Abstract
Calcium is an ambivalent signal: it is essential for the correct functioning of cell life, but may also become dangerous to it. The plasma membrane Ca2+ ATPase (PMCA) and the plasma membrane Na+/Ca2+ exchanger (NCX) are the two mechanisms responsible for Ca2+ extrusion. The NCX has low Ca2+ affinity but high capacity for Ca2+ transport, whereas the PMCA has a high Ca2+ affinity but low transport capacity for it. Thus, traditionally, the PMCA pump has been attributed a housekeeping role in maintaining cytosolic Ca2+, and the NCX the dynamic role of counteracting large cytosolic Ca2+ variations (especially in excitable cells). This view of the roles of the two Ca2+ extrusion systems has been recently revised, as the specific functional properties of the numerous PMCA isoforms and splicing variants suggests that they may have evolved to cover both the basal Ca2+ regulation (in the 100 nM range) and the Ca2+ transients generated by cell stimulation (in the μM range).
PMCA pumps and NCX exchangers both transport calcium out of cells. NCX counteracts large [Ca2+] variations in excitable cells. PMCA pumps were thought to have a housekeeping role but may also deal with large transients during cell stimulation.
Ca2+ controls critical cellular responses in all eukaryotic organisms. It controls both short-term biological processes that occur in milliseconds, such as muscle contraction, as well as long-term processes that require longer times, such as cell proliferation and organ development. The specificity of cellular Ca2+ signals is controlled by a sophisticated “toolkit” comprising numerous ion channels, pumps, and exchangers that drive the fluxes of Ca2+ ions across the plasma membrane and across the membranes of intracellular organelles (Berridge et al. 2003).
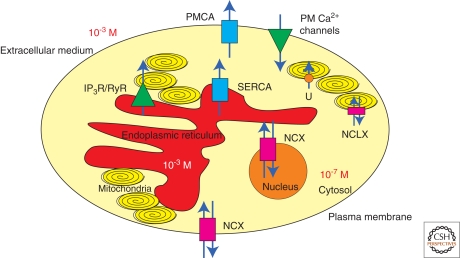
The plasma membrane contains several types of channels that mediate Ca2+ entry from the extracellular ambient, and two systems for Ca2+ extrusion: a low affinity, high capacity Na+/Ca2+ exchanger (NCX), and a high-affinity, low-capacity Ca2+-ATPase (the plasma membrane Ca2+ pump (PMCA)) (Fig. 1). The type of channels and the relative proportions of NCX and PMCA vary with the cell type, the NCX being particularly abundant in excitable tissues, e.g., heart and brain. The regulated opening of the Ca2+ channels by either voltage gating, interaction with ligands or the emptying of intracellular stores, allows a limited amount of Ca2+ to enter the cell to transmit signals to its designated targets. Thereafter, the Ca2+ transients must be dissipated: its extrusion from the cell is mediated by the NCX and the PMCA pump, but Ca2+ is also restored to basal levels by sequestration in the endo/sarcoplasmic reticulum via the SERCA pump and in the mitochondria by the electrophoretic uniporter. The NCX has also been found at the inner membrane of the nuclear envelope (NE) and has been proposed to mediate Ca2+ flux between the nucleoplasm and the NE (Xie et al. 2002), and then to the ER (Wu et al. 2009) in neuronal and certain other cell types. Ca2+ binding proteins also contributed to Ca2+ buffering: In this review, we will not cover them, as we will only discuss the systems that extrude Ca2+ out of the cell.
Figure 1.
A schematic representation of the structures involved in cellular Ca2+ homeostasis. The model shows a cell with its Ca2+-transporting systems: Ca2+-ATPases (plasma membrane and sarco/endoplasmic reticulum, PMCA and SERCA), plasma membrane (PM) Ca2+ channels, Na+/Ca2+ exchangers (NCX and NCLX), 1,4,5-triphosphate receptor (IP3R) and ryanodine receptor (RyR), the electrophoretic mitochondrial uptake uniporter (U). Mitochondria are drawn as yellow ellipses, nucleus as orange circle and endoplasmic reticulum is colored in red. The different Ca2+-transporting systems cooperate to maintain the Ca2+ concentration gradient between the extracellular and the intracellular ambient.
The PMCA pump is a minor component of the total protein of the plasma membrane (less than 0.1% of it). Quantitatively, it is overshadowed by the more powerful NCX in excitable tissue like heart; however, even cells in which the NCX predominates, the PMCA pump is likely to be the fine tuner of cytosolic Ca2+, as it can operate in a concentration range in which the low affinity NCX is relatively very inefficient.
The PMCA was discovered in erythrocytes (Schatzmann 1966), and was then described and characterized in numerous other cell types. It was purified in 1979 using a calmodulin affinity column (Niggli et al. 1979), and cloned about 10 years later (Shull and Greeb 1988; Verma et al. 1988). It shows the same essential membrane topology properties of the SERCA pump. Molecular modeling work using the structure of the SERCA pump as a template (Toyoshima et al. 2000) predicts the same general features of the latter, with 10 transmembrane domains and the large cytosolic headpiece divided into the three main cytosolic A, N, and P domains. The Na+/Ca2+ cotransport process was discovered at about the same time as PMCA by two independent groups working on heart (Reuter and Seitz 1968) and on the squid giant axon (Baker et al. 1969). The exchanger was cloned in 1990 (Nicoll et al. 1990). The sequence was initially predicted to correspond to a protein with 11 transmembrane domains and one large cytosolic loop linking transmembrane domain five and six but a revised model predicting only nine transmembrane domains is now generally accepted.
PLASMA MEMBRANE CALCIUM PUMP
Structural and Regulatory Characteristics
The PMCA pump belongs to the family of P-type ATPases, which are characterized by the temporary conservation of ATP energy in the form of a phosphorylated enzyme intermediate (hence P-type) formed between the γ − phosphate of hydrolyzed ATP and an invariant D-residue in a highly conserved sequence of the pump molecules: SDKTGT (L/I/V/M) (T/I/S). The pump operates with a 1:1 Ca2+/ATP stoichiometry as a Ca2+: H+ exchanger: the matter of Ca2+/H+ stoichiometry is still controversial (Niggli et al. 1982; Hao et al. 1994). The pump has high Ca2+ affinity and a multitude of agents that modulate it. The Kd of the pump for Ca2+, which is 10 to 20 µM in the resting state, decreases to less than 1 µM following calmodulin interaction (Brini and Carafoli 2009). Acidic phospholipids also increase the Ca2+ affinity of the pump. Even if the molecular mechanism of the activation by phospholipids is obscure, it could be physiologically meaningful: It has been calculated that in the membrane environment the pump is probably permanently activated by acidic phospholipids to about 50% of its maximal activity (Niggli et al. 1981).
