Abstract
Interleukin-8 (IL-8), a chemokine with a defining CXC amino acid motif, is known to possess tumorigenic and proangiogenic properties. Overexpression of IL-8 has been detected in many human tumors, including colorectal cancer, and is associated with poor prognosis. The goal of our study was to determine the role of IL-8 overexpression in colorectal cancer cells in vitro and in vivo. We stably transfected the IL-8 cDNA into two human colon cancer cell lines, HCT116 and Caco2, and selected IL-8-secreting transfectants. Real time RT-PCR confirmed that IL-8 mRNA was overexpressed in IL-8 transfectants with 45∼85-fold higher than parental cells. The IL-8-transfected clones secreted 19∼28-fold more IL-8 protein than control and parental cells as detected by ELISA. The IL-8 transfectants demonstrated increased cellular proliferation, cell migration and invasion based on functional assays. Growth inhibition studies showed that IL-8 overexpression lead to a significant resistance to oxaliplatin (P < 0.0001). Inhibition of IL-8 overexpression with small interfering RNA reversed the observed increases in tumorigenic functions and oxaliplatin resistance suggesting that IL-8 not only provides a proliferative advantage, but also promotes the metastatic potential of colon cancer cells. Using a tumor xenograft model, IL-8-expressing cells formed significantly larger tumors than the control cells with increased microvessel density. Together, these findings indicate that overexpression of IL-8 promotes tumor growth, metastasis, chemoresistance and angiogenesis, implying IL-8 to be an important therapeutic target in colorectal cancers.
Keywords: Interleukin-8, colon cancer, proliferation, angiogenesis and chemoresistance
Introduction
Colorectal cancer (CRC) is the leading cause of death from gastrointestinal malignancy and second common cause of cancer-related death in the United States, resulting in approximately 49,920 deaths in 20091. A number of pathways and genes have been implicated in the metastasis and angiogenesis of colorectal cancer, however, the precise mechanisms determining the directional migration and invasion of tumor cells into specific organs remain to be established. Recent studies showed that chemokines and their receptors act as key regulators of metastatic cancer, including colorectal cancer, and are involved in many neoplastic processes such as autonomous growth signaling, emergence of a vascular supply, and the acquisition of invasive/metastatic properties 2-4.
Interleukin 8 (IL-8), a member of the neutrophil-specific CXC subfamily of chemokines with ELR (Glu-Leu-Arg) motif, can act not only on leukocyte chemotaxis and inflammatory responses and infectious diseases 5, but also on endothelial cells via their receptors to promote migration, invasion, and proliferation, and in vivo angiogenesis 6, 7. The biological action of IL-8 is mediated through binding to its receptors, CXCR1 (IL-8RA) and CXCR2 (IL-8RB), which are members of the seven transmembrane G-protein-coupled receptor (GPCR) family 8, 9.
It has been suggested that tumor cells produce IL-8 as an autocrine growth factor, which promote tumor growth, tissue invasion and metastatic spread 7. Previous studies have revealed that highly metastatic solid tumors such as prostate, breast, melanoma and ovarian cancer constitutively express IL-8 7, 10, 11. In the colonic mucosa, upregulation of IL-8 occurs in proportion to the degree of inflammation in Crohn's disease and ulcerative colitis 12, 13. Moreover, IL-8 expression correlates with tumor cell growth and vascularity in gastric carcinoma 13. In human colon cancer cell lines, constitutive expression of IL-8 has been linked to metastatic potential 14, and has been suggested to play a role in the development of distant metastases. Our group has recently demonstrated that gene polymorphism of IL-8 and CXCR2 are associated with clinical outcome in patients with metastatic CRC treated with oxaliplatin-based chemotherapy 15, 16. However, the exact role of IL-8 in the progressive growth of colon cancer remains unclear.
The purpose of this study was to provide evidence for the role of IL-8 in determining the proliferative rate, metastasis, angiogenesis and chemosensitivity of human colon cancer. We hypothesized that constitutive expression of IL-8 increases proliferation, migration and angiogenesis and is correlated with oxaliplatin resistance in vitro and in vivo. This study may provide the basis for the development of new therapies for colon cancer to increase chemosensitivity and decrease proliferation, migration and angiogenesis.
Materials And Methods
Patients
Fifty colorectal cancer patients were enrolled into a serum collection protocol OS-99-10 at the University of Southern California / Norris Comprehensive Cancer Center (USC/NCCC) or the Los Angeles County / University of Southern California Medical Center (LAC/USCMC), between 2005 and 2008. Thirty-five patients had stage IV metastatic colorectal cancer, and fifteen patients with history of stage II and III colon cancer at time of serum collection had no evidence of disease (NED). This study was approved by the Institutional Review Board of the University of Southern California for Medical Sciences. All patients involved in the study signed informed consent.
Cell culture and Stable Transfection
HCT116 and Caco2 cell lines were grown in McCoy's 5A and DMEM media supplemented with 10% fetal bovine serum, 5% penicillin/streptomycin, sodium pyruvate and L-Glutamine. The IL-8 overexpression construct3 (pEF6/V5-His TOPO, Invitrogen, Carlsbad, CA) was a generous gift of Dr. Bal L. Lokeshwar, University of Miami Miller School of Medicine, Miami, Florida. The empty plasmid vector (pEF6/V5-His TOPO) or the plasmid vector containing IL-8 cDNA was transfected into HCT116 or Caco2 cells using ExpressFect™ transfection reagent (Denville Scientific Inc. South Plainfield, NJ). Multiple clones were selected in presence of 5 μg/ml blasticidin (Invitrogen). IL-8 transfected clones were screened for IL-8 expression. Clone E2 and IIIe were selected for further analysis and designated HCT116-E2 and Caco2-IIIe, with HCT116-vector or Caco2-vector as the vector only cell line.
ELISA
Parental cells and IL-8-transfected cells (1×106 cells/ well) were plated in 6-well plate and incubated at 37°C for 72 h. Equal volume of cell culture supernatants were collected. The quantification of IL-8 protein was determined using the Quantikine IL-8 ELISA kit (R&D systems, Minneapolis, MN). The concentration of IL-8 in culture supernatants was determined by comparing their optical density to the standard curve.
In Vitro Cell Proliferation Assay
Cells (5×103) were seeded on 24-well plates and cultured in media in the absence or presence of FBS. HCT116 and Caco2 cells also were treated with recombinant human IL-8 (2.5, 5, 10, 20, 40 ng/ml, R&D systems) for 72 h. Medium was changed every 48 h. Cells were fixed with 60% cold methanol (-20°C) after 1d, 2d, 4d, 6d, 7d and 8d. 0.2% Crystal violet solution was added for 20 min and cells were subsequently washed with PBS and allowed to dry overnight. Crystal violet was absorbed by adding 10% SDS to each well. Absorbance was measured on Spectra Max 190 micro-plate reader (Molecular Devices, Sunnyvale, CA) at A570 nm. Samples were analyzed in triplicate and mean absorbance presented. Error bars represent ± standard deviation.
