infection into a B6 mouse, as shown by the development of a proteinase K-resistant band pattern of PrP in the brain tissue of the mouse. The B6 mice also succumbed to disease after 163 days of infection, fitting with previous observations of PrP infection in B6 mice.
In summary, these results indicate that extraneural deposits of PrPsc occurred in multiple tissues including kidney, spleen, pancreas, eye, skeletal muscle, and gut. Two excreted sources, urine and feces from these infected mice, transmitted infectivity to donor mice, as did extraneural tissue expressing PrPsc. Lastly, PrPsc occurred as amyloid deposits in the gut. Following these studies, we focused our attention on the pancreas.
Pancreas deposits PrP and contains transmissible infectious material
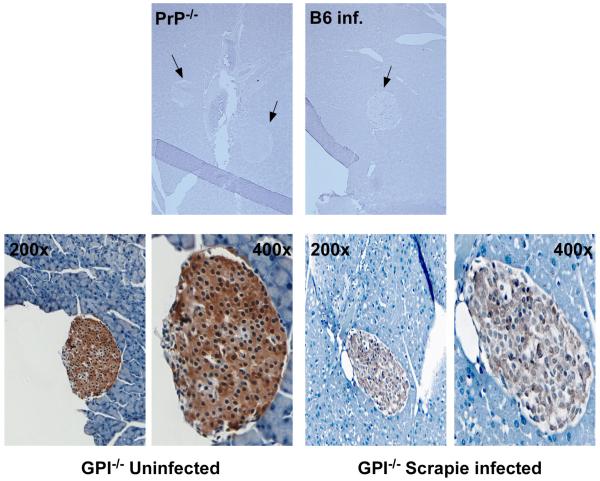
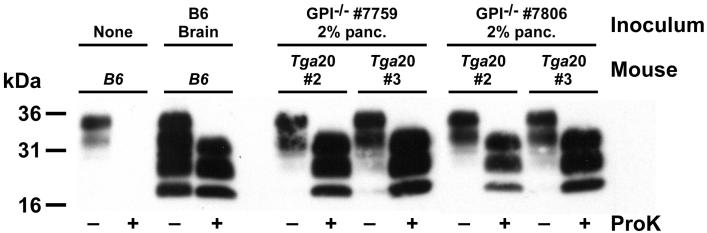
Heavy deposits of PrP were found in islets of Langerhans but not the acinar cells of the pancreas (Figure 3). In the mice, heavy concentrations of insulin were also noted (Figure 4). To address whether the PrP deposited in the pancreas is infectious, we harvested both brain (positive control) and pancreatic tissue from five GPI−/− PrP tg mice at 400 days post-scrapie infection. Tissue was homogenized and then injected intracranially into either tga20 mice, which express 4- to 6-fold higher levels of PrP than normal mice and develop clinical symptoms of prion disease 55-70 days post-infection, or into C57Bl/6 recipient mice that developed disease 155-170 days post-infection. With each of the five homogenates from the pancreas, both tga20 and C57Bl/6 mice exhibited clinical CNS manifestations of scrapie. As expected, brain tissue from these mice was also positive for PrPsc by Western blot (Figure 5) and transmitted infectious material.
Fig. 3.
Deposition of PrP in the islets of GPI−/− transgenic mice. Pancreas isolated from PrP knockout mice (PrP−/−), B6 mice, or GPI−/− transgenic mice infected with 2% scrapie intracranially was sectioned and stained for PrP as described in Materials and Methods. PrP was detected only in the islets of the pancreas, not in the acinar cells, in the GPI−/− PrP tg mice. No PrP was detected in the pancreas of PrP−/− mice or in B6 mice infected with scrapie at 160 days post-infection. Arrows indicate the Islets of Langerhans in the tissues. Images of the islets from GPI−/− PrP tg mice were taken at 200x and 400x magnification. Control tissues are shown at 50x magnification.