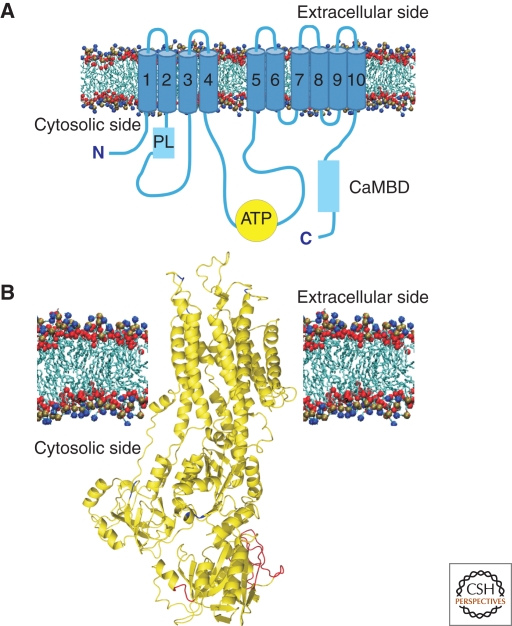
Structurally, the pump is predicted to consist of 10 transmembrane domains, two large intracellular loops, and of amino- and carboxy-terminal cytoplasmic tails (Fig. 2). The 90-residue amino-terminal tail contains a consensus-binding site for the 14-3-3 protein, which plays an inhibitory role on pump activity (Rimessi et al. 2005). The first cytosolic loop between transmembrane domains two and three is considered the “transducer domain.” It contains one of the two sites that mediate the activation by acididic phospholipids (Zvaritch et al. 1990) and one of the two sites for the autoinhibitory interaction with the calmodulin binding domain. This loop also contains site A, which is one of the two main sites of the alternative splicing that generates pump variants. Interestingly, the A-splice-site-insert also plays a key role in the targeting of PMCA to specialized portion of the plasma membrane of polarized cells (Chicka and Strehler 2003).
Figure 2.
(A) Topology model of PMCA. The pump is organized in the membrane with ten transmembrane domains connected on the external side by short loops. The cytosolic portion of the pump contains the catalytic center and other functionally important domains. The ATP binding site is indicated with a yellow circle. Acidic phospholipid binding domain (PL) and Calmodulin binding domain (CaMBD) are represented with pale blue boxes. (B) Deduced 3D structure of the PMCA pump. The three-dimensional structure of the PMCA pump has been obtained modeling it on that of the SERCA pump (Toyoshima et al. 2000). The image is a kind gift of Dr. M. Hilge (Nijmegen, Holland).
The cytosolic loop that connects transmembrane domains four and five contains the catalytic center that includes the ATP binding site and the aspartate residue that forms the acyl phosphate intermediate. It also contains the second binding site for the carboxy-terminal calmodulin-binding domain. The carboxy-terminal tail contains the main regulatory sites for the activity of the pump: the calmodulin binding domain (which also binds acidic phospholipids), consensus sites for protein kinases A (PKA), and C (PKC) and high affinity allosteric Ca2+ binding sites. The calmodulin-binding domain of the PMCA pump acts as an auto-inhibitory domain, binding to “receptor” sites in the first and second cytoplasmic loops of the pump. Calmodulin would remove the domain from the binding sites, freeing the pump from autoinhibition.
The carboxy-terminal region of the pump is also the target of isoform-specific phosphorylations by PKA and PKC. Phosphorylation could influence the regulation of the pumps either directly (e.g., by de-inhibiting it) or indirectly (e.g., via interference with calmodulin regulation). Recent studies have shown that PKC affects the various PMCA isoforms and splicing variants in different ways according to variations in the carboxy-terminal sequence: the phosphorylation may activate the pump, inhibit it, or have no effects (Strehler and Zacharias 2001). As for PKA, its consensus site has only been described in isoform one of the pump. The PMCA pump is also a substrate of intracellular proteases, as its carboxy-terminal tail contains target sequences for the Ca2+ dependent protease calpain (Guerini et al. 2003) and for caspase 1 and 3. Calpain cleaves the pump in the calmodulin binding sequence, leading to permanent activation of the pump. Both activation and inactivation have been described for caspases 1 and 3 action on PMCA2 and 4 (Schwab et al. 2002; Paszty et al. 2005).
The carboxy-terminal domain of the pump also contains an important site of alternative splicing, site C. C-site splicing occurs in all pump isoforms (albeit with varying degree of complexity) and causes the inclusion of one (or two) additional exons, or of portion of exons. The insertion has structural and functional consequences: its leads to the truncation of the pump because of a frame shift in the coding region, and alters, for instance, the pH sensitivity of the interaction of calmodulin with the calmodulin binding domain. In general, the truncated isoforms have much decreased affinity for calmodulin (Enyedi et al. 1994). Surprisingly, however, the full length and the truncated variants expressed in living cells appear to have the same ability to restore the cytosolic Ca2+ transient generated by cell stimulation: the finding suggests that in vivo, calmodulin may not be the only factor regulating PMCA activity (Brini et al. 2003).
The carboxy-terminal domain also mediates the interaction with PDZ domains of different proteins, and mediates PMCA dimerization (which also activates the pump (Kosk-Kosicka and Bzdega 1988; Vorherr et al. 1991)). The interaction with PDZ domains is apparently limited to the full-length splice isoforms, as the carboxy-terminally truncated variants lack the consensus site for its binding. PMCA has been reported to interact with PDZ domain-containing proteins involved in the recruitment and maintenance of membrane proteins in specific membrane domains, such as members of membrane associate guanylate kinase (MAGUK) family, i.e., SAP, CASK, the Na/H exchanger regulatory factor 2, PISP and the Ania3/Homer protein (reviewed in (Brini and Carafoli 2009)). These proteins could contribute to targeting and retention of PMCA in specialized domains. The increased local pump concentration could regulate Ca2+ signaling. The PDZ binding domain also directs the interaction of one of the PMCA-isoforms (4b) with nitric oxide synthase 1 (NOS-1) in a ternary complex with α-syntropin. The latter would bind to a domain further upstream in the pump sequence (Williams et al. 2006). The double interaction would thus down-regulate the production of NO by decreasing Ca2+ in the immediate vicinity of the synthase: i.e., the pump could function as a modulator of signal transduction, in addition to regulating cell Ca2+ (Cartwright et al. 2009).
Isoforms and Tissue Distribution
The four basic PMCA isoforms (the PMCA is the product of four separate genes) have tissue-specific expression (Brini and Carafoli 2009). Although PMCA1 and PMCA4 are expressed in most tissues, PMCA2 and 3 are found in a restricted number of tissues, among them in brain, striated muscle and the mammary gland. In particular, PMCA2 is abundant in specialized cells such as cerebellar Purkinje neurons and cochlear hair cells, and it is also present in uterus and in lactating mammary glands. It is also expressed in significant amounts in liver and kidney. PMCA3 distribution is more restricted, the choroid plexus expressing significant amounts of it.
