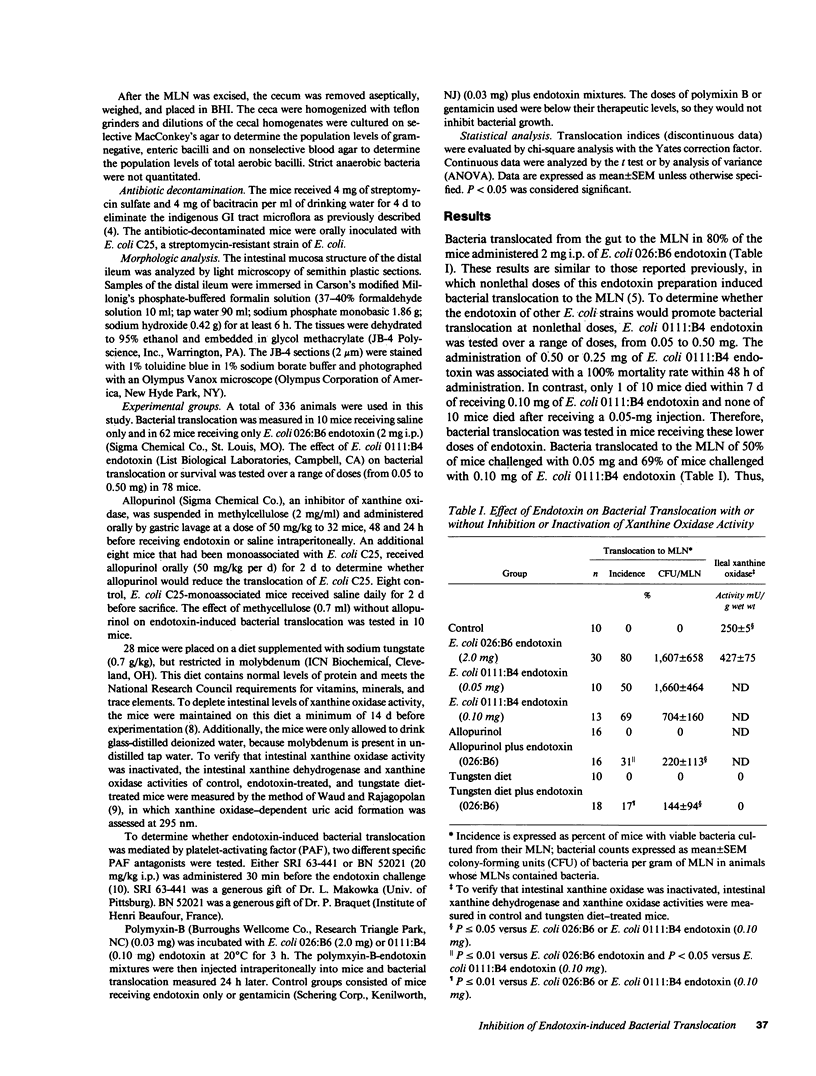
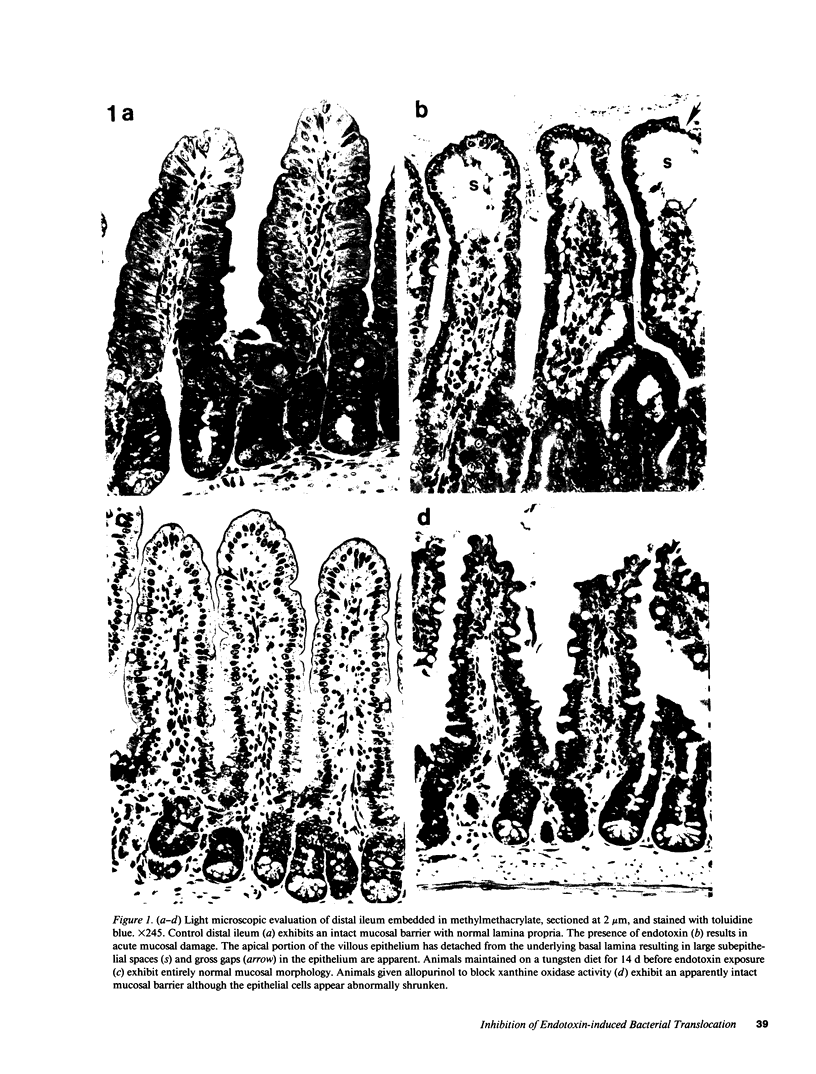
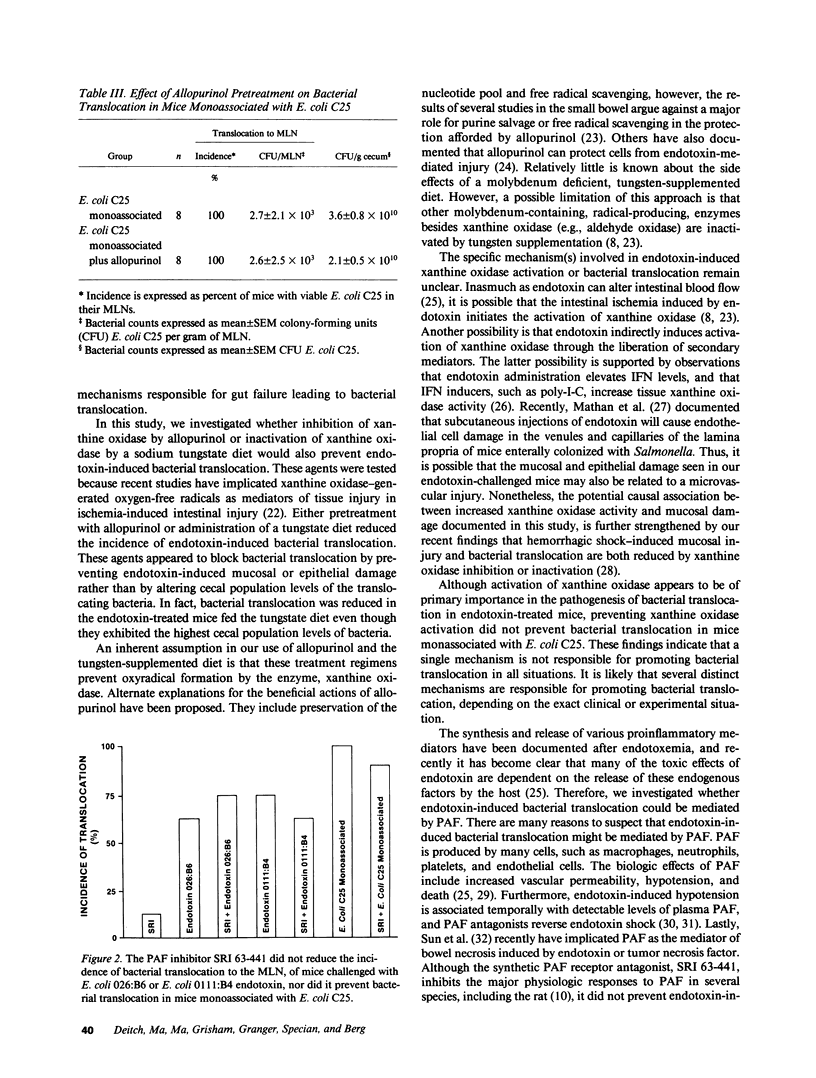
Abstract
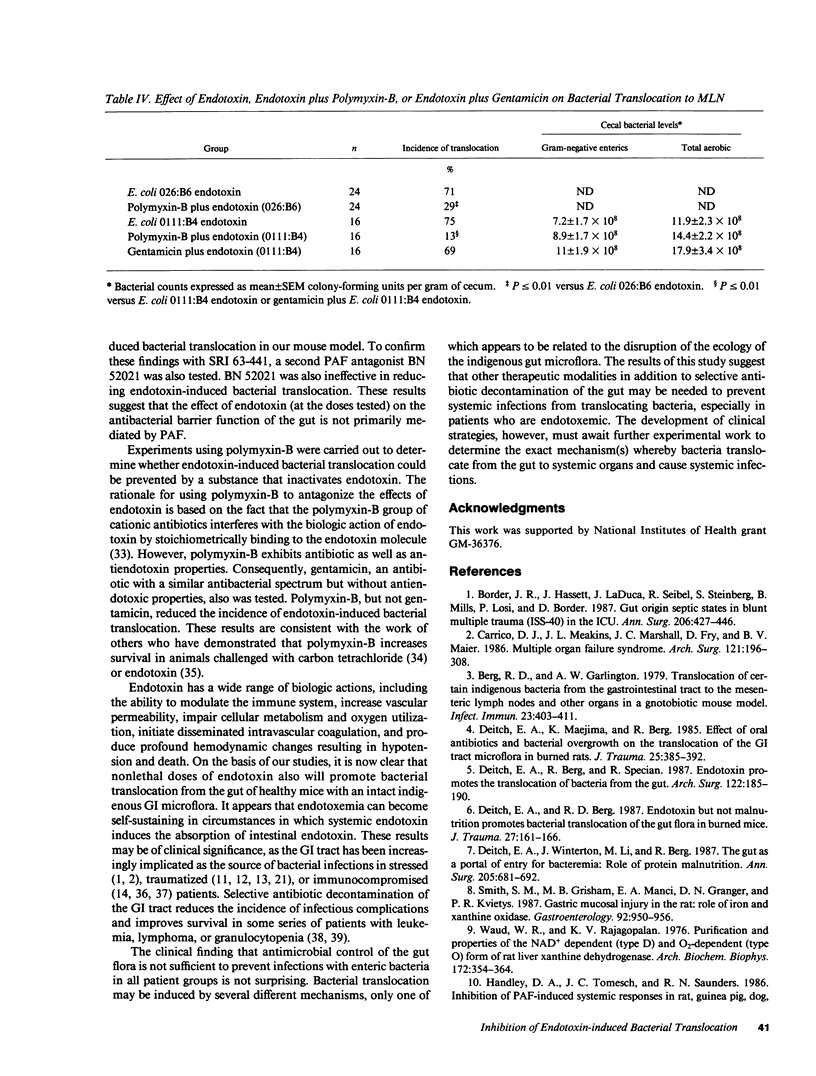
The primary functions of the gut are to absorb nutrients and exclude bacteria and their products. However, under certain circumstances the gut may lose its barrier function and serve as a reservoir for systemic microbial infections. These experiments were performed to determine the mechanisms whereby endotoxin causes bacteria to escape (translocate) from the gut. Bacteria translocated from the gut to the mesenteric lymph nodes of mice challenged with nonlethal doses of Escherichia coli 026:B6 or E. coli 0111:B4 endotoxin. Physical disruption of the gut mucosal barrier appears to be the primary mechanism whereby endotoxin promotes bacterial translocation. Mucosal injury and endotoxin-induced bacterial translocation were reduced by inhibition (allopurinol) or inactivation (tung-sten diet) of xanthine oxidase activity (P less than 0.01), but were not affected by the platelet-activation factor antagonists, SRI 63-441 or BN 52021. Because the inhibition or inactivation of xanthine oxidase activity reduced both the extent of mucosal injury and endotoxin-induced bacterial translocation, the effect of endotoxin on the gut appears to be mediated, at least to some degree, by xanthine oxidase-generated, oxygen-free radicals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P. Antibiotic prophylaxis in cancer patients: regimens of oral, nonabsorbable antibiotics for prevention of infection during induction of remission. Rev Infect Dis. 1981 Nov-Dec;3 Suppl:S259–S268. [PubMed] [Google Scholar]