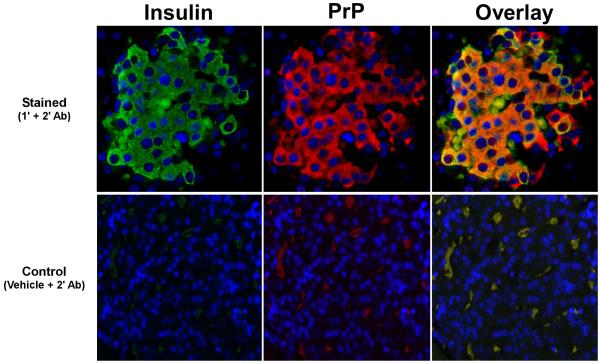
Fig. 4.
Insulin production occurs in the presence of PrPsc deposition. The expression of insulin in the islets of GPI−/− mice infected with scrapie was examined by immunofluorescence microscopy. As shown, despite the presence of large amounts of PrPsc in the islets (red), there is a significant amount of insulin production (green). The overlay panel shows colocalization (yellow) of both molecules. The top panels show staining with the primary and secondary antibodies for insulin or PrP, as indicated, while the bottom panels show background staining with the secondary antibody only. No non-specific background is noted. These observations were made on tissues from four independent GPI−/− PrP tg mice infected with scrapie. Images were taken at 400x magnification.
Fig. 5.
PrP deposition in the pancreas of scrapie-infected GPI−/− PrP tg mice is infectious. Pancreas or brain was taken from different mice (termed inoculum above) and injected into tga20 mice (shown) or B6 mice (not shown) intracranially. Mice were sacrificed when displaying clinical signs of prion disease and brain samples were analyzed by Western blot for PrP protein. C57Bl/6J (B6) mice are shown as both negative and positive controls for conversion of PrPc into PrPsc. Tga20 mice injected with inoculum from different GPI−/− infected mice exhibited clinical manifestations of prion disease, and brain isolates from these mice were positive for PrPsc by Western blot.
PrP deposits in the pancreatic islets of infected GPI−/− PrP tg mice
Fifteen infected GPI−/− PrP tg mice were sacrificed at 400 days post-infection and pancreatic tissue was stained for PrPsc using the human anti-PrP antibody D13. As controls, five PrP−/− tg mice and five GPI−/− PrP tg mice not infected with scrapie were also sacrificed at 400 days post-infection, as well as five B6 mice infected with scrapie and sacrificed at day 155 to 170 post-infection when they displayed progressive CNS signs. In the GPI−/− PrP tg mice, there is a large amount of PrP expressed in the islets of Langerhans but not in the acinar cells (Figure 3). Despite multiple attempts, we were unable to consistently totally remove PrPc by proteinase K digestion to ensure that there was PrPsc conversion. Despite using concentrations of proteinase K as high as 50 μg/mL for as long as 2 hours, all the PrPc was not removed. PrP was only detectable in GPI−/− PrP tg mice and we found no PrP staining in the infected B6 pancreas. There was no PrP found in the islets of PrP−/− mice. These findings, coupled with the infectivity data below (Figure 5), indicate the presence of PrPc in the islets of Langerhans and lead to the assumption that subsequent conversion of PrPc to PrPsc allows the presence of infectious transmissible material in the pancreas. Neither PrP nor infectious transmissible material was found in B6 mice, either uninfected or infected with RML scrapie, or in PrP−/− mice.
Deposition of PrP in islets does not affect insulin production or glucose metabolism
A possible consequence of PrPsc deposition in the islets is a reduction in the production of insulin. Using double immunofluorescence staining (Oldstone et al., 1983), we looked for both insulin and PrP in the islets of GPI−/− PrP tg mice infected with scrapie. As shown in Figure 4, there was significant production of insulin in the islets despite the presence of PrP. As a result, deposition of PrP in the islets did not lead to an imbalance of glucose metabolism. Glucose metabolism was tested by two methods. First, mice were bled 220 and 400 days after infection with scrapie to assess blood glucose levels. At 220 days, scrapie-infected GPI−/− PrP tg mice had blood glucose levels of 142 ± 8 mg/dL (n = 19, mean ± SEM), while uninfected GPI−/− PrP tg control littermates (n = 29) had blood glucose levels of 168 ± 11 mg/dL. By 400 days, the average blood glucose levels of uninfected and scrapie-infected GPI−/− PrP tg mice were equivalent (173 ± 13 or 173 ± 17 mg/dL, respectively). Glucose tolerance tests performed on eleven individual GPI−/− PrP tg mice, six infected with scrapie and five non-infected age- and sex-matched C57Bl/6 controls, showed less than a 2-fold increase in blood glucose levels over the pre-challenge level at 1, 4, 6, or 24 hours after challenge with 2 mg/kg of glucose intravenously, while in contrast, 3 of 3 NOD mice (positive controls) showed a greater than a 3-fold increase in glucose levels at the earlier time points. Given the lack of an abnormal increase in blood glucose levels and the presence of insulin in the islets during PrP infection, we determined that infection with PrPsc does not result in defects in glucose metabolism.