The transcript of each of the four genes encoding PMCA pumps is subject to alternative splicing: The sites in which it occurs are named A, B, and C. Of the large number of splice variants theoretically possible about 30 have being detected at the RNA or protein levels (Brini and Carafoli 2009). Alternative splicing at site A and C occurs for all four isoforms (however, PMCA1 is never spliced at site A). Alternative splicing at site B has been described only for human isoform one and four and it could be artifactual. Splice site A is located upstream of one of the regulatory binding sites for acidic phospholipids and involves up to three exons that can be optionally inserted or excluded. In PMCA3 and 4 a single exon may be included or excluded in the mature mRNA, thus generating the x and z splice variants, respectively. The situation in PMCA2 is more complex: in the human pump, three exons of 33, 60, and 42 nt, alternatively used, could in principle originate eight different variants. However, only four of them have so far been detected as mRNAs in a variety of tissues. Variant w includes all three exons, variant z excludes all three exons, variant x includes the 42 nt exon, and variant y includes the 33 and 60 nt exons. This alternative splicing was shown to be essential in the apical targeting of PMCA, and thus could also be involved in the regulation of the pump distribution in specialized portions of the plasma membrane (directly or through the interaction with other molecular determinants). Considering that splice site A is contiguous to one of the two phospholipid-binding domains, it could in principle effect the phospholipid regulation of PMCA isoforms by altering the accessibility of the binding domain to acidic phospholipids. As mentioned, the splicing at site B may be an artifact, as its occurrence in vivo has been a point of controversy. It has been proposed to remove a carboxy-terminal 108 nt segment, leading to the loss of the 10th transmembrane domain and causing a reorganization of the pump topology (Preiano et al. 1996). Splice site C is located in the carboxy-terminal of PMCA protein, within the calmodulin binding domain: the inclusion of a large exon (154–175 nt) alters the carboxy-terminal half of the domain in all isoforms, and changes the reading frame of the remaining carboxy-terminal tail. This splicing is thus responsible for the generation of variant a, truncated after the first 12 residues of the calmodulin binding domain. The variant has reduced responsiveness to calmodulin with respect to the full-length variant (termed variant b). Lower Ca-calmodulin affinity and a significantly elevated basal pumping activity are general characteristics of the a splice variants in comparison with the b variants (Preiano et al. 1996; Elwess et al. 1997; Caride et al. 2007).
Interestingly, the expression of the PMCA pumps is transcriptionally regulated by Ca2+ itself (Zacharias and Strehler 1996; Carafoli et al. 1999). The phenomenon has been studied on cultured neurons and is correlated to their maturation. In cerebellar granule cells, as Ca2+ concentration in the cytoplasm increases, the pattern of expression of the PMCA pumps undergoes a dramatic rearrangement: isoform two and three become strongly up-regulated, whereas isoform one undergoes a splicing switch that generates high levels of the truncated a variant. In contrast, PMCA4 disappears in a process that is calcineurin dependent (Guerini et al. 2000). The reprogramming of the transcription of PMCAs is essential to the survival of the developing neurons: the regulation of PMCA expression may thus be critical to the survival of cells exposed to pathological increases in intracellular Ca2+. Parallel amplification of Ca2+ influx and efflux pathways may enable differentiated neurons to precisely localize Ca2+ signals in time and space (Usachev et al. 2001). Similarly, in rat hippocampal neurons, transcripts for all isoforms of the PMCA pump have been found to be strongly up-regulated during the second week in culture, the overall increase in Ca2+ extrusion systems being accompanied by changes in the expression and cellular localization of different isoforms. The accumulation of PMCAs in dendrites and dendritic spines coincided with the functional maturation of these neurons, underlining the importance of the proper spatial organization of the Ca2+ extrusion systems in synaptic function and development (Kip et al. 2006).
Role in Physiology and Pathology
PMCA pumps are expressed ubiquitously, thus, they are unlikely candidates as molecules that could mediate cell specific processes. However, the existence of so many PMCA variants (basic isoforms and alternative spliced forms), the finding on knockout mice for the specific PMCA isoforms, and studies on the mechanisms modulating their cell/tissue specific distribution indicate that PMCA pumps may play important roles as signaling molecules, in addition to having a constitutive role as Ca2+ housekeeping enzymes.
The ablation of isoforms two and four in mice has indeed revealed that they are important in specialized biological processes. PMCA2 plays an essential role in the hearing process, because it dissipates with peculiar kinetics the Ca2+ transients generated by the opening of the mechanoelectrical transduction (MET) channels in the stereocilia of cochlear hair cells. Mutations in the PMCA2 pumps in hereditary deafness impair the longer-term export of Ca2+ from hair cells, but not their ability to respond to a sudden increase of Ca2+. This suggests that the mutated pumps reduce the extracellular Ca2+ gradient in the endolymph surrounding the hair cells, compromising the adaptation process responsible for the control of the opening of MET channels (Ficarella et al. 2007; Spiden et al. 2008). Interestingly, the PMCA2 isoform has another peculiar function in mammary gland: its activity is responsible for the high content of Ca2+ in the milk. PMCA2 is strongly up-regulated in lactating mammary gland, and PMCA2 null mice have 40% less Ca2+ in the milk (Reinhardt et al. 2004).
PMCA4 is crucial to male fertility: in mice, its ablation reduces sperm motility, probably resulting from Ca2+ overload and mitochondrial damage (Schuh et al. 2004). Interestingly, PMCA4 is also essential for the modulation of the activity of the calcium/calmodulin dependent neuronal nitric oxide synthase (nNOS) (Schuh et al. 2001) and thus, in turn, controls nitric oxide production, which is important in the regulation of excitation-contraction coupling of the heart. It has been shown that PMCA4b regulates cardiac contractility in vivo through its interaction with nNOS (Oceandy et al. 2007).
PMCA defects have been described in a large number of disease conditions, among them those linked to the oxidative stress in several tissues, particularly brain, in brain ischemia, diabetes, atherosclerosis, aging, neurodegenerative diseases, and various disturbances of Ca2+ metabolism. PMCA defects have also been reported in various cancer types (reviewed in Monteith et al. 2007), in line with accumulating evidence on the involvement of Ca2+ homeostasis dysfunction in the process of malignant transformation. The genetic diseases involving the PMCA pump are best characterized: a number of dysfunctions linked to the ablation, or to partial disruptions, including point mutations, of its genes, have been described in mice. So far, the only identified spontaneous human disease related to a PMCA pump (isoform 2) defect is the form of hereditary deafness described above (Schultz et al. 2005; Ficarella et al. 2007).
Genetic Manipulations
Knockout mice have been developed and the phenotypes studied for PMCA1, PMCA2, and PMCA4, but not yet for PMCA3. As this isoform is widely expressed in developing embryos, it may be essential for normal gestation.
The ablation of the PMCA1 gene has resulted in early embryonic lethality in homozygotes, underlying the essential role of PMCA1 in the early stages of development and in organogenesis, in line with its suggested housekeeping function (Okunade et al. 2004). Heterozygotes, by contrast, appeared healthy, however, loss of one copy of the PMCA1 gene in mice with a PMCA4 null background led to increased propensity to apoptosis in the smooth muscles of blood vessels, possibly because of Ca2+ overload (Okunade et al. 2004). The ablation of the PMCA2 gene revealed its involvement in the hearing process: the phenotype of the PMCA2 knockout mice (Kozel et al. 1998) was characterized by balance impairment and hearing loss. The study of the vestibular inner ear revealed the absence of the otoconia and the progressive degeneration of the hair cells beginning 10 days after birth. The PMCA2 pump is probably also important to general neuronal Ca2+ homeostasis because PMCA2 null mice showed a significant reduction in the number of spinal cord motor neurons (Kurnellas MP 2005) and abnormalities in Purkinje neurons (Kurnellas MP 2007). However, the balance defect was apparently not due, as could in principle have been expected, or was only partially due, to a cerebellar dysfunction. It was apparently related to the absence of the Ca-carbonate crystals of the otoconia embedded in the otolithic membrane overlying the sensory epithelium that sense gravity and linear acceleration in the inner ear. In keeping with the high level of expression of PMCA2 in the mammary gland, the ablation of its gene, in addition to generating the hearing loss phenotype, also strongly reduced the concentration of Ca2+ in the milk (Reinhardt et al. 2004).