- Border J. R., Hassett J., LaDuca J., Seibel R., Steinberg S., Mills B., Losi P., Border D. The gut origin septic states in blunt multiple trauma (ISS = 40) in the ICU. Ann Surg. 1987 Oct;206(4):427–448. doi: 10.1097/00000658-198710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B., Berry L. C., Jr, Repine J. E. Antioxidants protect cultured bovine lung endothelial cells from injury by endotoxin. J Appl Physiol (1985) 1987 Aug;63(2):840–850. doi: 10.1152/jappl.1987.63.2.840. [DOI] [PubMed] [Google Scholar]
- Caridis D. T., Reinhold R. B., Woodruff P. W., Fine J. Endotoxaemia in man. Lancet. 1972 Jun 24;1(7765):1381–1385. doi: 10.1016/s0140-6736(72)91108-7. [DOI] [PubMed] [Google Scholar]
- Carrico C. J., Meakins J. L., Marshall J. C., Fry D., Maier R. V. Multiple-organ-failure syndrome. Arch Surg. 1986 Feb;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- Chang S. W., Feddersen C. O., Henson P. M., Voelkel N. F. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A., Berg R. D. Endotoxin but not malnutrition promotes bacterial translocation of the gut flora in burned mice. J Trauma. 1987 Feb;27(2):161–166. doi: 10.1097/00005373-198702000-00012. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Berg R., Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987 Feb;122(2):185–190. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Bridges W., Baker J., Ma J. W., Ma L., Grisham M. B., Granger D. N., Specian R. D., Berg R. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery. 1988 Aug;104(2):191–198. [PubMed] [Google Scholar]
- Deitch E. A. Infection in the compromised host. Surg Clin North Am. 1988 Feb;68(1):181–197. doi: 10.1016/s0039-6109(16)44439-7. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Maejima K., Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma. 1985 May;25(5):385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Winterton J., Li M., Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987 Jun;205(6):681–692. doi: 10.1097/00000658-198706000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- Fry D. E., Klamer T. W., Garrison R. N., Polk H. C., Jr Atypical clostridial bacteremia. Surg Gynecol Obstet. 1981 Jul;153(1):28–30. [PubMed] [Google Scholar]
- Garrison R. N., Fry D. E., Berberich S., Polk H. C., Jr Enterococcal bacteremia: clinical implications and determinants of death. Ann Surg. 1982 Jul;196(1):43–47. doi: 10.1097/00000658-198207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Bianchi M., Mantovani A., Spreafico F., Salmona M. Enhanced xanthine oxidase activity in mice treated with interferon and interferon inducers. Biochem Biophys Res Commun. 1984 Feb 29;119(1):144–149. doi: 10.1016/0006-291x(84)91630-9. [DOI] [PubMed] [Google Scholar]
- Gurwith M. J., Brunton J. L., Lank B. A., Harding G. K., Ronald A. R. A prospective controlled investigation of prophylactic trimethoprim/sulfamethoxazole in hospitalized granulocytopenic patients. Am J Med. 1979 Feb;66(2):248–256. doi: 10.1016/0002-9343(79)90539-4. [DOI] [PubMed] [Google Scholar]
- Handley D. A., Tomesch J. C., Saunders R. N. Inhibition of PAF-induced systemic responses in the rat, guinea pig, dog and primate by the receptor antagonist SRI 63-441. Thromb Haemost. 1986 Aug 20;56(1):40–44. [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977 Jan;118(1):21–27. [PubMed] [Google Scholar]
- Jones R. J., Roe E. A. Measurement of endotoxins with the limulus test in burned patients. J Hyg (Lond) 1979 Aug;83(1):151–156. doi: 10.1017/s0022172400025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan V. I., Penny G. R., Mathan M. M., Rowley D. Bacterial lipopolysaccharide-induced intestinal microvascular lesions leading to acute diarrhea. J Clin Invest. 