PrP deposits primarily in the α-cells of the islets of Langerhans with some β-cell involvement
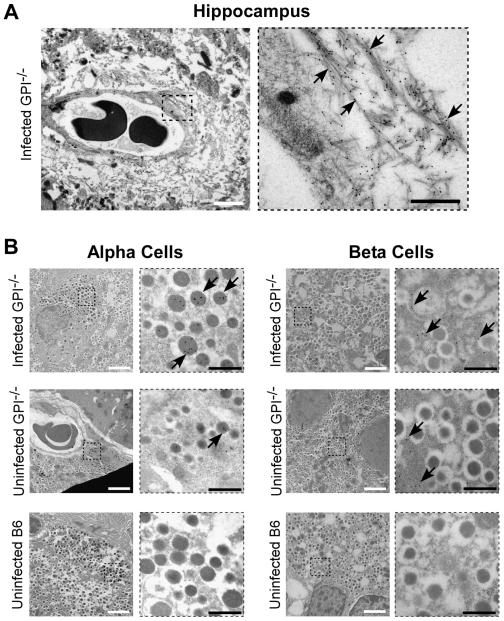
In the last series of studies we used high-resolution electron microscopy to identify which islet cells contained PrP deposits. GPI−/− PrP tg mice were sacrificed at 500 days post-infection and brain (positive control for amyloid and PrP deposits) and pancreas sections were prepared for immuno-electron microscopy as in Materials and Methods. The sections were labeled with the D18 monoclonal antibody to PrP and visualized with protein A-coupled 10 nm gold particles. For the brain as a positive control, we investigated PrP staining and amyloid deposition in the hippocampus (Figure 6A). For the pancreas, we investigated PrP staining in the islets of Langerhans with uninfected B6 and GPI−/− mice as negative controls (Figure 6B). The 10 nm gold particles appear as black dots in the electron micrographs indicating the presence of PrP. Large areas of extracellular fibrillar material composed of prion protein were observed in the hippocampus of the infected GPI−/− PrP tg mice brains (Figure 5A). These aggregates were located mainly around small blood vessels and capillaries. No amyloid was found in uninfected transgenic GPI−/− PrP tg mice or B6 mice (data not shown). The acquired high-resolution data confirms the histological findings previously reported (Chesebro et al., 2005; Trifilo et al., 2008). Fibrillar aggregates of PrP were also found peri-nuclear in hippocampal cells (not shown). The close proximity (100 nm) to the nucleus and the frequency of this finding suggest that PrP aggregation may not only occur extracellularly but also intracellularly, possibly in the secretory and/or endocytotic pathways of the cell.
Fig. 6.
Electron microscopy of PrP in brain and islet cells of the pancreas. (A) PrP stained with D18 monoclonal antibody coupled to immunogold is visible in the hippocampus of GPI−/− mice infected with RML scrapie i.c. The right panel is a magnification of the region outlined with a dotted square on the left. Arrows point to PrP-labeled amyloid fibrils. (B) PrP is seen predominantly in the alpha cells of the pancreas in infected GPI−/− mice with deposition also noted in the beta cells (top panels). Little or no deposition is noted in uninfected mice, either GPI−/− (middle panels) or wild-type B6 (lower panels). The panels to the right are magnifications of the region outlined with a dotted square in each figure. Arrows point to gold-labeled PrP in the cells of the islets. The white scale bars are 2 um, while the black scale bars are 500 nm.
