Abstract
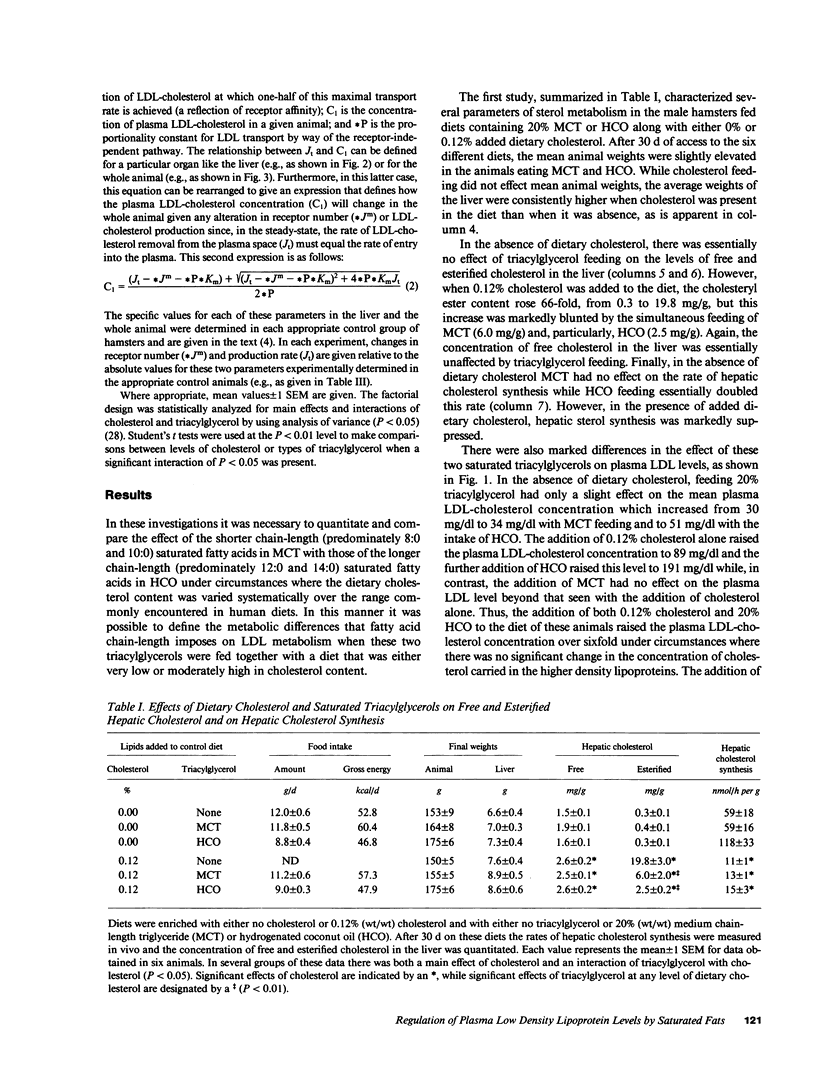
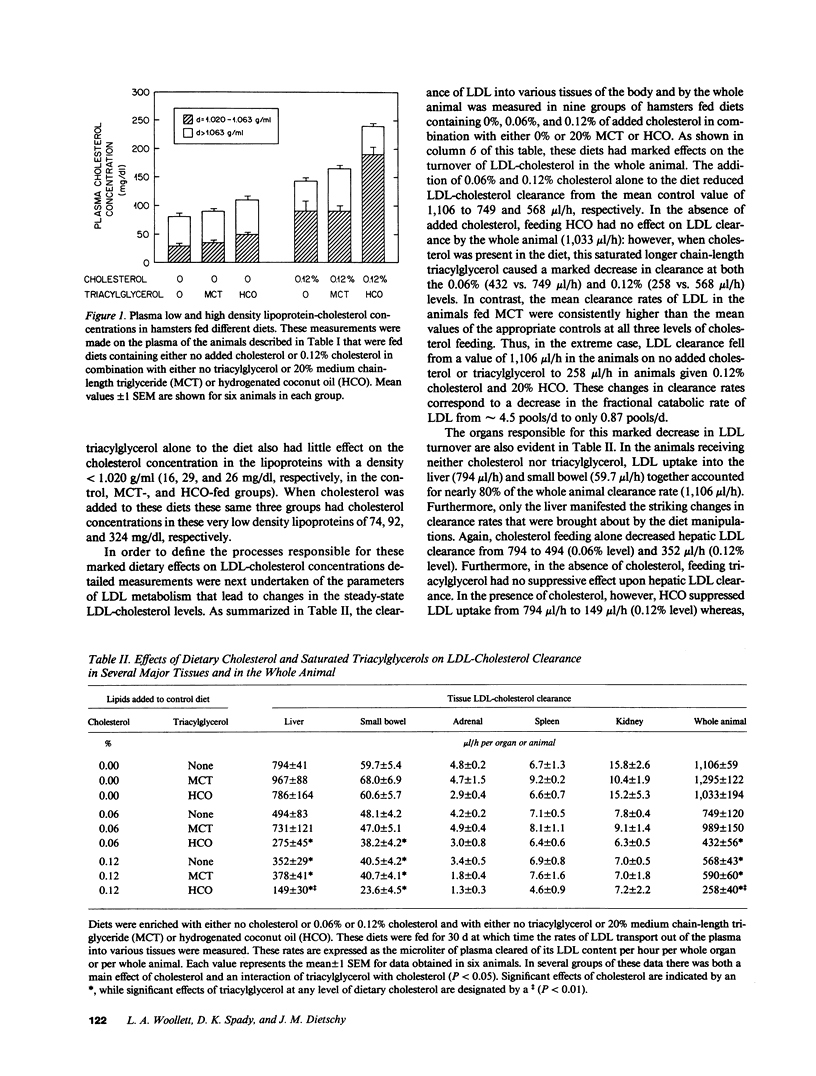
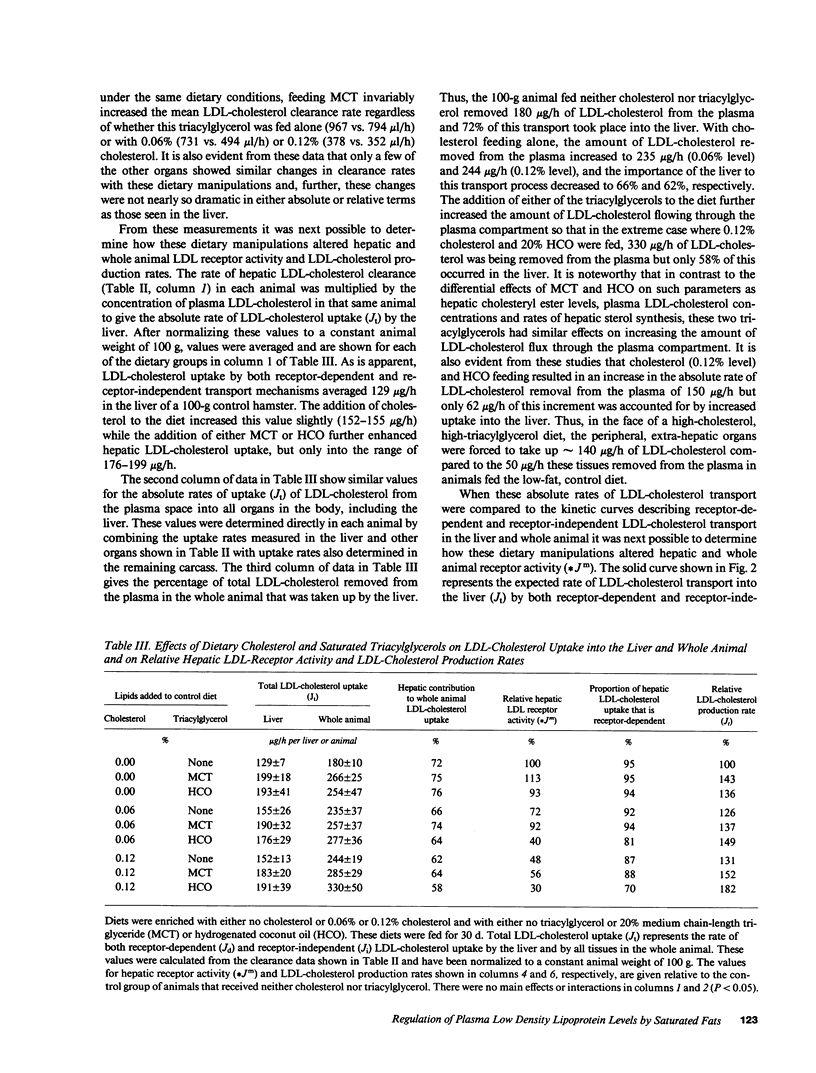
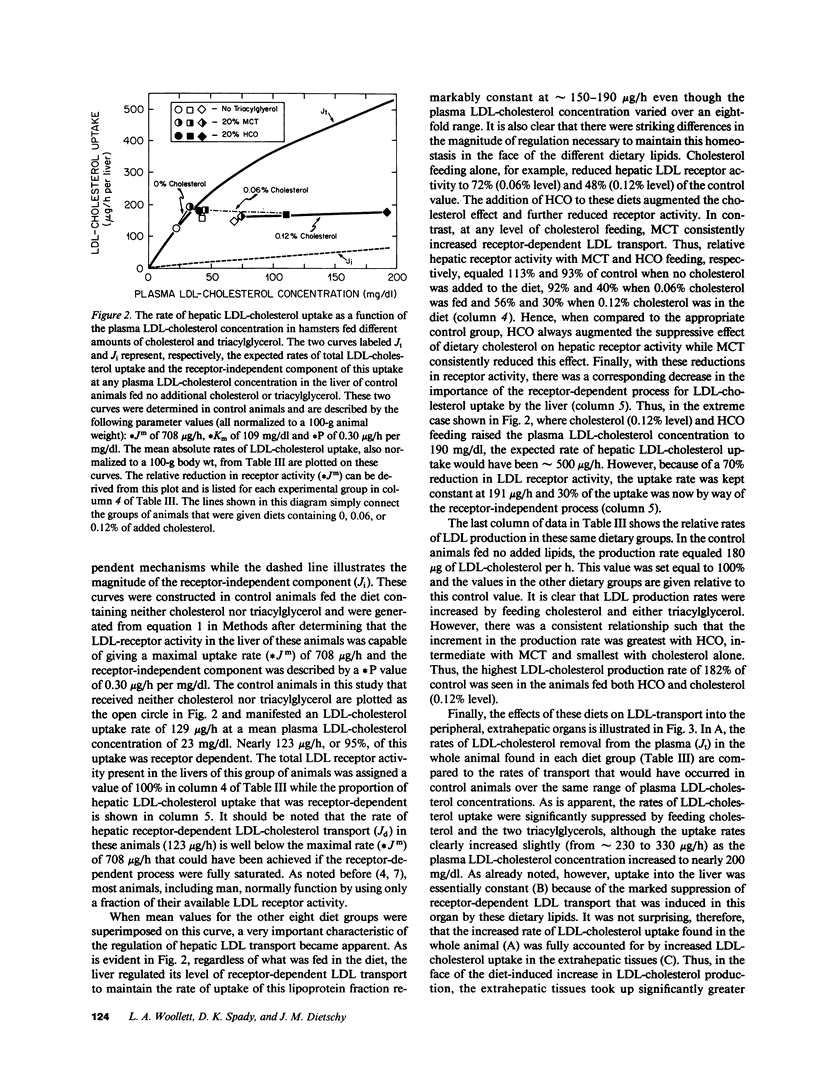
These studies were designed to elucidate how shorter (MCT) and longer (HCO) chain-length saturated triacylglycerols and cholesterol interact to alter steady-state plasma LDL-cholesterol levels. When either MCT or HCO was fed in the absence of cholesterol, there was little effect on receptor-dependent LDL transport but a 36-43% increase in LDL-cholesterol production. Cholesterol feeding in the absence of triacylglycerol led to significant suppression of receptor-dependent LDL transport and a 26-31% increase in LDL-cholesterol production. However, when the longer chain-length saturated triacylglycerol was fed together with cholesterol there was a marked increase in the suppression of receptor-dependent LDL transport and an 82% increase in production rate. Together, these two alterations accounted for the observed eightfold increase in plasma LDL-cholesterol concentration. In contrast, feeding the shorter chain-length saturated triacylglycerol with cholesterol actually enhanced receptor-dependent LDL transport while also causing a smaller increase (52%) in the LDL-cholesterol production rate. As a result of these two opposing events, MCT feeding had essentially no net effect on plasma LDL-cholesterol levels beyond that induced by cholesterol feeding alone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Absolute rates of cholesterol synthesis in extrahepatic tissues measured with 3H-labeled water and 14C-labeled substrates. J Lipid Res. 1979 Aug;20(6):740–752. [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Bersot T. P., Mahley R. W., Brown M. S., Goldstein J. L. Interaction of swine lipoproteins with the low density lipoprotein receptor in human fibroblasts. J Biol Chem. 1976 Apr 25;251(8):2395–2398. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A. Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med. 1977 Aug 18;297(7):371–377. doi: 10.1056/NEJM197708182970707. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Spady D. K. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984 Dec 15;25(13):1469–1476. [PubMed] [Google Scholar]
- Fukuda N., Azain M. J., Ontko J. A. Altered hepatic metabolism of free fatty acids underlying hypersecretion of very low density lipoproteins in the genetically obese Zucker rats. J Biol Chem. 1982 Dec 10;257(23):14066–14072. [PubMed] [Google Scholar]
- Glass C. K., Pittman R. C., Keller G. A., Steinberg D. Tissue sites of degradation of apoprotein A-I in the rat. J Biol Chem. 1983 Jun 10;258(11):7161–7167. [PubMed] [Google Scholar]
- Goldstein J. L., Sobhani M. K., Faust J. R., Brown M. S. Heterozygous familial hypercholesterolemia: failure of normal allele to compensate for mutant allele at a regulated genetic locus. Cell. 1976 Oct;9(2):195–203. doi: 10.1016/0092-8674(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Vega G. L., Bilheimer D. W. Kinetic mechanisms determining variability in low density lipoprotein levels and rise with age. Arteriosclerosis. 1985 Nov-Dec;5(6):623–630. doi: 10.1161/01.atv.5.6.623. [DOI] [PubMed] [Google Scholar]
- Havel R. J. Functional activities of hepatic lipoprotein receptors. Annu Rev Physiol. 1986;48:119–134. doi: 10.1146/annurev.ph.48.030186.001003. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Ide T., Ontko J. A. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem. 1981 Oct 25;256(20):10247–10255. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Basu S. K., Bilheimer D. W., Goldstein J. L. Saturation and suppression of hepatic lipoprotein receptors: a mechanism for the hypercholesterolemia of cholesterol-fed rabbits. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1396–1400. doi: 10.1073/pnas.78.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha R. S., Barnwell G. M., Carey K. D., McGill H. C., Jr Metabolism of apoprotein B in selectively bred baboons with low and high levels of low density lipoproteins. J Lipid Res. 1986 May;27(5):497–507. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Melchior G. W., Innerarity T. L., Holcombe K. S. Inhibition of receptor-mediated clearance of lysine and arginine-modified lipoproteins from the plasma of rats and monkeys. Proc Natl Acad Sci U S A. 1980 Jan;77(1):225–229. doi: 10.1073/pnas.77.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. E. Why does plasma low density lipoprotein concentration in adults increase with age? Lancet. 1984 Feb 4;1(8371):263–267. doi: 10.1016/s0140-6736(84)90135-1. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Attie A. D., Witztum J. L., Watanabe Y., Steinberg D. Receptor-dependent and receptor-independent degradation of low density lipoprotein in normal rabbits and in receptor-deficient mutant rabbits. J Biol Chem. 1982 Jul 25;257(14):7994–8000. [PubMed] [Google Scholar]
- Spady D. K., Bilheimer D. W., Dietschy J. M. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Interaction of aging and dietary fat in the regulation of low density lipoprotein transport in the hamster. J Lipid Res. 1989 Apr;30(4):559–569. [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Interaction of dietary cholesterol and triglycerides in the regulation of hepatic low density lipoprotein transport in the hamster. J Clin Invest. 