Cell Migration Assay and Invasion Assay
Cell migration assays were performed on modified Boyden chambers as previously described 17. Cells (1.25 × 105) were allowed to migrate for 48 h through 8-micron pore cell culture inserts (Becton Dickinson Labware, Franklin Lakes, NJ). Non-migratory cells were removed by Q-tip. Migratory cells were stained with 0.2% crystal violet in 10% ethanol. Invasion was measured using 8-micron pore BD BioCoat Matrigel Invasion Chambers (BD Biosciences) according to the manufacturer's instructions. Cells (2.5 × 105) were added to chambers and incubated for 48 h at 37°C. Matrigel and noninvasive cells were removed and chambers were stained as described above. To quantitate invasive cells, three independent fields of migratory or invasive cells per well were photographed under phase contrast microscopy. The number of cells per field were counted and averaged. Each migration or invasion assay was done on at least 3 independent occasions.
Growth Inhibition Assay
Growth inhibition was measured as previously described 18. Cells were trypsinized and seeded at 3 × 103 cells/well in 96-well tissue culture plates. After 24 h, cells were exposed to increasing concentrations of oxaliplatin (Sigma). After 72 h incubation, 10 μl of CellTiter 96 AQueous One Solution (Promega, Madison, WI) was added to each well, and then incubated at 37°C for 4 h. Triplicate plates were analyzed on a Spectra Max 190 micro-plate reader at A490, and the IC50 values for each cell line were calculated from sigmoidal-dose-response curve fits of the data using the Prism statistical software (GraphPad Software, San Diego, CA). Results are representative of three independent experiments.
Clonogenicity Assay
Clonogenicity assay were performed as previously described18. Cells were seeded at 500 cells/well in 6-well plates and allowed to adhere for 24 h. Cells were subsequently treated with oxaliplatin (0.5, 1, 2, 2.5 and 5 μM) for a period of 72 h after which, media was aspirated, cells were washed and incubated in drug-free media for approximately 3 weeks to allow colony formation. Colonies were then stained and solubilized by crystal violet and 10% SDS. Plates were analyzed on Spectra Max 190 micro-plate reader A570. All experiments were performed in triplicate.
mRNA Detection
Total RNA was extracted with Qiagen Kit (Invitrogen), and cDNA was generated from 2 μg of sample RNA using High Capacity cDNA Archive kit according to the instructions of the manufacturer (Applied Biosciences, Foster City, CA). Q-PCR was conducted using the ABI 7500 (Applied Biosystems) and Plexor™ multiplex (Promega), Probe sets (Biosearch Technologies, Novato, CA) used for qPCR analysis were as follows: IL-8 reverse, FAM™-iso-dC 5′-CACTCTCAATCACTCTCAGTTCTTTGAT-3′; 5′- CTTCCTGATTTC TGCAGCTCT GT -3′. CXCR2 reverse, 5′- Cal Fluor® Orange 560-iso-dC-ACTGTAATTACTAAGATCTTCACCTTTCCAGA-3′; 5′-GGAGGTGTCCTACAGGTGAAAAG-3′. CXCR1 reverse, 5′- Cal Fluor® Orange 560-iso-dC-GCA AGG AGT TCT TGG CAC GTC ATT -3′; β-actin is a pre-validated housekeeping gene purchased with the Plexor™ multiplex system and was detected as Cal Fluor® Red 610. Standard curves with >0.95R2 and PCR-efficiency at 100 ± 2% were confirmed for each primer set within the multiplex. Threshold cycle values (CT) were determined from three independently isolated RNA samples and performed in triplicate. IL-8, CXCR1 and CXCR2 mRNA expression levels were determined by normalizing against β-actin expression using the 2−ΔΔCT method 19. Statistical significance was determined using a two-tailed unpaired Student's t-test.
siRNA Analysis
siRNA against IL-8 was purchased from Ambion (Austin, TX). Two different siRNA oligonucleotides (siRNA#2261 and siRNA#2247) were tested (Supplement figure 2) and siRNA#2261 was used in the described knockdown experiments. Ambion silencer β-actin siRNA controls (which includes β-actin siRNA and a negative control siRNA) were used as siRNA controls. At 50% confluency, HCT116 and HCT116-E2 cells were mock transfected on 10 cm plates or transfected with 50 nmol/L of IL-8 siRNA by using Lipofectamine™ RNAiMAX (Invitrogen). siRNA-treated cells were compared (normalized) to negative control siRNA (control siRNA) for Q-PCR. For migration and invasion assays, 24 h after transfection, 2×105 cells were placed in Boyden chambers or Matrigel as described.
Luciferase Reporter Assay
PGL4.32 [luc2P/NF-κB-RE/Hygro] vector (Promega) contains five copies of an NF-κB response element (NF-κB-RE) that drives transcription of the luciferase reporter gene luc2P. Cells were cotransfected with pGL-luc2P/NF-κB-RE and pGL-hRluc (Renilla reniformis) using ExpressFect™ transfection reagent (Denville Scientific Inc.). The reporter activity was assayed 48 h after transfection using the Dual-Glo assay kit (Promega). Relative luciferase light units were normalized to Renilla luminescence.
Immunoblot Analysis
Total cellular proteins were separated by SDS-PAGE and transferred to PVDF membranes, probed with rabbit monoclonal antibodies from cell signaling technology (Beverly, MA), such as anti-phospho-NF- κB (#3033), anti-phospho-p44/42 MAP kinase (#9101), anti-phospho-Akt (#4060), anti-NF-κB (#4764S), anti-p44/42 MAP kinase (#4695), and anti-Akt (#4691), or rabbit anti-β-tubulin (#sc-9104, Santa Cruz Biotechnology), and incubated with HRP-conjugated anti-rabbit IgG secondary antibody.
In Vivo Studies
BALB/c nu/nu mice (Charles Rivers Laboratories, Wilmington, MA) aged 6-8 weeks were housed in laminar flow cabinets under specific pathogen-free conditions in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care in accordance with the current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Service and NIH. Subcutaneous tumors were generated by subcutaneous injection of 5×106 (HCT116 and HCT116-E2) and 3.5×106 (Caco2 and Caco2-IIIe) tumor cells in the right and left flank of mice (4 mice per group, 2 subcutaneous tumors per mouse). The subcutaneous tumors were measured using digital caliper and tumor volumes were calculated according to the equation: tumor volume = (short diameter) 2 × (long diameter) × 0.5. At necropsy, blood was collected to measure IL-8 levels.