The ablation of the PMCA4 gene failed to cause a very evident general pathological phenotype (Schuh et al. 2004), but local defects were present. This is interesting, as PMCA4 is also widely expressed, and has also been proposed to play a housekeeping role. However, it has now become evident that PMCA4 plays more specialized roles, and is not only essential for the general function of controlling Ca2+ homeostasis in all cells. One prominent defect caused by PMCA4 dysfunction was male infertility, reflecting the dominance of the PMCA4 pump in the testis, where it represents more than 90% of the total PMCA protein (Prasad et al. 2004). The ablation of the PMCA4 gene produced other localized dysfunctions, for instance in the modulation of the Ca2+ signals in B-lymphocytes (Chen et al. 2004), and in the contractility of vascular or bladder smooth muscles (Okunade et al. 2004).
Studies on mice overexpressing PMCA4 isoform had revealed other important functions for this isoform. Early studies, in which the PMCA4 pump had been expressed specifically in the myocardium of the rat, and in vascular smooth muscle cells in mice, had indicated a role in the modulation of myocardial growth and hypertrophy (Hammes et al. 1998). They have also indicated a role in the regulation of the peripheral vascular tone, as the mice displayed increased peripheral blood pressure (Gros et al. 2003; Schuh et al. 2003).
SODIUM/CALCIUM EXCHANGER
Structural and Regulatory Characteristics
NCX accomplishes Ca2+ extrusion by using the electrochemical gradient of Na+: during each cycle three Na+ ions enter the cell and one Ca2+ ion is extruded against its gradient (for comprehensive reviews see Blaustein and Lederer [1999]; Lytton [2007]). In addition to being transported, cytoplasmic Na+ and Ca2+ ions also regulate exchanger activity. Binding of Ca2+ ions to sites located in the cytosolic loop generally activate the exchanger, whereas binding of Na+ ions has been shown to deactivate it: the physiological importance and molecular mechanisms underlying the regulation remain unclear. The operation of NCX is fully reversible, and the direction of the movement of the transported ions depends entirely on the electrochemical gradients of Na+ and Ca2+ and on the number of ions that bind to the molecule and are transported. Under resting cellular ionic condition and membrane potential, the NCX acts to extrude Ca2+ from the cytoplasm. However, in the heart, when the plasma membrane becomes depolarized during systole, and the Na+ levels rise because of the opening of the plasma membrane voltage–operated Na+ channels, the exchanger reverses its operation and mediates Ca2+ entry. The influx of Ca2+ may play an important regulatory role in the excitation-contraction process by influencing the gating of voltage-operated Ca2+ channels and by altering the SR Ca2+ load.
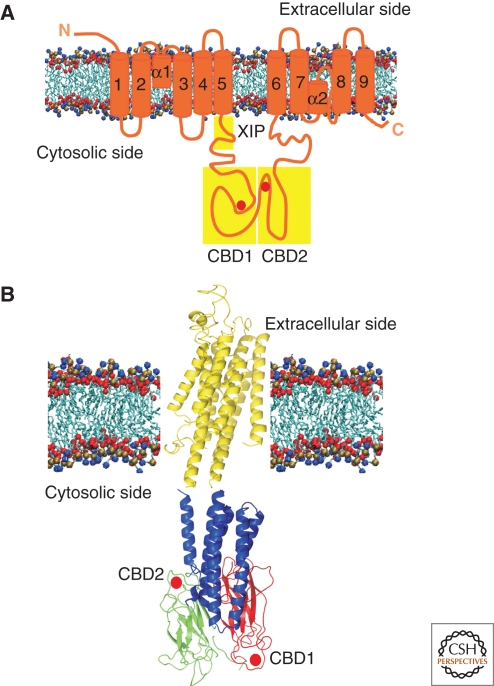
A membrane topology model based on isoform one of the NCX now predicts nine transmembrane α-helices that divide the molecule in a amino-terminal portion composed of the first five transmembrane domains, and a carboxy-terminal portion composed of the last four transmembrane domains (Fig. 3A). These two portions are separated by a cytosolic loop of about 500 residues that contains the site for NCX1 regulation, and is thus the target for the development of inhibitory compounds. NCX1 also contains two regions of internal repeats: the α repeats (α1 and α2 in Fig. 3A) are involved in ion binding and transport; the β repeats in the large intracellular loop are involved in binding of regulatory Ca2+ (CBD1 and CBD2 in Fig. 3). The β repeats have been structural defined by crystallization (Nicoll et al. 2006) and NMR studies (Hilge et al. 2006).
Figure 3.
(A) Topology model of NCX. The nine transmembrane domains comprise the two putative transport repeat regions: α-1 and α-2. XIP region, CBD1 and CBD2 are indicated by yellow boxes. The red spheres indicate the Ca2+ binding sites position (B) Hypothetical structural model of the intact NCX. The nine transmembrane domains are shown as yellow α-helices, the CLD as blue α-helices and the CBD1 and CBD2 β repeats as red and green β strands, respectively. The red spheres indicate the Ca2+ binding sites position. The image is a kind gift of Dr. M. Hilge (Nijmegen, Holland).
The first portion of the large cytosolic loop (close to transmembrane domain five) is an amphipathic sequence, called XIP (exchanger inhibitor peptide) because the addition of a peptide corresponding to this sequence inhibits the exchanger. This first portion of the region is responsible for the regulation by Na+ and the acidic phospholipids. The second portion of the loop contains the two Ca2+ binding domains (CBD1 and CBD2) that are arranged in an antiparallel fashion, and connected through a third domain designated as CLD (α-catenin like domain) to the membrane portion of the NCX (Fig. 3B). The CBD1 is the primary Ca2+ sensor that detects small cytosolic Ca2+ increases and undergoes large structural changes that activate the exchanger. The CBD2 undergoes instead modest structural alterations and binds Ca2+ only at elevated concentrations (Hilge et al. 2006; Nicoll et al. 2006). The carboxy-terminal end of the large cytosolic loop contains a hydrophobic and proline-rich sequence that had been originally modeled as a transmembrane domain. Recent evidence has positioned it on the cytosolic side of the membrane.
In addition to the two transported species, other regulatory agents of the exchanger include the intracellular pH, metabolic components (e.g., ATP, phosphatydyl inositol 4,5 bisphosphate), protein kinases PKA and PKC, redox agents, hydroxyl radicals, H2O2, dithiothreitol, O2−, Fe3+, Fe2+, Cu2+, and OH− (Doering and Lederer 1993; Matsuoka et al. 1995; Matsuoka et al. 1997; Iwamoto et al. 1998; He et al. 2000).
