Abstract
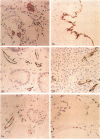
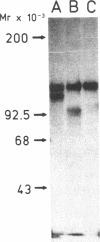
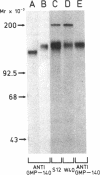
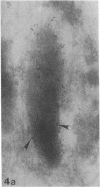
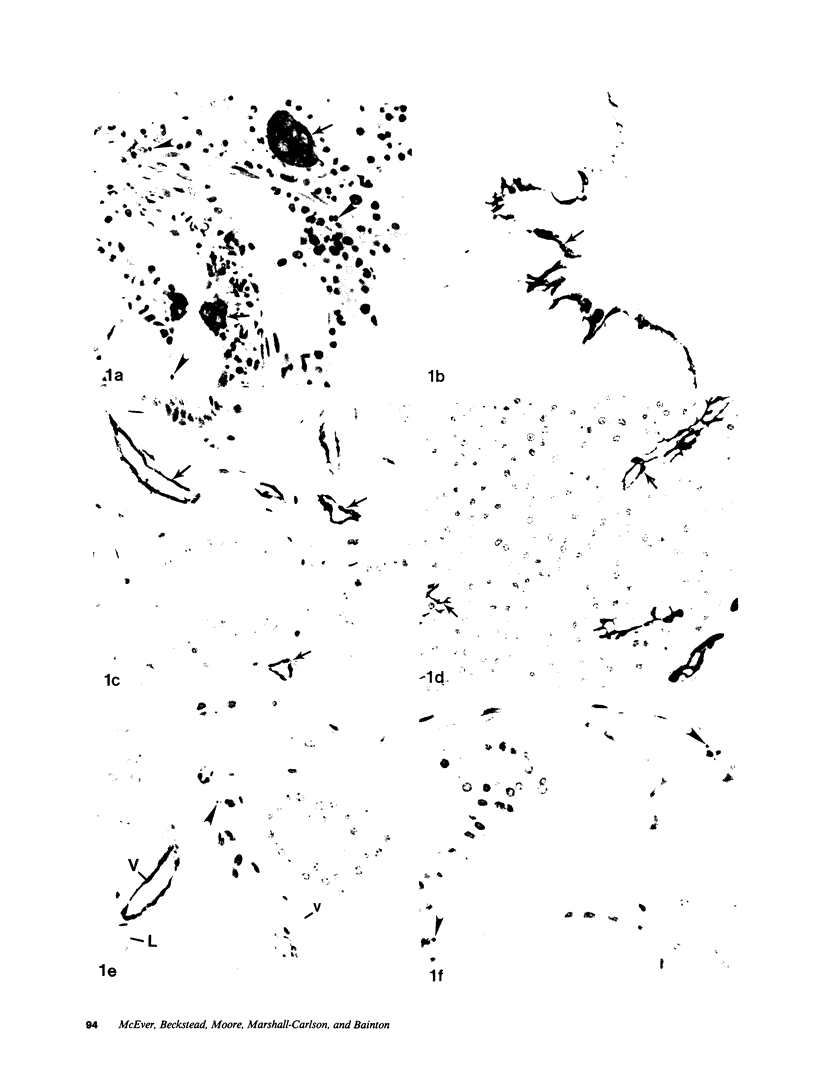
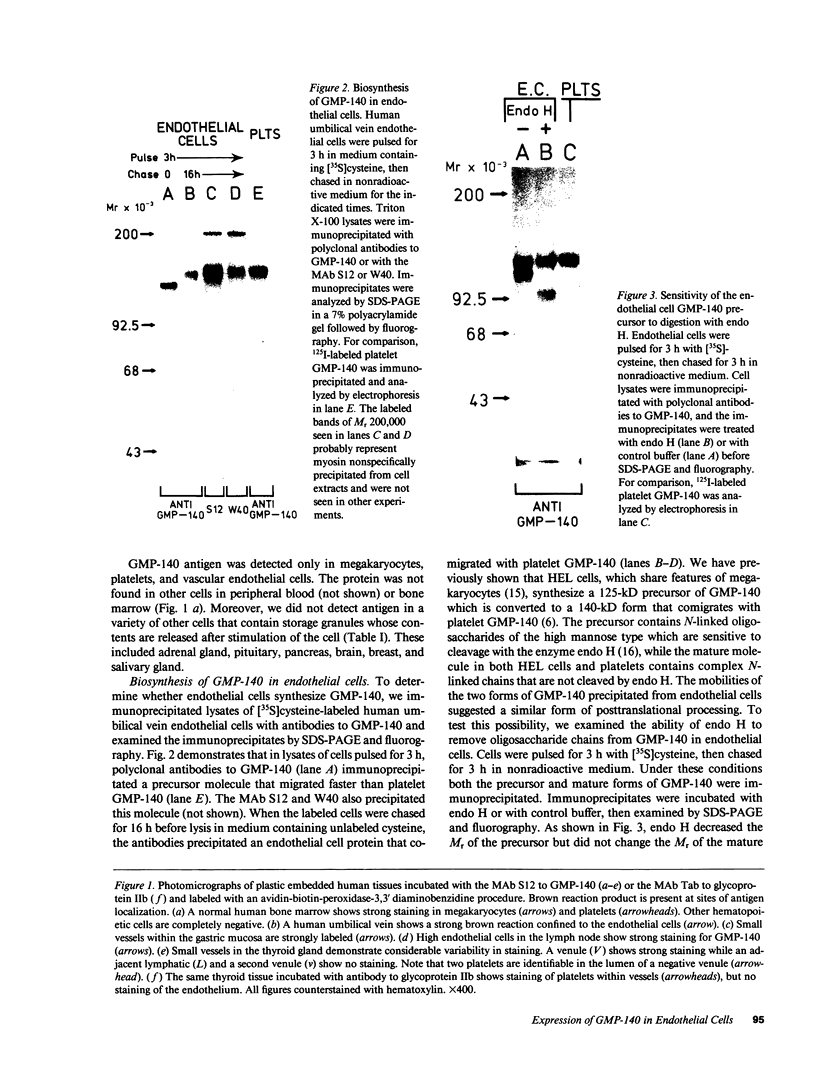
We used an immunoperoxidase procedure to examine the tissue distribution of the platelet alpha-granule membrane protein, GMP-140. In addition to its presence in megakaryocytes and platelets, GMP-140 antigen was found in vascular endothelial cells of diverse human organs, but it was not detected in other types of secretory cells. [35S]Cysteine-labeled human umbilical vein endothelial cells synthesized a GMP-140 molecule containing complex N-linked oligosaccharides similar to those previously demonstrated in platelets and the megakaryocytic HEL cell line. Using an immunogold procedure on frozen thin sections of endothelial cells, we found GMP-140 antigen to be localized to membranes of electron-dense storage granules. In double-label experiments there was colocalization of GMP-140 with vWf, indicating that these granules are Weibel-Palade bodies. When endothelial cells were stimulated with histamine, GMP-140 rapidly redistributed to the plasma membrane. Immunoassays of cell lysates indicated that, relative to total cell protein, less GMP-140 is present in human umbilical vein endothelial cells than in platelets. The restricted expression of GMP-140 in secretory granules of platelets and endothelium suggests that it has a specific function in the vascular system rather than a general role related to inducible secretion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckstead J. H., Stenberg P. E., McEver R. P., Shuman M. A., Bainton D. F. Immunohistochemical localization of membrane and alpha-granule proteins in human megakaryocytes: application to plastic-embedded bone marrow biopsy specimens. Blood. 1986 Feb;67(2):285–293. [PubMed] [Google Scholar]
- Berman C. L., Yeo E. L., Wencel-Drake J. D., Furie B. C., Ginsberg M. H., Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986 Jul;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bray P. F., Rosa J. P., Johnston G. I., Shiu D. T., Cook R. G., Lau C., Kan Y. W., McEver R. P., Shuman M. A. Platelet glycoprotein IIb. Chromosomal localization and tissue expression. J Clin Invest. 1987 Dec;80(6):1812–1817. doi: 10.1172/JCI113277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewenstein B. M., Warhol M. J., Handin R. I., Pober J. S. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987 May;104(5):1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D. S., Marlar R. A., Levin E. G. Human endothelial cells synthesize protein S. Blood. 1986 Apr;67(4):1168–1171. [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Pickett E. B., Saucerman S., McEver R. P., Kunicki T. J., Kieffer N., Newman P. J. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest. 1986 Aug;78(2):340–348. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. K., Sims P. J. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987 Feb;79(2):600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., McEver R. P., Sims P. J. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989 May 25;264(15):9053–9060. [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Isenberg W. M., McEver R. P., Shuman M. A., Bainton D. F. Topographic distribution of a granule membrane protein (GMP-140) that is expressed on the platelet surface after activation: an immunogold-surface replica study. Blood Cells. 1986;12(1):191–204. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Johnston G. I., Kurosky A., McEver R. P. Structural and biosynthetic studies of the granule membrane protein, GMP-140, from human platelets and endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1816–1823. [PubMed] [Google Scholar]
- Johnston G. I., Pickett E. B., McEver R. P., George J. N. Heterogeneity of platelet secretion in response to thrombin demonstrated by fluorescence flow cytometry. Blood. 1987 May;69(5):1401–1403. [PubMed] [Google Scholar]
- Loesberg C., Gonsalves M. D., Zandbergen J., Willems C., van Aken W. G., Stel H. V., Van Mourik J. A., De Groot P. G. The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim Biophys Acta. 1983 Sep 22;763(2):160–168. doi: 10.1016/0167-4889(83)90039-3. [DOI] [PubMed] [Google Scholar]
- Maruyama I., Bell C. E., Majerus P. W. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985 Aug;101(2):363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- McNiff J. M., Gil J. Secretion of Weibel-Palade bodies observed in extra-alveolar vessels of rabbit lung. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1284–1286. doi: 10.1152/jappl.1983.54.5.1284. [DOI] [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Reinders J. H., De Groot P. G., Gonsalves M. D., Zandbergen J., Loesberg C., Van Mourik J. A. Isolation of a storage and secretory organelle containing Von Willebrand protein from cultured human endothelial cells. Biochim Biophys Acta. 1984 Jul 20;804(3):361–369. doi: 10.1016/0167-4889(84)90140-x. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Sporn L. A., Chavin S. I., Marder V. J., Wagner D. D. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985 Sep;76(3):1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D., Brett J., Harris K., Nawroth P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: the synthesis and release of protein S. J Cell Biol. 1986 May;102(5):1971–1978. doi: 10.1083/jcb.102.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer T., Ando B., Sims P. J. Complement C5b-9-stimulated platelet secretion is associated with a Ca2+-initiated activation of cellular protein kinases. J Biol Chem. 1987 Oct 5;262(28):13674–13681. [PubMed] [Google Scholar]
- Wiedmer T., Esmon C. T., Sims P. J. On the mechanism by which complement proteins C5b-9 increase platelet prothrombinase activity. J Biol Chem. 1986 Nov 5;261(31):14587–14592. [PubMed] [Google Scholar]