Immunohistochemistry and Quantitation of Microvascular Density
Formalin-fixed and snap-frozen fragments of tumor specimens were sectioned to 4 μm thicknesses. Primary antibodies were added in the following dilutions: Rat anti-mouse CD31 (1:100. BD Pharmingen, San Diego, CA), Rabbit anti-human IL-8 (1:100, Biosource, Camarillo, CA). The sections were incubated overnight at 4°C, after which the appropriate biotinylated anti-rat secondary antibody (Vector Laboratories, Burlingame, CA) was added. Following secondary antibody incubation, the VectaStain Elite ABC Reagent (Vector Laboratories) was applied, and the subsequent antibody/enzyme conjugate was developed with DAB (Vector Laboratories). All sections were counterstained with hematoxylin. Sections from peripheral and central regions of a tumor were imaged at 10× magnification, and areas of viable tumor tissue and necrosis were evaluated using the Zeiss Axiovision software program. Blood vessel density was quantified by counting the total number of CD31-positive vessels across the whole section of tumors. For these analyses, six different tumor samples were evaluated per experimental condition.
Statistical Analysis
For all analyses, the difference between each two-cell line compared and/or treatment groups were evaluated using a two-tailed t-test from GraphPad software. Any P-value below 0.05 was considered statistically significant.
Results
IL-8 stable transfection in colon cancer cells
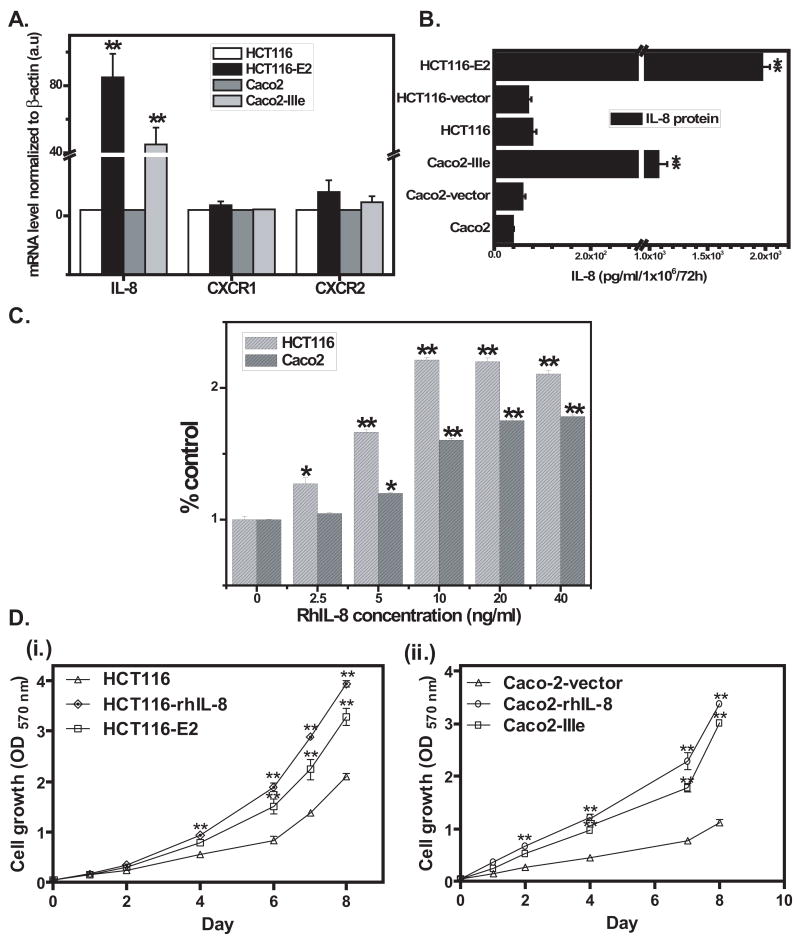
We quantified the mRNA expression of IL-8, CXCR1 and CXCR2 in a panel of colon cancer cell lines. HCT116 and Caco2 cell lines have lower expression of IL-8 in comparison with other colon cancer cell lines (LoVo, SW620, HT29 and HCT8), which have more metastatic characterization. (Supplement figure 1). However, all cell lines express mRNAs for CXCR1 and CXCR2 at comparable levels (Data not shown). HCT116 or Caco2 cells were stably transfected with full-length IL-8 expression construct (pEF6/V5-His TOPO) or the vector-only construct. Eight HCT116 and six Caco2 IL-8 secreting stable clones were initially selected for evaluation. Q-PCR revealed that IL-8 mRNA expression in the stable IL-8 transfected clone E2 (HCT116-E2) was 85-fold (p<0.005) more than HCT116 cells, and in the stable IL-8 transfected clone IIIe (Caco2-IIIe) was 45-fold (p<0.005) more than Caco2 cells. CXCR1 mRNA level were the same in both parental and IL-8-transfected cell lines (Figure 1A). CXCR2 mRNA level was 4-fold (p=0.0383) higher in HCT116-E2 cells than HCT116 cells, and was 2.3-fold (p=0.0503) higher in Caco2-IIIe cells than Caco2 cells (Figure 1A). Analysis of IL-8 secretion in the cell culture media using ELISA confirmed that HCT116-E2 cells secrete 1971 pg/ml, 28-fold more IL-8 than empty vector (HCT-116-vector) (70 pg/ml) and 25-fold more IL-8 than HCT116 parental (75 pg/ml) cells (Figure.1B), and Caco2-IIIe cells secrete 1074 pg/ml, 19-fold more IL-8 than empty vector (Caco2-vector) (58.5 pg/ml) and 27-fold more IL-8 than Caco2 parental (38.5 pg/ml) cells (Figure.1B).
Figure 1. Detection of IL-8 in stably transfected colon cancer cells and growth rate of IL-8-expressing clones and parental cells.
A, Real time RT-PCR determined the level of IL-8, CXCR1 and CXCR2 mRNA in parental colon cancer cell lines (HCT116 and Caco2) and IL-8 transfected cell lines (HCT116-E2 and Caco2-IIIe). Normalized transcript levels were calculated as 2−ΔΔCT ×100. Data shown are expressed in arbitrary units (a.u.). B, ELISA measured IL-8 production from IL-8-transfected, vector-transfected and parental colon cancer cells. The results were normalized to cell numbers. C, In Vitro proliferation assay. HCT116 and Caco2 cells (5×103 cells/well) were treated with rhIL-8 (2.5, 5, 10, 20, 40 ng/ml) for 72 h, then fixed with 60% cold methanol, cells were stained with crystal violet, then solubilized by adding 10% SDS, and read absorbance at A570nm on plate reader. D, (i.) HCT116 cells with or w/o treatment rhIL-8 (10 ng/ml) and HCT116-E2 cells and (ii.) Caco2-vector cells with or w/o treatment rhIL-8 (10 ng/ml), Caco2-IIIe in a 12-well culture plate (5×103 cells/well) were incubated with media for 1, 2, 4, 6, 7 and 8 d. Then fixed, stained and solubilized as mentioned as above. In A, B, C and D, the results are representative of a minimum of three independent experiments. Data shown represent the mean ±standard deviation (*, P< 0.05, **, P<0.005.).