As is the case for the PMCA, a number of cytoskeletal proteins have been shown to interact with the NCX. The large cytosolic loop interacts with several proteins: the 14-3-3 protein, phosphorylated PLM (phospholemman, a member of a family of transport regulators, best known as modulators of Na, K-ATPase activity) and calcineurin: they all have inhibitory function on NCX activity (Katanosaka et al. 2005; Pulina et al. 2006; Wang et al. 2006; Zhang et al. 2006). Other proteins have also been shown to interact with the NCX: ankyrin (Li et al. 1993), the filamentous actin network (Condrescu and Reeves 2006), caveolin 3 (in the heart) (Bossuyt et al. 2002; Camors et al. 2006), and caveolin 1 and 2 (in neuronal cells) (Cha et al. 2004). These interactions have been proposed to regulate NCX activity by modulating the membrane NCX localization and by recruiting it to specialized portions of the plasma membrane as a part of macromolecular complexes (Schulze et al. 2003; Lencesova et al. 2004), similarly to PMCA, which can be recruited through the interaction with nNOS to form a specialized Ca2+ concentration microdomain.
The phosphorylation of NCX1 remains a topic of much controversy: several studies have claimed that PKA and/or PKC, operating through adrenergic stimulation, could modulate NCX function. The regulation could also be indirect and mediated by interactions with other proteins in macromolecular complexes.
Isoforms and Tissue Distribution
The NCX family belongs to a larger superfamily of related Ca2+/cation antiporter genes that has five major branches. Three of them relate to the Na+/Ca2+ transport in mammals: NCX (SLC8 family), NCKX (Na+/Ca2+-K+ exchangers, SLC24 family) and the CCX (Ca2+/anion exchangers), which contains the Na+/Ca2+-Li+ exchanger (NCLX). NCKX proteins play a crucial role in regulating Ca2+ fluxes during photoreceptors adaptation, synaptic plasticity, and skin pigmentation (for a comprehensive review see Lytton [2007]). NCLX is the single mammalian member of the phylogenetically ancestral CCX family. It has been initially characterized as a novel plasma membrane Na+/Ca2+ exchanger that catalyzes Na+ or Li+-dependent Ca2+ transport, but very recent evidence indicates that it is the long elusive mitochondrial Na+/Ca2+ exchanger (Palty 2009).
The SLC8 family includes three basic proteins, NCX1, NCX2, and NCX3, encoded by three separate genes. A fourth member, described in teleost fish, is not present in the mammalian genome (Marshall et al. 2005). NCX1 was originally characterized and cloned from heart, but its expression is almost ubiquitous, with high levels in brain and kidney. NCX2 and NCX3 are selectively expressed in the brain and skeletal muscle, and in some neuronal populations, respectively. NCX1 is the best-characterized member of the family. Its expression is controlled by alternative promoters in a tissue-specific and transcription factor-specific manner: one of them is specific to heart, one to kidney, and one to the other tissues. The transcripts of NCX1 gene are alternatively spliced at two sites: the first is located in the 5′-untranslated region and does not change the structure of the mature protein. The second is located within the coding region and leads to the introduction of two mutually exclusive exons (exon A in excitable tissues such as brain and muscle, and exon B in nonexcitable tissues), and of four other exons, thus potentially encoding a large number of NCX1 isoforms with differences in the cytosolic loop. Interestingly, giant excised patch clamp experiments have shown that exon A containing isoforms, in contrast to exon B containing variants, may not have intracellular Na+-dependent inactivation (Dyck et al. 1999). NCX3, but probably not NCX2, is alternatively spliced to generate variability in the cytoplasmic region corresponding to that of NCX1. No distinctive properties have been described for the different splicing isoforms, suggesting that their specific function is probably related to different spatial targeting.
Importantly, as is the case for the PMCA pump, the transcription of NCX genes is also differentially regulated by Ca2+ during development, and also in adult neurons (Carafoli et al. 1999). NCX2 and NCX3 transcripts are down-regulated and up-regulated in cerebellar granule neurons, respectively, under the conditions of intracellular Ca2+-increase that reprogram the pattern of transcription of the PMCA pumps. The down-regulation of NCX2 transcription in cerebellar granules is calcineurin dependent (Li et al. 2000), whereas that of NCX3 in model neuroblastoma neurons is regulated by Ca2+ through the transcriptional repressor DREAM (Gomez-Villafuertes et al. 2005).
Role in Physiology and Pathology
As the NCX is not an enzyme, the qualitative measurement of its activity has always been difficult and the role of NCX in physiological conditions has been mostly inferred from theoretical considerations and from observations obtained on isolated systems in artificial conditions, or with invasive approaches such as giant patch techniques. More recently, the availability of selective inhibitors (Iwamoto 2007) and genetic manipulation approaches have permitted the dissection of many physiological aspects of NCX activity. The role of NCX has so far been best characterized in the heart: contraction is initiated by a small influx of Ca2+ from the extracellular ambient that induces a larger release of Ca2+ from the SR; relaxation requires Ca2+ removal, which is achieved mainly by the extrusion activity of NCX1 and the pumping back of Ca2+ in the SR lumen by the SERCA pump. Ca2+ extrusion through NCX1 also generates an inward depolarizing current (because of Na+ entry and to the uneven charge translocation) which may contribute to the shaping of the action potential, and/or counteracts the Ca2+ entry pathway by reducing its driving force. The Ca2+ entry during the reverse mode operation of NCX1 (induced by signals that generated Na+ entry, i.e., through ionotropic glutamate receptors) has been described to contribute to Ca2+ influx. These actions probably do not modify the single action potential, but they have been suggested to play a possible role in the refilling of the stores with Ca2+, thanks to the formation of protein complexes and functional coupling between plasma membrane microdomains and the ER lumen mediated by local Ca2+ gradients (Blaustein and Golovina 2001). In addition to the heart, where its function has received great attention, numerous studies have underlined the role of the NCX in other tissues as well. The NCX1 has been shown to play a special role in the kidney, where the Ca2+ filtered at the glomerulus is reabsorbed passively along the proximal nephron. However, at the distal nephron level, vitamin D3 and parathyroid hormone regulate Ca2+ transport. NCX1 is highly expressed in the basolateral membrane of the distal nephron, and helps regulating the active transcellular Ca2+ reabsorption, thus contributing to the regulation of systemic Ca2+ levels rather than to the intracellular levels. The role of the exchanger in other tissues has not been analyzed in the same detail as it has in the heart (or kidney); however, in the last few years its role in the brain has received increasing attention, particularly as a means to protect neurons from ischemic damage. The specific contribution of each of the three basic NCX isoforms cannot be established with certainly, because they are coexpressed in brain neurons. However, the widespread and abundant expression of NCX2 has indicated a key role for it in neuronal Ca2+ homeostasis. NCX3 also appears to be important to neurons: its specific cleavage during brain ischemia and in neurons undergoing excitotoxicity has shown that it plays a critical role in protecting them from ischemic insults (Bano et al. 2005; Annunziato et al. 2007). However, the issue still has controversial aspects: evidence has been provided that during brain ischemia changes in membrane potential and unregulated Ca2+ entry could activate the NCX Ca2+ entry-mode, and thus contribute to Ca2+ overload and eventual neurodegeneration (Kintner et al. 2007). Other work has suggested instead that during excitotoxic insults, the activation of calpain leads to the cleavage of NCX and thus to the impairment of the Ca2+ extrusion process, culminating in excitotoxic death. Thus, the inhibition (or the down-regulation) of the exchanger could be neuroprotective (Luo et al. 2007), but could also transform a Ca2+ transient elicited by nonexcitotoxic stimulation into a lethal Ca2+ overload (Bano et al. 2005; Annunziato et al. 2007).