When the endocrine portion of the islets of Langerhans in the pancreas was studied with respect to PrP, immunolabeling was detected in both α- and β-cells in the islets but in different cellular compartments. In α-cells, a very specific and strong labeling was observed in the core of the secretory granules while reactivity was detected only in the cytosol of β-cells (Figure 6B, top panels). No signs of cell apoptosis or amyloid deposits were observed in the islets, consistent with negative thioflavin S and Congo Red stains in the islets of GPI−/− PrP tg mice infected with scrapie, and the islets of uninfected GPI−/− PrP tg mice showed less labeling for PrP, while uninfected B6 mice did not label for PrP (Figure 6B, bottom panels). Owing to the conditions required to obtain high tissue/cell resolution for immuno-electron microscopy, we could not treat the tissue with proteinase K to determine whether the PrP observed in the positive α- or β-cells is PrPc, PrPsc, or both. Attempts to do so using histoblots of infected tissue were inconclusive, with positive staining for PrPsc found in the brain but not reliably in the pancreas of infected mice.
DISCUSSION
In this study, we report three novel observations. First, extraneural manifestations of PrPsc occur in the kidney, pancreas, eye, skeletal muscle, and gut of scrapie-infected mice that have an anchorless PrP. Second, focus on the gut and kidney revealed deposition of PrP and infectious material in these tissues resulted in infectious transmissible material in the corresponding excreta, feces and urine. Third, in the pancreas PrP is found exclusively in the islets of Langerhans, primarily in the α-cells but also in the β-cells of the islets and not in the acinar cells. The pancreas also contains infectious transmissible material. While RML scrapie induces amyloid deposits in brain, heart and gut, it fails to do so in the islets of Langerhans. Despite PrP involvement in the kidneys and islets, neither abnormal proteinuria nor abnormal glucose metabolism occurred.
Deposition of PrPsc in the GPI−/− anchorless mouse following scrapie infection appeared in several peripheral tissues, consistent with previous observations using 22L scrapie in the GPI model system (Race et al., 2008), and with studies of scrapie-induced diabetes in hamsters (Srinivasappa et al., 1989). Levels of PrPsc vary depending on the tissue, as observed in all tissues tested except the thymus. The consequences of deposits of PrPsc and amyloid in the brain and heart in GPI−/− PrP tg mice infected with scrapie lead to abnormal neuron conduction and cognitive function in the CNS (Trifilo et al., 2008) and left ventricular failure when fluid load was increased in the heart (Trifilo et al., 2006). In agreement with our findings in GPI−/− PrP tg mice infected with scrapie, PrPsc deposition in the kidney leads to infectious material being shed into the urine and that PrPsc in the gut leads to fecal infectivity, urine, feces, and milk have been shown to be able to transmit scrapie in cows, sheep, goats, deer, and hamsters (Didier et al., 2008; Haley et al., 2009; Kariv-Inbal et al., 2006; Lacroux et al., 2008; Maddison et al., 2009; Safar et al., 2008). The recent report of GPI-anchorless prion protein in human patients (Jansen et al., 2010) suggests that it may be possible for similar extraneural manifestations to occur in man.
PrP deposited in the islets of Langerhans but was not detected in acinar cells. Using electron microscopy with immunogold labeling of PrP, we determined that in GPI−/− PrP tg mice, PrP labeling occurs primarily within the α-cells of the islet, also with β-cell involvement. Despite PrP localization solely in the islets, we observed no effect on either insulin production or glucose metabolism in PrP-infected mice, a result inconsistent with infection in hamsters that results in significant alterations in both (Srinivasappa et al., 1989). It appears that the extraneural deposition of PrPsc in the islets does not have the same functional consequence as does deposition in the brain and heart. Of interest is that amyloid deposits associated with the CNS and heart in PrPsc deposition did not occur in the islets of Langerhans. In the islets, incorrectly folded PrPsc may undergo degradation or be secreted into the blood, preventing a critical concentration needed for fibril formation to occur.