Since IL-8 has been reported to be an autocrine growth factor 6, we examined the role of IL-8 in the progressive growth of human colon cancer cells. First, treatment of HCT116 and Caco2 cells with rhIL-8 for 72 h resulted in a dose-dependent increase in the growth of the cells (Figure.1C). In both cell lines, the maximal induction of proliferation was observed following the treatment with 10 ng/ml rhIL-8 (1.2 fold and 60% increase) (Figure.1C). Based on this observation, 10 ng/ml of rhIL-8 as a concentration was used in all next experiments. To compare the in vitro growth of parental or vector (HCT116 and Caco2-vector) and IL-8-transfected (HCT116-E2 and Caco2-IIIe) cells, 5×103 cells were seeded on 24-well plates and incubated at 37°C, then fixed after 1, 2, 4, 6, 7 and 8 d. IL-8-transfected cells showed higher cellular proliferation as compared to parental or vector control cells [Figure 1D, (i) and (ii)]. Similarly, the addition of rhIL-8 (10 ng/ml) to the medium also led to an increase of proliferation of HCT116 and Caco2 cells (Figure 1D).
IL-8 is involved in cell migration and invasion in colon cancer cells
To determine the role of constitutive IL-8 expression in HCT116-E2 cells, the metastatic potential of cells was investigated by using Boyden chamber migration assay and in vitro Matrigel invasion assay with HCT116 and HCT116-E2 cells. Expression of IL-8 increased migration through cell culture inserts by 2.1-fold over parental control cells in the HCT116-E2 clones (P<0.005, Figure 2A). Invasive potential was measured using cell culture inserts coated with artificial basement membrane (Matrigel). HCT116-E2 cells exhibited a 1.95-fold increase in invasion above HCT116 parental cells (P<0.005, Figure 2B). Therefore, IL-8 overexpression is sufficient to increase the rate of colon cancer cell migration and in vitro invasion.
Figure 2. Overexpressed IL-8 is involved in cell migration and invasion in colon cancer cells.
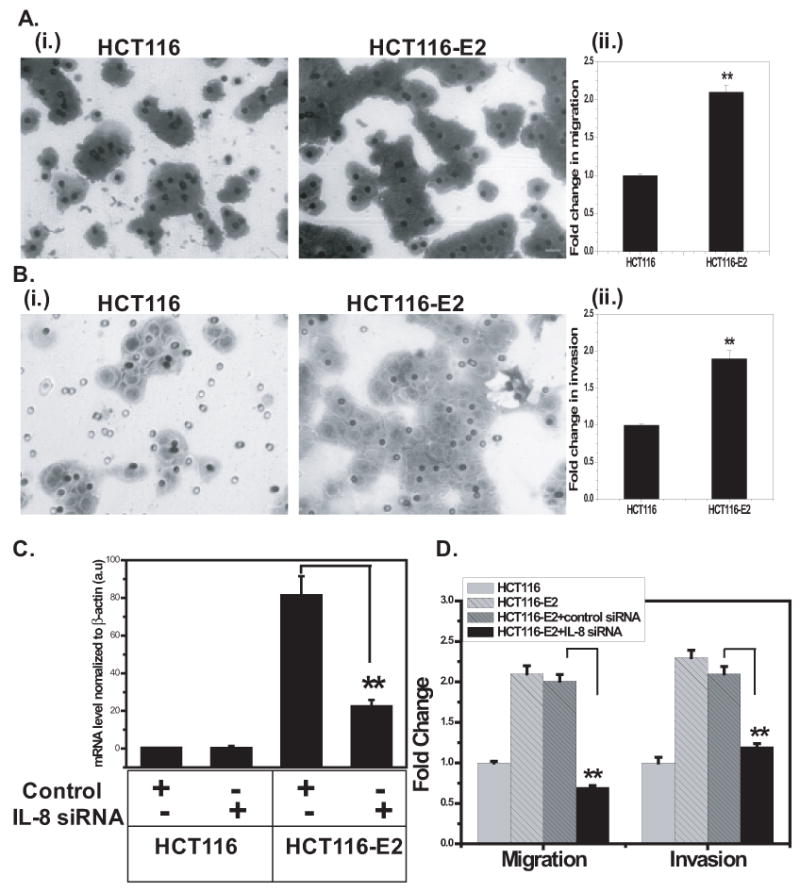
A, Migration assay. (i.) HCT116 and HCT116-E2 cells (2×105 cells/well) in a 24-well Boyden chambers with feeder tray were incubated with media for 48 h. Cell migration was determined by staining with crystal violet as mentioned above. Cells were photographed. Dark staining were migratory cells (original magnification, ×200). (ii.) Migration assay was quantitated. Columns shows fold change over the number of HCT116 control cells that migrated (**, P<0.005). B, Invasion assay. (i.) HCT116 and HCT116-E2 cells (2×105 cells/well) were plated on porous cell culture inserts which coated with Matrigel and allowed to invade for 48 h. Dark staining were invasive cells (original magnification, ×200). (ii.) Invasion assay was quantitated. Columns shows fold change over the number of HCT116 control cells that invaded (**, P<0.005). C, Q-PCR analysis of IL-8 expression knockdown in HCT116 and HCT116-E2 cells. All siRNA experiments were done with the following cells: untransfected cells, mock-transfected cells (control siRNA), siRNA-transfected cells (siRNA #2261). D, Quantitation of migration and invasion after siRNA transfection. HCT116, HCT116-E2, control-transfected HCT116-E2, and siRNA transfected HCT116-E2 cells were incubated on Boyden chambers or Matrigel for 48 h as described in Materials and Methods. Columns shows fold change over the number of HCT116 control cells that migrated or invaded. The results are representative of a minimum of three independent experiments. Data shown represent the mean ± standard deviation (**, P<0.005).