1988 Nov;82(5):1714–1721. doi: 10.1172/JCI113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Nolan J. P., Leibowitz A. I. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978 Sep;75(3):445–449. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Smith S. M., Grisham M. B., Manci E. A., Granger D. N., Kvietys P. R. Gastric mucosal injury in the rat. Role of iron and xanthine oxidase. Gastroenterology. 1987 Apr;92(4):950–956. doi: 10.1016/0016-5085(87)90969-3. [DOI] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D., Currie C. A., Geheber C. E., Cuzzell J. Z. Candida sepsis: pathogenesis and principles of treatments. Ann Surg. 1974 May;179(5):697–711. doi: 10.1097/00000658-197405000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. M., Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988 May;81(5):1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I. The contribution of intestinal endotoxin to mortality in hosts with compromised resistance: a review. Exp Hematol. 1978 Feb;6(2):172–184. [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976 Feb;172(2):354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Podzorski R. P., Peterson P. K., Ramsay N. K., Simmons R. L., Rhame F. S. Incidence of trimethoprim-sulfamethoxazole-resistant enterobacteriaceae among transplant recipients. J Infect Dis. 1984 Nov;150(5):699–706. doi: 10.1093/infdis/150.5.699. [DOI] [PubMed] [Google Scholar]
- de Vries-Hospers H. G., Sleijfer D. T., Mulder N. H., van der Waaij D., Neiweg H. O., van Saene H. K. Bacteriological aspects of selective decontamination of the digestive tract as a method of infection prevention in granulocytopenic patients. Antimicrob Agents Chemother. 1981 May;19(5):813–820. doi: 10.1128/aac.19.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]