Extraneural evidence that PrPsc can occur as an amyloid deposit was found in the gut (Figure 2). We showed that excreta from these mice contain PrPsc (Figure 2D) and transmitted infectious material as did tissue from the pancreas of infected GPI−/− PrP tg mice (Figures 2B, 5). However, since their weights and survival were no different than uninfected GPI−/− PrP tg mice and their feces was not abnormal, any defect in scrapie-infected GPI−/− PrP tg mice likely would be modest. It has been suggested that PrP infection may be transferred horizontally in the excreta of mice (Seeger et al., 2005), as has been demonstrated in cervid models of chronic wasting disease (Haley et al., 2009; Mathiason et al., 2006). We demonstrate that mouse excreta can harbor infectious PrPsc, which confirms previous results with other rodent transgenic models (Miller et al., 2004), and demonstrates that anchorless PrP has the potential to be a transferrable scrapie agent.
Amyloid deposits do occur in other diseases of the pancreas, most notably type 2 diabetes (Clark et al., 1987; Lorenzo et al., 1994). Deposition of amyloid fibrils in the pancreatic islets of Langerhans is a common pathological manifestation associated with Type 2 diabetes and is called islet amyloidosis. The fibrillogenic peptide amylin/IAPP (islet amyloid polypeptide) is produced and secreted in concert with insulin by the β-cells and aggregation of the peptide is associated with β- cell death and progression of hyperglycaemia (Hoppener and Lips, 2006; Westermark et al., 1986). Islet amyloid occurs during prolonged beta-cell stress and no or little amyloid can be detected in healthy subjects even though amylin is very amyloidogenic, suggesting a highly developed cellular capacity to handle misfolding and aggregating proteins and peptides (Clark et al., 1995; Murray et al., 2010; Narita et al., 1992). The finding of PrP in the islets and transmission of infectious material without evidence of amyloid fibrils was unexpected given that PrP deposits readily as amyloid in other tissues (Chesebro et al., 2005; Trifilo et al., 2006). In addition there were no signs of apoptosis or recruitment of macrophages or lymphocytes to the islets of GPI−/− PrP tg mice infected with scrapie. Perhaps the failure of amyloid formation in GPI−/− PrP tg mice infected by RML scrapie is prevented by cellular mechanisms for disaggregation by the highly specialized hormone producing islet cells (Clark et al., 1995; Murray et al., 2010; Narita et al., 1992).
Jansen and colleagues recently detailed two cases of human prion protein misfolding diseases that coincided with a loss of the GPI anchor via a stop codon mutation (Jansen et al., 2010). Their findings not only support use of the GPI−/− PrP tg mouse model to assess the effects of prion infection but suggest that extraneural manifestations may also occur in human cases. While this has been reported for one case of dilated cardiomyopathy (Ashwath, Dearmond, and Culclasure, 2005), it has not yet been analyzed in the human genetic mutations described by Jansen et. al. (Jansen et al., 2010)
MATERIALS AND METHODS
Scrapie inoculation of mice
A transgenic mouse line heterozygous for PrP (Tg line 44) lacking a GPI anchor (GPI−/− PrP tg mice), was created, genotyped, and infected as described previously (Chesebro et al., 2005). When infected with murine scrapie, these mice developed PrPsc amyloid deposits and infectious PrP material but not clinical neurodegenerative disease. C57BL/6, C57Bl/10 (wild-type), and tga20 mice were obtained from the Rodent Breeding Colony at The Scripps Research Institute. The tga20 mice (PrP over-expressers) were used for infectivity studies and as controls. Infection was induced by inoculating transgenic, wild-type, and Tga20 mice intracerebrally at 6 weeks of age with 30 μl of a 1% suspension of brain tissue or pancreatic from GPI−/− PrP tg mice and GPI+/+ PrP mice infected with RML (Chandler) scrapie. Only wild type and Tga20 mice exhibit signs of CNS disease and dysfunction following scrapie infection at 150-160 days and 50-60 days post-infection, respectively. These clinical signs include weakness, lassitude, ataxia, and paralysis, with death resulting from scrapie infection shortly after the onset of these symptoms. These CNS signs of scrapie infection are absent in the GPI−/− PrP tg mice during their lifespan.