To further validate IL-8 as an important role in colon cancer cell migration and invasion, we used IL-8 siRNA to suppress IL-8 expression by RNAi in HCT116-E2 cells. Q-PCR analyses revealed that transfection of IL-8 siRNA reduced the expression of IL-8 about 72% in HCT116-E2 cells compared to HCT116 control cells (P<0.005, Figure 2C). HCT116-E2 cells transfected with control siRNA had no evidence of IL-8 knockdown (Figure 2C). Having confirmed IL-8 knockdown, we examined if the reversal of overexpressed IL-8 in HCT116-E2 cells can reduce the increased cell migration and invasion previously observed. Boyden chamber migration assay and Matrigel invasion assay were done on cells transfected with negative control siRNA or IL-8 siRNA. The cell migration was decreased 65% (P<0.005, Figure 2D) and cell invasion was decreased 45% (P<0.005, Figure 2D) in HCT116-E2 with IL-8 siRNA cells compared with HCT116-E2 with control siRNA cells. These data demonstrated that siRNA-mediated knockdown of IL-8 abrogates the increased cell migration and invasion observed in the HCT116-E2 cells. These findings indicate that IL-8 may play an important role in colon cancer cell migration and invasion. Similar results were also observed in Caco2-IIIe cells (Supplement figure 3).
IL-8 increases NF-κB activity in colon cancer cells
Recent studies have reported that induction of IL-8 signaling increased NF-κB transcriptional activity 20, 21. Based on this, we sought to evaluate the potential role of NF-κB signaling and activity in the presence of IL-8 overexpression. Further experiments were focused on whether NF-κB was a downstream effector of IL-8 signaling in these colon cancer cells. We used a luciferase reporter assay to measure the transcriptional activity of NF-κB in HCT116-vector, HCT116-E2, Caco2-Vector and Caco2-IIIe cell lines. After 48 h transfection with a NF-κB-reporter construct, pluc/NF-κB luciferase activities in HCT116-E2 and Caco2-IIIe cells were 3.4-fold and 2.3-fold higher when compared with HCT116-vector and Caco2-vector (Figure 3A). Next, we investigated NF-κB activation in IL-8 overexpressed cells compared with control cells by Western blot. As shown in Figure 3B, phospho-NF-κB-p65 level was increased in HCT116-E2 and Caco2-IIIe when compared with HCT116-vector and Caco2-vector cells. It has been reported that exogenous IL-8 activates the phosphoinositide-3-kinase (PI3K) and mitogen-activated protein (MAP) kinase (MAPK) cascade 22, 23. Therefore, we tested that whether autocrine expression of IL-8 can stimulate AKT and MAPK activity. In Figure 3C, the levels of phospho-Akt and phospho-p42/44 MAP kinase were up-regulated in HCT116-E2 and Caco2-IIIe cells when compared with parental vectors. These data suggest that constitutive IL-8 expression is strongly increased NF-κB activation, which may be related with activation of Akt and MAPK signaling.
Figure 3. Overexpressed IL-8 increases NF-κB, Akt and MAPK activities in colon cancer cells.

A, HCT116, HCT116-E2 and Caco2 transfectants were transiently transfected with pGL4.32 [luc2P/NF-κB-RE/Hygro] vector and cultured in media for 48 h. Promoter activity was measured by luciferase assay and normalized against Renilla activity (*, P<0.05). B and C, Cell lysates of each cell line were polyacrylamide gel electrophoresis and immunoblotted with anti-phospho-NF-κB-p65, Akt (60 kDa), and p44/42 MAP kinase or anti-total NF-κB-p65, Akt and p44/42 MAP kinase antibodies. In B and C, β-tubulin as a loading control.
IL-8 increases resistance to oxaliplatin in colon cancer cells
Having confirmed that IL-8 overexpression in our colon cancer cells increased NF-κB activity, previous reports indicated that increased NF-κB is associated with chemoresistance, such as Oxaliplatin 21, 24. Oxaliplatin is a platinum-based chemotherapy drug and is typically administered in combination with 5-FU in a chemotherapeutic regimen commonly referred to as FOLFOX for the treatment of colorectal cancer 25. To investigate whether IL-8 is associated with chemosensitivity, the effects of IL-8 expression on oxaliplatin sensitivity in IL-8 transfected and parental cells were tested using growth inhibition analyses. The growth profiles of HCT116, HCT116-E2, HCT116-rhIL-8 and HCT116-E2 treated with siRNA were analyzed after treatment with a range of increasing concentrations of oxaliplatin for 72 h by MTS growth inhibition assay. As shown in figure 4A with oxaliplatin treatment, in HCT116-E2 cells, the oxaliplatin IC50 (72h) concentrations increased significantly 1.8-fold compared with the value obtained for the HCT116 parental cells. Interestingly, a ∼2-fold increase in IC50 (72h) was noted in HCT116 cells after treatment with recombinant human IL-8 (10 ng/ml), which is similar to the HCT116-E2 cells. In contrast, when IL-8 overexpression was suppressed in HCT116-E2 cells using siRNA, there was no significant difference in oxaliplatin sensitivity compared with HCT116 in the IC50 (72h) concentrations. These results suggest that IL-8 overexpression in HCT116 cells decreased sensitivity to the cytotoxic effects of oxaliplatin.
Figure 4. Growth inhibition assay and clonogenicity analysis of IL-8-transfected cells treated with oxaliplatin.

A, Growth inhibition assay. HCT116, HCT116-E2, HCT116 treated with rhIL-8 and HCT116-E2 transfected with IL-8 siRNA cells were measured for growth inhibition using CellTiter 96 AQueous One solution. After treatment for 72 h with oxaliplatin, IC50 were calculated using GraphPad Prism software. Data are presented as the mean ± standard deviation of three experiments carried out in triplicate. B, Clonogenicity assay. All cells mentioned above were treated with oxaliplatin at different concentration 0.5, 1, 1.5, 2.5, and 5 μM for 72 h. Colonies were fixed with 60% methanol after 3 weeks incubation with drug-free medium, then colonies were staining with crystal violet, (i.), Colonies were quantitated, (ii.), Wells were scanned. Results are representative of three independent experiments. Data shown represent the mean ± standard deviation.
To determine whether alterations in sensitivity to oxaliplatin observed by growth inhibition assay translate to changes in the ability of cells to recover from drug treatment, clonogenicity analyses were performed. In HCT116 and HCT116-E2 cells, there was a correlation between IL-8 overexpression and increased number of colonies formed in the presence of 0.5, 1, 2, 2.5 and 5 μM oxaliplatin (Figure 4B, i). In HCT116-E2 cells treated with different dose of oxaliplatin for 72 h, there was 1.8 and 6.4-fold increase at 1 μM compared with HCT116 parental cells and HCT116-E2-IL-8siRNA cells and 2.2 and 8.2-fold increase in colony formation at 2 μM (Figure 4B, ii). These results indicate overexpression of IL-8 may contribute to chemoresistance to oxaliplatin. In addition, HCT116 cells incubated with rhIL-8 (10 ng/ml) displayed a similar result to HCT116-E2 in their clonogenic cell survival.