Considering the essential role of NCX in regulating Ca2+ homeostasis in a variety of tissues, the importance of understanding how changes in its activity could contribute to pathological situations is obvious. No genetic disease has so far been associated to NCX mutations, but a number of pathologies have been related to its malfunctioning. Again, the best documented studies are related to heart dysfunctions: NCX deficiencies have been claimed to play a role both in cardiac arrhythmias and in the ischemia/reperfusion injury. They can contribute to the former in two ways: through changes in the balance between the forward-mode of Ca2+ efflux and the reverse-mode of Ca2+ influx consequent on the deregulation of intracellular Na+, or through conditions that could lead to Ca2+ overload in the SR, and thus to spontaneous Ca2+ release from it that could generate extrasystolic events: the extra Ca2+ release could enhance NCX activity, thus generating a depolarizing current that can contribute to the elongation of the action potential. Up-regulation of NCX expression at the transcriptional level has been described in cardiac hypertrophy, ischemia, and failure (Kent et al. 1993). However, it is difficult to establish whether the up-regulation is the cause of the dysfunction or rather an adaptive mechanism that, in the end, leads to cardiac hypertrophy and heart failure.
In ischemia/reperfusion injury the action of NCX is still linked to its reverse mode of operation: although other factors could contribute, the removal of H+ ions that accumulate during the ischemic period by the Na+/H+ exchanger could enhance the intracellular Na+, thus activating Ca2+ entry, and eventually causing SR Ca2+ overload.
Genetic Manipulations
In the last few years, various NCX knockout mice have been generated. Homozygous NCX1 KO mice were not vital and died during embryonic development, possibly because of heart failure (Wakimoto et al. 2000). However, the cardiac-specific knockout of NCX1 was not lethal (Henderson et al. 2004) suggesting that the cause of lethality in NCX1 KO may have been extra-cardiac, and that NCX2 and 3 could not compensate for the absence of NCX1. The cardiac-specific NCX1 KO mice presented only a mild deficit in cardiac function that culminated in hypertrophy and heart failure only as the mice aged. Ca2+ measurements on myocytes revealed that the reduced Ca2+ clearance in the absence of NCX1 led to enhanced Ca2+ dependent inactivation of L-type Ca2+ channels. These myocytes also displayed a shorter action potential because of hyperpolarization caused probably by the increase in the expression of K+ channel subunits. The outcome of these modifications was a reduction of about 80% of Ca2+ fluxes through the plasma membrane: however, this reduction did not impair the Ca2+ release from the SR and contractility because of compensation mechanisms involving the gain of excitation-contraction coupling.
NCX2 KO mice displayed impairment in several hippocampal-dependent learning and memory tasks (Jeon et al. 2003). Larger presynaptic Ca2+ transients evoked an increase in the neurotransmitter release, and the increased postsynaptic Ca2+ transient enhanced long-term potentiation: the findings are consistent with the predominant role of NCX2 in pre- and postsynaptic Ca2+ clearance.
Mice lacking the NCX3 gene, which is highly expressed in cerebellum and in the peripheral nervous system, showed reduced motor activity and weakness of forelimb muscles (Sokolow et al. 2004). In addition, skeletal muscle defects were also observed, in line with the finding of abundant NCX3 expression in skeletal muscle.
CONCLUDING REMARKS
To respond dynamically to the changing needs of Ca2+ signaling, cells must be able to precisely control the type, amount, localization, and activation of Ca2+ transporters. This control is performed in two ways: through the variable expression of Ca2+ transporters with different biochemical characteristics, and through the fine modulation of their function by expressing differently active isoforms in response to the local or temporal cell demands. The cell also modulates the activity of its transporters by choosing specific molecular partners. This increases the complexity of the controlling machinery, but protects the cells from “the Ca2+ damage” that would inevitably follow the general failure of the Ca2+ controlling operation. The absence, or malfunction, of specific Ca2+ transport proteins would induce a confined defect: although generating functional discomfort, it would frequently still be compatible with cell life.
ACKNOWLEDGMENTS
The original work described in this review by the authors has been supported over the years by grants from the Italian Ministry of University and Research (FIRB2001 to E.C., PRIN 2003 and 2005 to M.B), the Telethon Foundation (Project GGP04169 to M.B.), the FP6 program of the European Union (FP6 Network of Excellence NeuroNe, LSH-2003-2.1.3-3 to E.C. and Integrated Project Eurohear LSHG-CT-20054-512063, to E.C.), the Human Frontier Science Program Organization to E.C., the Italian National Research Council (CNR) to M.B.
Footnotes
Editors: Martin D. Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Annunziato L, Pignataro G, Boscia F, Sirabella R, Formisano L, Saggese M, Cuomo O, Gala R, Secondo A, Viggiano D, et al. 2007. ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann N Y Acad Sci 1099: 413–426 [DOI] [PubMed] [Google Scholar]
- Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA 1969. The influence of calcium on sodium efflux in squid axons. J Physiol 200: 431–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P 2005. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120: 275–285 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529 [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA 2001. Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci 24: 602–608 [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ 1999. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854 [DOI] [PubMed] [Google Scholar]
- Bossuyt J, Taylor BE, James-Kracke M, Hale CC 2002. Evidence for cardiac sodium-calcium exchanger association with caveolin-3. FEBS Lett 511: 113–117 [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E 2009. Calcium pumps in health and disease. Physiol Rev 89: 1341–1378 [DOI] [PubMed] [Google Scholar]
- Brini M, Coletto L, Pierobon N, Kraev N, Guerini D, Carafoli E 2003. A comparative functional analysis of plasma membrane Ca2+ pump isoforms in intact cells. J Biol Chem 278: 24500–24508 [DOI] [PubMed] [Google Scholar]
- Camors E, Charue D, Trouve P, Monceau V, Loyer X, Russo-Marie F, Charlemagne D 2006. Association of annexin A5 with Na+/Ca2+ exchanger and caveolin-3 in non-failing and failing human heart. J Mol Cell Cardiol 40: 47–55 [DOI] [PubMed] [Google Scholar]
- Carafoli E, Genazzani A, Guerini D 1999. Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem Biophys Res Commun 266: 624–632 [DOI] [PubMed] [Google Scholar]
- Caride AJ, Filoteo AG, Penniston JT, Strehler EE 2007. The plasma membrane Ca2+ pump isoform 4a differs from isoform 4b in the mechanism of calmodulin binding and activation kinetics: implications for Ca2+ signaling. J Biol Chem 282: 25640–25648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright EJ, Oceandy D, Neyses L 2009. Physiological implications of the interaction between the plasma membrane calcium pump and nNOS. Pflugers Arch 457: 665–671 [DOI] [PubMed] [Google Scholar]