Immunohistochemistry and immunofluorescence staining
Immunohistochemistry and immunofluorescence staining were performed essentially as described (Chesebro et al., 2005). Briefly, tissues were fixed in a neutral-buffered 4% formaldehyde solution for 3-5 days, dehydrated, embedded in paraffin and cut into 4-μM sections on glass slides using a Leica microtome. Slides were deparaffinized and rehydrated in xylene and alcohol rinses, and autoclaved for 15 minutes at 20 p.s.i. and 120 °C for retrieval of PrP antigen. Samples were incubated with 25 μg/mL proteinase K for 1 hour at room temperature to remove PrPc, blocked in 2% BSA and stained for PrPsc using 3 μg/mL D13 antibody (Chesebro et al., 2005; Matsunaga et al., 2001). For double immunofluorescence staining (Oldstone et al., 1983), a FITC-conjugated donkey antibody to mouse insulin and the mouse antibody D13 (Matsunaga et al., 2001) conjugated to rhodamine were used. After overnight incubation with the primary antibody, slides were rinsed with buffer and stained with a 1:200 solution of biotinylated goat anti-human antiserum. Streptavidin-conjugated rhodamine or horseradish peroxidase followed by Ventana aminoethylcarbazol was used for immunofluorescence or light microscopy, respectively. Staining for amyloid was performed using 1% w/v Thioflavin S (MP Biomedicals) in 50% ethanol. Slides were rinsed twice in 50% ethanol and twice in water prior to mounting and coverslipping. Staining of PrPsc and amyloid was observed using an Axiovert S100 microscope (Zeiss).
Immuno-gold electron microscopy
GPI−/− PrP tg mice infected with RML scrapie i.c. were sacrificed 500 days post-infection, along with age matched uninfected GPI−/− PrP tg and B6 mice. Brain and pancreatic tissue were harvested from these mice and immediately fixed in a PBS/2% paraformaldehyde/0.25% glutaraldehyde solution for 24 hours. Following fixation, the tissue was washed in PBS and dehydrated through a series of graded ethanol solutions followed by propylene oxide. The tissue was embedded in an Epon/Araldite mixture (Electron Microscopy Sciences, Hatfield, PA) for sectioning. The resin-embedded tissue was sectioned at 70 nm and mounted on uncoated 400 mesh nickel grids (Electron Microscopy Sciences, Hatfield, PA) for immunolabeling. Antigen retrieval was performed by incubating samples in a saturated aqueous solution of sodium m-periodate for 10 minutes followed by a wash in Tris-buffered saline (TBS; 50 mM Tris-HCl, pH 7.4, 150 mM NaCl). Tissue sections were blocked in 3% bovine serum albumin (BSA) in TBS for 30 minutes, followed by an overnight incubation at room temperature with a 1:50 dilution of D18 monoclonal antibody (human anti-PrP, (Matsunaga et al., 2001)) in TBS/1% BSA. Sections were washed 3x in TBS and blocked a second time in TBS/3% BSA for 30 minutes, followed by a 2-hour incubation at room temperature in a 1:100 solution of protein A conjugated to 10 nm gold particles (PAG10 BB International, Cardiff, UK) in TBS/1% BSA. The sections were rinsed 3 times in TBS, 3 times in water, air dried, and then contrasted with 2% uranyl acetate in 50% ethanol for 10 min and in Reynold’s lead citrate solution (120 mmol/l sodium citrate, 25 mmol/l lead nitrate) for 1.5 min. Specimens were studied in a Jeol 100CX electron microscope (Jeol, Akishima, Tokyo, Japan) at 100 kV, and electron micrographs were taken with a Mega View III CCD camera linked to Analysis Pro v3.2 digital micrograph software (Soft Imaging System GmbH, Muenster, Germany).