Enhanced angiogenic activity in IL-8-transfected xenografts
To test whether IL-8 transfected cells display enhanced tumor growth and angiogenic activity, HCT116, HCT116-E2, Caco2 and Caco2-IIIe cells were injected subcutaneously into nude mice to establish colon cancer xenografts. Tumor latency (time to develop palpable tumor), and growth rate were monitored for 19 days (HCT116) and 25 days (Caco2). Figure 5A showed that IL-8-transfected tumors (Caco2-IIIe and HCT116-E2) grew significant faster (36% and 63%) than parental cells. Immunohistochemical analysis confirmed the increased expression of IL-8 at the cellular level in HCT116-E2 cells when compared to HCT116 parental cells. IL-8 immunoreactivity within tumors was primarily limited to HCT116-E2 cells (Figure 5B, right). In HCT116 cells, IL-8 staining demonstrated minimal staining intensity (Figure 5B, left). Measurement of circulating IL-8 levels in serum which were collected at the time of necropsy showed a significant increase in serum IL-8 (235±68 pg/ml) in HCT116-E2 tumor-bearing mice compared with HCT116 tumor-bearing mice (70±16 pg/ml) (Figure 5C).
Figure 5. Examination of IL-8 level and detection of microvessel density in xenograft of colon cancer cells.

A, Examination of tumor growth in xenograft of colon cancer cells. HCT116, HCT116-E2, Caco2 and Caco2-IIIe subcutaneously injected into nude mice. The graphs indicate the mean tumor growth rates ±SD of three animals per experimental condition. P < 0.05 was obtained by ANOVA. B, Sera IL-8 productions were measured by ELISA, and the results were normalized to whole blood volume. C and D (bottom panel), Immunohistochemistry analysis of IL-8 expression and localization of microvessel in tumor tissue slides. In HCT116 and HCT116-E2 tumor specimens, rabbit anti-human IL-8 or rat anti-mouse CD31 antibodies were added to tissue sections. All sections imaged by Zeiss Axiovision software program. The images are presented at 200× magnification. D (top panel), Blood vessel density was quantified by counting the total number of CD31-positive vessels across the whole section of tumors. For these analyses, six different tumor samples were evaluated per experimental condition. Data shown represent the mean ± standard deviation (*, P<0.05; **, P<0.005).
To establish whether the increased tumorigenicity and growth of tumors was associated with increased angiogenesis, we performed IHC against CD31 (mouse endothelial cell-specific) and evaluated microvessel density (MVD) in the tumor specimens. As shown in figure 5D, there was a significant increase in MVD in HCT116-E2 tumors, when compared with HCT116 tumors. MVD in HCT116 tumors was 2.4-fold higher than in HCT116 tumors. These results demonstrate that IL-8 overexpression resulted in an increase in the development of the angiogenic response.
IL-8 expression in patient with metastatic disease
In order to test whether IL-8 overexpression in vitro and in vivo have clinical relevance, we measured IL-8 serum concentrations in patients with metastatic CRC and patients (patients of stage II and III with chemotherapy after surgical resection of the tumor)with no evidence (NED) of CRC disease. Serum samples from 15 patients with NED and 35 patients with stage IV metastatic disease were analyzed. ELISA revealed significant differences in IL-8 protein level between no evidence of disease and stage IV patients; stage IV patients express significant higher levels of IL-8 (800-1300 pg/ml) than NED patients (80-140 pg/ml) (Table 1, P<0.05). The IL-8 expression levels measured in serum from patients with metastatic disease were similar to concentration measured in our generated stable IL-8 overexpressing cell line HCT116-E2. This result suggests that our observations in vitro and in vivo may have clinical relevance based on our analysis of patient IL-8 serum levels.
Table 1. Relationship between IL-8 expression and stages of human colorectal cancer.
ELISA quantitated IL-8 protein level in patients' serum. The significance in expression level was determined by the GraphPad Prism software. Data shown represent the mean ± standard deviation. P <0.05 was considered significant. Stage of colorectal cancer was classified according to TNM staging system. Stage IV, presence of metastasis.
| Stage | No. of patients | IL-8 expression (pg/ml, Serum) |
|---|---|---|
| NED § | 15 | 79 ± 56 |
| IV | 35 | 1089 ±311 |
P-value: 0.04
No evidence of disease: patients of stage II and III with chemotherapy after surgical resection of the tumor
Discussion
IL-8 is produced by a wide panel of human cancer cells including melanoma, prostate, ovary, breast or colon 10, 26-28. Our goal was to evaluate whether IL-8 is involved in proliferation, metastasis, angiogenesis and sensitivity to chemotherapeutics in human colon cancer cell line models. The key findings of this study are that IL-8 overexpression in colon cancer cells was associated with increased metastatic and angiogenic potential, and resistance to oxaliplatin, suggesting that IL-8 is a promising therapeutic target.
Overexpression of IL-8 has been shown to be associated with a poor outcome in patients with colorectal cancer. Recently, our group demonstrated that high-expression variants of IL-8 T-251A polymorphism were found to be significantly associated with risk of recurrence in stage III CRC patients, gastric and ovarian cancer patients 15, 29, 30. Our clinical results prompted us to investigate the role of IL-8 in the growth, metastasis and angiogenesis of two human colon cancer cells in vitro and in vivo. To our knowledge, this is the first study that demonstrates that overexpression of IL-8 is associated with progression, angiogenesis and chemoresistance in colon cancer from cell lines and animal models.
In our study, stable IL-8-transfectants were successfully generated for the first time in colon cancer cell lines. Either the exogenous addition of recombinant IL-8, or constitutive expression of IL-8, significantly stimulated the growth of HCT116 and Caco2 cells. Furthermore, constitutive overexpression of IL-8 in HCT116 cells increased cell migration and invasion, which was reversed by silencing IL-8 overexpression in HCT116-E2 cells using siRNA. Our findings suggest that expression of IL-8 plays an important role in modulating cell proliferation, migration, invasion in colon cancer cells.
The previous study has been reported that the receptors of IL-8 (CXCR1 and CXCR2) are expressed in colon cancer cells with different metastatic potentials 14. However, in our study, overexpression of IL-8 seems to play a dominant effect rather than the IL-8 receptors (CXCR1 and CXCR2) in modulating cellular functions. This observation is supported by other studies, which have shown that CXCR1 and CXCR2 are expressed in most cancer cells with no correlation with the stage of the tumor 3, 31.
Several mechanism are involved in NF-κB activation to induce IL-8 transcription, such as Ras 32, MAPK signaling pathway 33. However, it is not clear how constitutive IL-8 expression caused increased activation of NF-κB. One of our key observations was increased NF-κB activity in the presence of IL-8 overexpression. Moreover, overexpressed IL-8 caused phosphorylation of Akt and MAPK. Therefore, it is interesting to speculate that overexpressed IL-8 causes activation of Akt and MAPK signaling resulting in increased activation of NF-κB.