- Cha SH, Shin SY, Jung SY, Kim YT, Park YJ, Kwak JO, Kim HW, Suh CK 2004. Evidence for Na+/Ca2+ exchanger 1 association with caveolin-1 and -2 in C6 glioma cells. IUBMB Life 56: 621–627 [DOI] [PubMed] [Google Scholar]
- Chen J, McLean PA, Neel BG, Okunade G, Shull GE, Wortis HH 2004. CD22 attenuates calcium signaling by potentiating plasma membrane calcium-ATPase activity. Nat Immunol 5: 651–657 [DOI] [PubMed] [Google Scholar]
- Chicka MC, Strehler EE 2003. Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem 278: 18464–18470 [DOI] [PubMed] [Google Scholar]
- Condrescu M, Reeves JP 2006. Actin-dependent regulation of the cardiac Na(+)/Ca(2+) exchanger. Am J Physiol Cell Physiol 290: C691–C701 [DOI] [PubMed] [Google Scholar]
- Doering AE, Lederer WJ 1993. The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells. J Physiol 466: 481–499 [PMC free article] [PubMed] [Google Scholar]
- Dyck C, Omelchenko A, Elias CL, Quednau BD, Philipson KD, Hnatowich M, Hryshko LV 1999. Ionic regulatory properties of brain and kidney splice variants of the NCX1 Na(+)-Ca(2+) exchanger. J Gen Physiol 114: 701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwess NL, Filoteo AG, Enyedi A, Penniston JT 1997. Plasma membrane Ca2+ pump isoforms 2a and 2b are unusually responsive to calmodulin and Ca2+. J Biol Chem 272: 17981–17986 [DOI] [PubMed] [Google Scholar]
- Enyedi A, Verma AK, Heim R, Adamo HP, Filoteo AG, Strehler EE, Penniston JT 1994. The Ca2+ affinity of the plasma membrane Ca2+ pump is controlled by alternative splicing. J Biol Chem 269: 41–43 [PubMed] [Google Scholar]
- Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, et al. 2007. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci 104: 1516–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Villafuertes R, Torres B, Barrio J, Savignac M, Gabellini N, Rizzato F, Pintado B, Gutierrez-Adan A, Mellstrom B, Carafoli E, et al. 2005. Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J Neurosci 25: 10822–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R, Afroze T, You XM, Kabir G, Van Wert R, Kalair W, Hoque AE, Mungrue IN, Husain M 2003. Plasma membrane calcium ATPase overexpression in arterial smooth muscle increases vasomotor responsiveness and blood pressure. Circ Res 93: 614–621 [DOI] [PubMed] [Google Scholar]
- Guerini D, Pan B, Carafoli E 2003. Expression, purification, and characterization of isoform 1 of the plasma membrane Ca2+ pump: focus on calpain sensitivity. J Biol Chem 278: 38141–38148 [DOI] [PubMed] [Google Scholar]
- Guerini D, Wang X, Li L, Genazzani A, Carafoli E 2000. Calcineurin controls the expression of isoform 4CII of the plasma membrane Ca(2+) pump in neurons. J Biol Chem 275: 3706–3712 [DOI] [PubMed] [Google Scholar]
- Hammes A, Oberdorf-Maass S, Rother T, Nething K, Gollnick F, Linz KW, Meyer R, Hu K, Han H, Gaudron P, et al. 1998. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circ Res 83: 877–888 [DOI] [PubMed] [Google Scholar]
- Hao L, Rigaud JL, Inesi G 1994. Ca2+/H+ countertransport and electrogenicity in proteoliposomes containing erythrocyte plasma membrane Ca-ATPase and exogenous lipids. J Biol Chem 269: 14268–14275 [PubMed] [Google Scholar]
- He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD 2000. Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol 278: C661–C666 [DOI] [PubMed] [Google Scholar]
- Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, et al. 2004. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604–611 [DOI] [PubMed] [Google Scholar]
- Hilge M, Aelen J, Vuister GW 2006. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol Cell 22: 15–25 [DOI] [PubMed] [Google Scholar]
- Iwamoto T 2007. Na+/Ca2+ exchange as a drug target–insights from molecular pharmacology and genetic engineering. Ann N Y Acad Sci 1099: 516–528 [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Pan Y, Nakamura TY, Wakabayashi S, Shigekawa M 1998. Protein kinase C-dependent regulation of Na+/Ca2+ exchanger isoforms NCX1 and NCX3 does not require their direct phosphorylation. Biochemistry 37: 17230–17238 [DOI] [PubMed] [Google Scholar]
- Jeon D, Yang YM, Jeong MJ, Philipson KD, Rhim H, Shin HS 2003. Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38: 965–976 [DOI] [PubMed] [Google Scholar]
- Katanosaka Y, Iwata Y, Kobayashi Y, Shibasaki F, Wakabayashi S, Shigekawa M 2005. Calcineurin inhibits Na+/Ca2+ exchange in phenylephrine-treated hypertrophic cardiomyocytes. J Biol Chem 280: 5764–5772 [DOI] [PubMed] [Google Scholar]
- Kent RL, Rozich JD, McCollam PL, McDermott DE, Thacker UF, Menick DR, McDermott PJ, Cooper Gt 1993. Rapid expression of the Na(+)-Ca2+ exchanger in response to cardiac pressure overload. Am J Physiol 265: H1024–H1029 [DOI] [PubMed] [Google Scholar]
- Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D 2007. Role of Na+-K+-Cl- cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol 292: C1113–C1122 [DOI] [PubMed] [Google Scholar]
- Kip SN, Gray NW, Burette A, Canbay A, Weinberg RJ, Strehler EE 2006. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus 16: 20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosk-Kosicka D, Bzdega T 1988. Activation of the erythrocyte Ca2+-ATPase by either self-association or interaction with calmodulin. J Biol Chem 263: 18184–18189 [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, et al. 1998. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem 273: 18693–18696 [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Lee AK, Li H, Deng L, Ehrlich DJ, Elkabes S 2007. Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons. Mol Cell Neurosci 34: 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnellas MP, Nicot A, Shull GE, Elkabes S 2005. Plasma membrane calcium ATPase deficiency causes neuronal pathology in the spinal cord: a potential mechanism for neurodegeneration in multiple sclerosis and spinal cord injury. FASEB J 19: 298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencesova L, O'Neill A, Resneck WG, Bloch RJ, Blaustein MP 2004. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem 279: 2885–2893 [DOI] [PubMed] [Google Scholar]
- Li L, Guerini D, Carafoli E 2000. Calcineurin controls the transcription of Na+/Ca2+ exchanger isoforms in developing cerebellar neurons. J Biol Chem 275: 20903–20910 [DOI] [PubMed] [Google Scholar]
- Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD 1993. The cardiac Na+-Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem 268: 11489–11491 [PubMed] [Google Scholar]
- Luo J, Wang Y, Chen X, Chen H, Kintner DB, Shull GE, Philipson KD, Sun D 2007. Increased tolerance to ischemic neuronal damage by knockdown of Na+-Ca2+ exchanger isoform 1. Ann N Y Acad Sci 1099: 292–305 [DOI] [PubMed] [Google Scholar]
- Lytton J 2007. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406: 365–382 [DOI] [PubMed] [Google Scholar]