1988 Feb;81(2):300–309. doi: 10.1172/JCI113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Huettinger M., Bilheimer D. W., Dietschy J. M. Role of receptor-independent low density lipoprotein transport in the maintenance of tissue cholesterol balance in the normal and WHHL rabbit. J Lipid Res. 1987 Jan;28(1):32–41. [PubMed] [Google Scholar]
- Spady D. K., Meddings J. B., Dietschy J. M. Kinetic constants for receptor-dependent and receptor-independent low density lipoprotein transport in the tissues of the rat and hamster. J Clin Invest. 1986 May;77(5):1474–1481. doi: 10.1172/JCI112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. J Lipid Res. 1985 Apr;26(4):465–472. [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Receptor-independent low density lipoprotein transport in the rat in vivo. Quantitation, characterization, and metabolic consequences. J Clin Invest. 1985 Sep;76(3):1113–1122. doi: 10.1172/JCI112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange E. F., Dietschy J. M. Age-related decreases in tissue sterol acquisition are mediated by changes in cholesterol synthesis and not low density lipoprotein uptake in the rat. J Lipid Res. 1984 Jul;25(7):703–713. [PubMed] [Google Scholar]
- Stange E. F., Dietschy J. M. Cholesterol synthesis and low density lipoprotein uptake are regulated independently in rat small intestinal epithelium. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5739–5743. doi: 10.1073/pnas.80.18.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S. D., Andersen J. M., Dietschy J. M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981 May;22(4):551–569. [PubMed] [Google Scholar]
- Vessey D. A., Zakim D. Membrane fluidity and the regulation of membrane-bound enzymes. Horiz Biochem Biophys. 1974;1:138–174. [PubMed] [Google Scholar]