Elevated serum IL-8 levels in patients with prostate, ovarian, melanoma, esophageal, or breast cancers correlate with a poor initial response to chemotherapy, such as oxaliplatin, paclitaxel or 5-FU 21, 34-37. To determine whether constitutive IL-8 overexpression may alter the sensitivity of colon cancer cells to oxaliplatin, the effect of stimulating HCT116 cells with 10 ng/ml rhIL-8, and then measure the growth inhibitory effects of oxaliplatin was characterized. We found that rhIL-8 increased cell growth and resistance to oxaliplatin in HCT116 cells. This observation was consistent with the effect of IL-8 overexpression as observed in our HCT116-E2 cells. Moreover, we determined that siRNA-mediated suppression of IL-8 reversed the resistance to oxaliplatin associated with IL-8 overexpression in HCT116-E2 cells. IL-8 transcription has been shown to be regulated by NF-κB 4, 20, 32, and the sensitivity of colorectal cancer cells to oxaliplatin-induced death is also associated with NF-κB activity 21, 38. Therefore, these data suggested that IL-8 may increase resistance to oxaliplatin, at least, partially by dysregulated NF-κB activity. However, further investigations are warranted to elucidate the mechanism of oxaliplatin sensitivity mediated through NF-κB activity.
It is widely accepted that the continued growth of solid tumors is dependent on the development of an intact tumor vasculature. Vascular endothelial growth factor (VEGF) and IL-8 have been established as playing a role in promoting tumor angiogenesis 7, 39, 40. In this study, we found that HCT116 cells expressing IL-8 when injected subcutaneously into nude mice produce rapidly growing tumors with increased angiogenic activity from high MVD. However, there was no significant change in the protein levels for VEGF between HCT116 and HCT116-E2 (data not shown). This finding demonstrated that overexpression of IL-8 induced an increased VEGF-independent angiogenic response in this xenograft experimental model. Similar with our findings, overexpression of IL-8 has been found to be associated with VEGF-independent angiogenesis, advanced disease stage, poor prognosis and tumor recurrence in several different malignancies, including colorectal, ovarian and non-small-cell lung cancer 15, 30, 41-43. Future in vivo studies using mouse models that recapitulate the in vivo progression of arising human colon tumors are warranted to determine the significance of our in vitro observations, such as the inhibition of IL-8 either using neutralizing antibodies or siRNA strategies to prove the hypothesis that IL-8 represents a valid therapeutic target in colon cancer.
The clinical significance of our results from in vitro and in vivo studies are supported by IL-8 serum level in CRC patients with metastatic disease being similar to the IL-8 level measured in the IL-8 transfected cell line. Serum IL-8 concentrations have been associated with clinical stage of CRC, bowel wall invasion and liver metastasis 44, 45. The association between high tumor load and circulating IL-8 was confirmed in other tumors, such as melanoma 46, breast 47, ovarian 48, 49, and high IL-8 was consistently found as being significantly associated with progression.
In conclusion, our studies provide significant evidence that IL-8 overexpression contributes to colon cancer cell proliferation, migration, invasion, angiogenesis and leads to resistance to oxaliplatin. These findings may provide the evidence which support the development of novel IL-8-targeted therapies in colon cancer treatment.
Supplementary Material
The analysis of IL-8 protein expression by ELISA in 6 colon cancer cell lines (LoVo, SW620, HT29, HCT8 Caco2 and HCT116). The results were normalized to cell numbers. The results are representative of a minimum of three independent experiments. Data shown represent the mean ±standard deviation.
A, To knock down IL-8 expression in HCT116-E2 cells, the cells were mock-transfected or transfected with two different siRNA oligos against IL-8 (siRNA#2247, siRNA #2261). RNA was collected 48h and 72 h after transfection. RT-PCR was done for the IL-8 mRNA (top) and β-actin mRNA (bottom) on RNA collected from the transfected cells after the indicated time points. B, All siRNA experiments were done with the following cells: untransfected cells, mock-transfected cells (negative control), siRNA-transfected cells (siRNA #2261, #2247). Forty-eight and 72 hours after transfection, cells were collected and Q-PCR was done for IL-8 mRNA (*, P<0.05).
A. Quantitation of migration and invasion after siRNA transfection. Caco2, Caco2-IIIe, control-transfected Caco2-IIIe, and siRNA transfected Caco2-IIIe cells were incubated on Boyden chambers or Matrigel for 48 h as described in Materials and Methods. Columns shows fold change over the number of Caco2 control cells that migrated or invaded. B. Growth inhibition assay. Caco2 and Caco2-IIIe were measured for growth inhibition using CellTiter 96 AQueous One solution. After treatment for 72 h with oxaliplatin, IC50 were calculated using GraphPad Prism software. Data are presented as the mean ± standard deviation of three experiments carried out in triplicate (**, P<0.005).
Acknowledgments
Philipp Manegold was supported by grant MA 4403/1-1 from the Deutsche Forschungs gemeischaft (DFG), Bonn, Germany.