- Marshall CR, Pan TC, Le HD, Omelchenko A, Hwang PP, Hryshko LV, Tibbits GF 2005. cDNA cloning and expression of the cardiac Na+/Ca2+ exchanger from Mozambique tilapia (Oreochromis mossambicus) reveal a teleost membrane transporter with mammalian temperature dependence. J Biol Chem 280: 28903–28911 [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Nicoll DA, He Z, Philipson KD 1997. Regulation of cardiac Na(+)-Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109: 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD 1995. Regulation of the cardiac Na(+)-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca(2+)-binding domain. J Gen Physiol 105: 403–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ 2007. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7: 519–530 [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD 1990. Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Science 250: 562–565 [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Sawaya MR, Kwon S, Cascio D, Philipson KD, Abramson J 2006. The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif. J Biol Chem 281: 21577–21581 [DOI] [PubMed] [Google Scholar]
- Niggli V, Adunyah ES, Carafoli E 1981. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+-ATPase. J Biol Chem 256: 8588–8592 [PubMed] [Google Scholar]
- Niggli V, Penniston JT, Carafoli E 1979. Purification of the (Ca2+-Mg2+)-ATPase from human erythrocyte membranes using a calmodulin affinity column. J Biol Chem 254: 9955–9958 [PubMed] [Google Scholar]
- Niggli V, Sigel E, Carafoli E 1982. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem 257: 2350–2356 [PubMed] [Google Scholar]
- Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, Alatwi N, Venetucci L, Schuh K, Williams JC, et al. 2007. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation 115: 483–492 [DOI] [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, et al. 2004. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279: 33742–33750 [DOI] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, et al. 2009. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci 107: 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszty K, Antalffy G, Penheiter AR, Homolya L, Padanyi R, Ilias A, Filoteo AG, Penniston JT, Enyedi A 2005. The caspase-3 cleavage product of the plasma membrane Ca2+-ATPase 4b is activated and appropriately targeted. Biochem J 391: 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad V, Okunade GW, Miller ML, Shull GE 2004. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun 322: 1192–1203 [DOI] [PubMed] [Google Scholar]
- Preiano BS, Guerini D, Carafoli E 1996. Expression and functional characterization of isoforms 4 of the plasma membrane calcium pump. Biochemistry 35: 7946–7953 [DOI] [PubMed] [Google Scholar]
- Pulina MV, Rizzuto R, Brini M, Carafoli E 2006. Inhibitory interaction of the plasma membrane Na+/Ca2+ exchangers with the 14-3-3 proteins. J Biol Chem 281: 19645–19654 [DOI] [PubMed] [Google Scholar]
- Reinhardt TA, Lippolis JD, Shull GE, Horst RL 2004. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem 279: 42369–42373 [DOI] [PubMed] [Google Scholar]
- Reuter H, Seitz N 1968. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol 195: 451–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimessi A, Coletto L, Pinton P, Rizzuto R, Brini M, Carafoli E 2005. Inhibitory interaction of the 14-3-3{epsilon} protein with isoform 4 of the plasma membrane Ca(2+)-ATPase pump. J Biol Chem 280: 37195–37203 [DOI] [PubMed] [Google Scholar]
- Schatzmann HJ 1966. ATP-dependent Ca++-extrusion from human red cells. Experientia 22: 364–365 [DOI] [PubMed] [Google Scholar]
- Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, et al. 2004. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J Biol Chem 279: 28220–28226 [DOI] [PubMed] [Google Scholar]
- Schuh K, Quaschning T, Knauer S, Hu K, Kocak S, Roethlein N, Neyses L 2003. Regulation of vascular tone in animals overexpressing the sarcolemmal calcium pump. J Biol Chem 278: 41246–41252 [DOI] [PubMed] [Google Scholar]
- Schuh K, Uldrijan S, Telkamp M, Rothlein N, Neyses L 2001. The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J Cell Biol 155: 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, et al. 2005. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med 352: 1557–1564 [DOI] [PubMed] [Google Scholar]
- Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM 2003. Sodium/calcium exchanger (NCX1) macromolecular complex. J Biol Chem 278: 28849–28855 [DOI] [PubMed] [Google Scholar]
- Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, Tam J, Xu D, Xanthoudakis S, Nicholson DW, et al. 2002. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ 9: 818–831 [DOI] [PubMed] [Google Scholar]
- Shull GE, Greeb J 1988. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem 263: 8646–8657 [PubMed] [Google Scholar]
- Sokolow S, Manto M, Gailly P, Molgo J, Vandebrouck C, Vanderwinden JM, Herchuelz A, Schurmans S 2004. Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J Clin Invest 113: 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiden SL, Bortolozzi M, Di Leva F, de Angelis MH, Fuchs H, Lim D, Ortolano S, Ingham NJ, Brini M, Carafoli E, et al. 2008. The novel mouse mutation Oblivion inactivates the PMCA2 pump and causes progressive hearing loss. PLoS Genet 4: e1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler EE, Zacharias DA 2001. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev 81: 21–50 [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Nakasako M, Nomura H, Ogawa H 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405: 647–655 [DOI] [PubMed] [Google Scholar]
- Usachev YM, Toutenhoofd SL, Goellner GM, Strehler EE, Thayer SA 2001. Differentiation induces up-regulation of plasma membrane Ca(2+)-ATPase and concomitant increase in Ca(2+) efflux in human neuroblastoma cell line IMR-32. J Neurochem 76: 1756–1765 [DOI] [PubMed] [Google Scholar]
- Verma AK, Filoteo AG, Stanford DR, Wieben ED, Penniston JT, Strehler EE, Fischer R, Heim R, Vogel G, Mathews S, et al. 1988. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem 263: 14152–14159 [PubMed] [Google Scholar]
- Vorherr T, Kessler T, Hofmann F, Carafoli E 1991. The calmodulin-binding domain mediates the self-association of the plasma membrane Ca2+ pump. J Biol Chem 266: 22–27 [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro OM, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, et al. 2000. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem 275: 36991–36998 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang XQ, Ahlers BA, Carl LL, Song J, Rothblum LI, Stahl RC, Carey DJ, Cheung JY 2006. Cytoplasmic tail of phospholemman interacts with the intracellular loop of the cardiac Na+/Ca2+ exchanger. J Biol Chem 281: 32004–32014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Armesilla AL, Mohamed TM, Hagarty CL, McIntyre FH, Schomburg S, Zaki AO, Oceandy D, Cartwright EJ, Buch MH, et al. 2006. The sarcolemmal calcium pump, α-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J Biol Chem 281: 23341–23348 [DOI] [PubMed] [Google Scholar]
- Wu G, Xie X, Lu ZH, Ledeen RW 2009. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci 106: 10829–10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wu G, Lu ZH, Ledeen RW 2002. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem 81: 1185–1195 [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Strehler EE 1996. Change in plasma membrane Ca2(+)-ATPase splice-variant expression in response to a rise in intracellular Ca2+. Curr Biol 6: 1642–1652 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY 2006. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaritch E, James P, Vorherr T, Falchetto R, Modyanov N, Carafoli E 1990. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry 29: 8070–8076 [DOI] [PubMed] [Google Scholar]