Western blot analysis of PrP infection
Brain, kidney, spleen, thymus, eye, skeletal muscle, gut, heart, and pancreatic tissues were harvested from GPI−/− PrP tg mice infected with RML scrapie at day 400 post-infection. A 2% homogenate of each was made in PBS and prepared for Western blot as described (Chesebro et al., 2005; Trifilo et al., 2008; Trifilo et al., 2006). To assay transmission of infectivity, Tga20 mice were inoculated intracranially with 30 μL of the 2% homogenate of the tissues, and brain tissue was harvested from these mice when clinical manifestations of prion disease became apparent (50-70 days post-infection). The presence of conversion of PrPc to PrPsc was confirmed by both Western blot and immunohistochemistry (Chesebro et al., 2005; Oldstone et al., 1983; Trifilo et al., 2008; Trifilo et al., 2006). Briefly, for Western blots, tissues were homogenized and subjected to digestion with proteinase K (50 μg/mL) for 1 hour at 37 °C Total protein in the processed homogenate was quantified by a colorimetric BCA assay (Pierce), and 20 μg of total protein was loaded per lane in a 16% SDS-PAGE gel. Samples were electrophoresed, blotted to PVDF membrane, and probed for PrP with a 3 μg/mL solution of D13 human anti-PrP antibody (Matsunaga et al., 2001) followed by a peroxidase-coupled anti-human IgG secondary antibody. Blots were developed using Super Signal ECL detection (Pierce).
Blood glucose measurements
Blood samples were obtained from the retroorbital plexus of mice. Plasma glucose was determined using a One-Touch Ultra glucose meter (Life Scan) as reported (Horwitz et al., 2009). Glucose tolerance tests were performed on mice deprived of food for 18 hours. Mice were pre-bled and then given 2 mg/kg glucose intravenously and bled at 1, 4, 6, and 24 hours following glucose injection as previously reported (Oldstone et al., 1984).
ACKNOWLEDGMENTS
This is publication number 20693 from the Department of Immunology and Microbial Science at The Scripps Research Institute in La Jolla, CA. We thank Talya Bordin-Wosk and Arineh Vartanian for technical assistance. This work was supported by U.S. Public Health Grants (AI09484, AI45927) to M.B.A.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ashwath ML, Dearmond SJ, Culclasure T. Prion-associated dilated cardiomyopathy. Arch. Intern. Med. 2005;165(3):338–40. doi: 10.1001/archinte.165.3.338. [DOI] [PubMed] [Google Scholar]
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73(7):1339–47. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, Caughey B, Masliah E, Oldstone M. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308(5727):1435–9. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- Clark A, Cooper GJ, Lewis CE, Morris JF, Willis AC, Reid KB, Turner RC. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;2(8553):231–4. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Clark A, de Koning EJ, Hattersley AT, Hansen BC, Yajnik CS, Poulton J. Pancreatic pathology in non-insulin dependent diabetes (NIDDM) Diabetes Res. Clin. Pract. 1995;28(Suppl):S39–47. doi: 10.1016/0168-8227(95)01075-o. [DOI] [PubMed] [Google Scholar]
- Didier A, Gebert R, Dietrich R, Schweiger M, Gareis M, Martlbauer E, Amselgruber WM. Cellular prion protein in mammary gland and milk fractions of domestic ruminants. Biochem. Biophys. Res. Commun. 2008;369(3):841–4. doi: 10.1016/j.bbrc.2008.02.108. [DOI] [PubMed] [Google Scholar]
- Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One. 2009;4(3):e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppener JW, Lips CJ. Role of islet amyloid in type 2 diabetes mellitus. Int. J. Biochem. Cell Biol. 2006;38(5-6):726–36. doi: 10.1016/j.biocel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Horwitz MS, Efrat S, Christen U, von Herrath MG, Oldstone MB. Adenovirus E3 MHC inhibitory genes but not TNF/Fas apoptotic inhibitory genes expressed in beta cells prevent autoimmune diabetes. Proc. Natl. Acad. Sci. U. S. A. 2009;106(46):19450–4. doi: 10.1073/pnas.0910648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C, Parchi P, Capellari S, Vermeij AJ, Corrado P, Baas F, Strammiello R, van Gool WA, van Swieten JC, Rozemuller AJ. Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP. Acta Neuropathol. 2010;119(2):189–97. doi: 10.1007/s00401-009-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariv-Inbal Z, Ben-Hur T, Grigoriadis NC, Engelstein R, Gabizon R. Urine from scrapie-infected hamsters comprises low levels of prion infectivity. Neurodegener Dis. 2006;3(3):123–8. doi: 10.1159/000094770. [DOI] [PubMed] [Google Scholar]