This study was funded by the NIH grant 5 p30CA14089-271. The support was also provided by the Kroha/Casner Family Foundation, Dhont Family Foundation.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 3.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, Lokeshwar BL. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 4.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 5.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–64. [PubMed] [Google Scholar]
- 6.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–65. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 9.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Fidler IJ. Interleukin 8: an autocrine growth factor for human ovarian cancer. Oncol Res. 2000;12:97–106. doi: 10.3727/096504001108747567. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–34. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlan JM, Seth R, Vautier G, Robins RA, Scott BB, Hawkey CJ, Jenkins D. Interleukin-8 and inducible nitric oxide synthase mRNA levels in inflammatory bowel disease at first presentation. J Pathol. 1997;181:87–92. doi: 10.1002/(SICI)1096-9896(199701)181:1<87::AID-PATH736>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Kido S, Kitadai Y, Hattori N, Haruma K, Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y, Chayama K. Interleukin 8 and vascular endothelial growth factor -- prognostic factors in human gastric carcinomas? Eur J Cancer. 2001;37:1482–7. doi: 10.1016/s0959-8049(01)00147-2. [DOI] [PubMed] [Google Scholar]
- 14.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–304. [PubMed] [Google Scholar]
- 15.Lurje G, Zhang W, Schultheis AM, Yang D, Groshen S, Hendifar AE, Husain H, Gordon MA, Nagashima F, Chang HM, Lenz HJ. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol. 2008;19:1734–41. doi: 10.1093/annonc/mdn368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Stoehlmacher J, Park DJ, Yang D, Borchard E, Gil J, Tsao-Wei DD, Yun J, Gordon M, Press OA, Rhodes K, Groshen S, et al. Gene polymorphisms of epidermal growth factor receptor and its downstream effector, interleukin-8, predict oxaliplatin efficacy in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2005;5:124–31. doi: 10.3816/ccc.2005.n.025. [DOI] [PubMed] [Google Scholar]
- 17.Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, Stack MS, Hudson LG. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–13. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehler SE, Ladner RD. Small interfering RNA-mediated suppression of dUTPase sensitizes cancer cell lines to thymidylate synthase inhibition. Mol Pharmacol. 2004;66:620–6. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O'Sullivan JM, Wilson RH, Johnston PG, Waugh DJ. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther. 2008;327:746–59. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- 22.Dobreva I, Waeber G, James RW, Widmann C. Interleukin-8 secretion by fibroblasts induced by low density lipoproteins is p38 MAPK-dependent and leads to cell spreading and wound closure. J Biol Chem. 2006;281:199–205. doi: 10.1074/jbc.M508857200. [DOI] [PubMed] [Google Scholar]
- 23.Lane HC, Anand AR, Ganju RK. Cbl and Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated chemotaxis. Int Immunol. 2006;18:1315–25. doi: 10.1093/intimm/dxl064. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7:2649–61. doi: 10.1158/1535-7163.MCT-08-0148. [DOI] [PubMed] [Google Scholar]
- 25.Oxaliplatin (Eloxatin) for advanced colon cancer. Med Lett Drugs Ther. 2003;45:7–8. [PubMed] [Google Scholar]
- 26.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–75. [PubMed] [Google Scholar]
- 27.Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997;72:937–41. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Greene GF, Kitadai Y, Pettaway CA, von Eschenbach AC, Bucana CD, Fidler IJ. Correlation of metastasis-related gene expression with metastatic potential in human prostate carcinoma cells implanted in nude mice using an in situ messenger RNA hybridization technique. Am J Pathol. 1997;150:1571–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Lurje G, Husain H, Power D, Yang D. Genetic variations in angiogenesis pathway genes associated with clinical outcome in localized gastric adenocarcinoma. Ann Oncol. 2009 doi: 10.1093/annonc/mdp280. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultheis AM, Lurje G, Rhodes KE, Zhang W, Yang D, Garcia AA, Morgan R, Gandara D, Scudder S, Oza A, Hirte H, Fleming G, et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res. 2008;14:7554–63. doi: 10.1158/1078-0432.CCR-08-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 32.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann E, Thiefes A, Buhrow D, Dittrich-Breiholz O, Schneider H, Resch K, Kracht M. MEK1-dependent delayed expression of Fos-related antigen-1 counteracts c-Fos and p65 NF-kappaB-mediated interleukin-8 transcription in response to cytokines or growth factors. J Biol Chem. 2005;280:9706–18. doi: 10.1074/jbc.M407071200. [DOI] [PubMed] [Google Scholar]
- 34.Uslu R, Sanli UA, Dikmen Y, Karabulut B, Ozsaran A, Sezgin C, Muezzinoglu GG, Omay SB, Goker E. Predictive value of serum interleukin-8 levels in ovarian cancer patients treated with paclitaxel-containing regimens. Int J Gynecol Cancer. 2005;15:240–5. doi: 10.1111/j.1525-1438.2005.15210.x. [DOI] [PubMed] [Google Scholar]
- 35.Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–22. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Latif MM, O'Riordan JM, Ravi N, Kelleher D, Reynolds JV. Activated nuclear factor-kappa B and cytokine profiles in the esophagus parallel tumor regression following neoadjuvant chemoradiotherapy. Dis Esophagus. 2005;18:246–52. doi: 10.1111/j.1442-2050.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 37.De Larco JE, Wuertz BR, Manivel JC, Furcht LT. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001;61:2857–61. [PubMed] [Google Scholar]
- 38.Rakitina TV, Vasilevskaya IA, O'Dwyer PJ. Additive interaction of oxaliplatin and 17-allylamino-17-demethoxygeldanamycin in colon cancer cell lines results from inhibition of nuclear factor kappaB signaling. Cancer Res. 2003;63:8600–5. [PubMed] [Google Scholar]
- 39.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 40.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 41.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–78. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Gordon MA, Gil J, Lu B, Zhang W, Yang D, Yun J, Schneider S, Groshen S, Iqbal S, Press OA, Rhodes K, Lenz HJ. Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation. Pharmacogenomics. 2006;7:67–88. doi: 10.2217/14622416.7.1.67. [DOI] [PubMed] [Google Scholar]
- 43.Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY, Kuo SH, Luh KT. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am J Respir Crit Care Med. 2000;162:1957–63. doi: 10.1164/ajrccm.162.5.2002108. [DOI] [PubMed] [Google Scholar]
- 44.Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M, Michalski W, Chechlinska M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I--an independent prognostic factor. Tumour Biol. 2005;26:186–94. doi: 10.1159/000086951. [DOI] [PubMed] [Google Scholar]
- 45.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–9. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 46.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–83. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 47.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, Vermeulen PB, Dirix LY. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 48.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, Landen CN, Jennings N, De Geest K, Langley RR, Villares G, Sanguino A, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100:359–72. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokoe T, Iino Y, Morishita Y. Trends of IL-6 and IL-8 levels in patients with recurrent breast cancer: preliminary report. Breast Cancer. 2000;7:187–90. doi: 10.1007/BF02967458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The analysis of IL-8 protein expression by ELISA in 6 colon cancer cell lines (LoVo, SW620, HT29, HCT8 Caco2 and HCT116). The results were normalized to cell numbers. The results are representative of a minimum of three independent experiments. Data shown represent the mean ±standard deviation.
A, To knock down IL-8 expression in HCT116-E2 cells, the cells were mock-transfected or transfected with two different siRNA oligos against IL-8 (siRNA#2247, siRNA #2261). RNA was collected 48h and 72 h after transfection. RT-PCR was done for the IL-8 mRNA (top) and β-actin mRNA (bottom) on RNA collected from the transfected cells after the indicated time points. B, All siRNA experiments were done with the following cells: untransfected cells, mock-transfected cells (negative control), siRNA-transfected cells (siRNA #2261, #2247). Forty-eight and 72 hours after transfection, cells were collected and Q-PCR was done for IL-8 mRNA (*, P<0.05).
A. Quantitation of migration and invasion after siRNA transfection. Caco2, Caco2-IIIe, control-transfected Caco2-IIIe, and siRNA transfected Caco2-IIIe cells were incubated on Boyden chambers or Matrigel for 48 h as described in Materials and Methods. Columns shows fold change over the number of Caco2 control cells that migrated or invaded. B. Growth inhibition assay. Caco2 and Caco2-IIIe were measured for growth inhibition using CellTiter 96 AQueous One solution. After treatment for 72 h with oxaliplatin, IC50 were calculated using GraphPad Prism software. Data are presented as the mean ± standard deviation of three experiments carried out in triplicate (**, P<0.005).