- Lacroux C, Simon S, Benestad SL, Maillet S, Mathey J, Lugan S, Corbiere F, Cassard H, Costes P, Bergonier D, Weisbecker JL, Moldal T, Simmons H, Lantier F, Feraudet-Tarisse C, Morel N, Schelcher F, Grassi J, Andreoletti O. Prions in milk from ewes incubating natural scrapie. PLoS Pathog. 2008;4(12):e1000238. doi: 10.1371/journal.ppat.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368(6473):756–60. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Maddison BC, Baker CA, Rees HC, Terry LA, Thorne L, Bellworthy SJ, Whitelam GC, Gough KC. Prions are secreted in milk from clinically normal scrapie-exposed sheep. J. Virol. 2009;83(16):8293–6. doi: 10.1128/JVI.00051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 1994;8(2-3):121–7. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314(5796):133–6. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y, Peretz D, Williamson A, Burton D, Mehlhorn I, Groth D, Cohen FE, Prusiner SB, Baldwin MA. Cryptic epitopes in N-terminally truncated prion protein are exposed in the full-length molecule: dependence of conformation on pH. Proteins. 2001;44(2):110–8. doi: 10.1002/prot.1077. [DOI] [PubMed] [Google Scholar]
- Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 2004;10(6):1003–6. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AN, Solomon JP, Wang YJ, Balch WE, Kelly JW. Discovery and characterization of a mammalian amyloid disaggregation activity. Protein Sci. 2010;19(4):836–46. doi: 10.1002/pro.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita R, Toshimori H, Nakazato M, Kuribayashi T, Toshimori T, Kawabata K, Takahashi K, Masukura S. Islet amyloid polypeptide (IAPP) and pancreatic islet amyloid deposition in diabetic and non-diabetic patients. Diabetes Res. Clin. Pract. 1992;15(1):3–14. doi: 10.1016/0168-8227(92)90060-5. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Fujinami RS, Tishon A, Finney D, Powell HC, Lampert PW. Mapping of the major histocompatibility complex and viral antigens on the plasma membrane of a measles virus-infected cell. Virology. 1983;127(2):426–37. doi: 10.1016/0042-6822(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Southern P, Rodriquez M, Lampert P. Virus persists in beta cells of islets of Langerhans and is associated with chemical manifestations of diabetes. Science. 1984;224(4656):1440–3. doi: 10.1126/science.6203172. [DOI] [PubMed] [Google Scholar]
- Race B, Meade-White K, Oldstone MB, Race R, Chesebro B. Detection of prion infectivity in fat tissues of scrapie-infected mice. PLoS Pathog. 2008;4(12):e1000232. doi: 10.1371/journal.ppat.1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F, Dearmond SJ, Prusiner SB. Transmission and detection of prions in feces. J. Infect. Dis. 2008;198(1):81–9. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310(5746):324–6. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J, Asher DM, Pomeroy KL, Murphy LJ, Wolff AV, Yoon JW, Gajdusek DC, Notkins AL. Scrapie-induced diabetes mellitus in hamsters. Microb. Pathog. 1989;7(3):189–94. doi: 10.1016/0882-4010(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Trifilo MJ, Sanchez-Alavez M, Solforosi L, Bernard-Trifilo J, Kunz S, McGavern D, Oldstone MB. Scrapie-induced defects in learning and memory of transgenic mice expressing anchorless prion protein are associated with alterations in the gamma aminobutyric acid-ergic pathway. J. Virol. 2008;82(20):9890–9. doi: 10.1128/JVI.00486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilo MJ, Yajima T, Gu Y, Dalton N, Peterson KL, Race RE, Meade-White K, Portis JL, Masliah E, Knowlton KU, Chesebro B, Oldstone MB. Prion-induced amyloid heart disease with high blood infectivity in transgenic mice. Science. 2006;313(5783):94–7. doi: 10.1126/science.1128635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem. Biophys. Res. Commun. 1986;140(3):827–31. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]