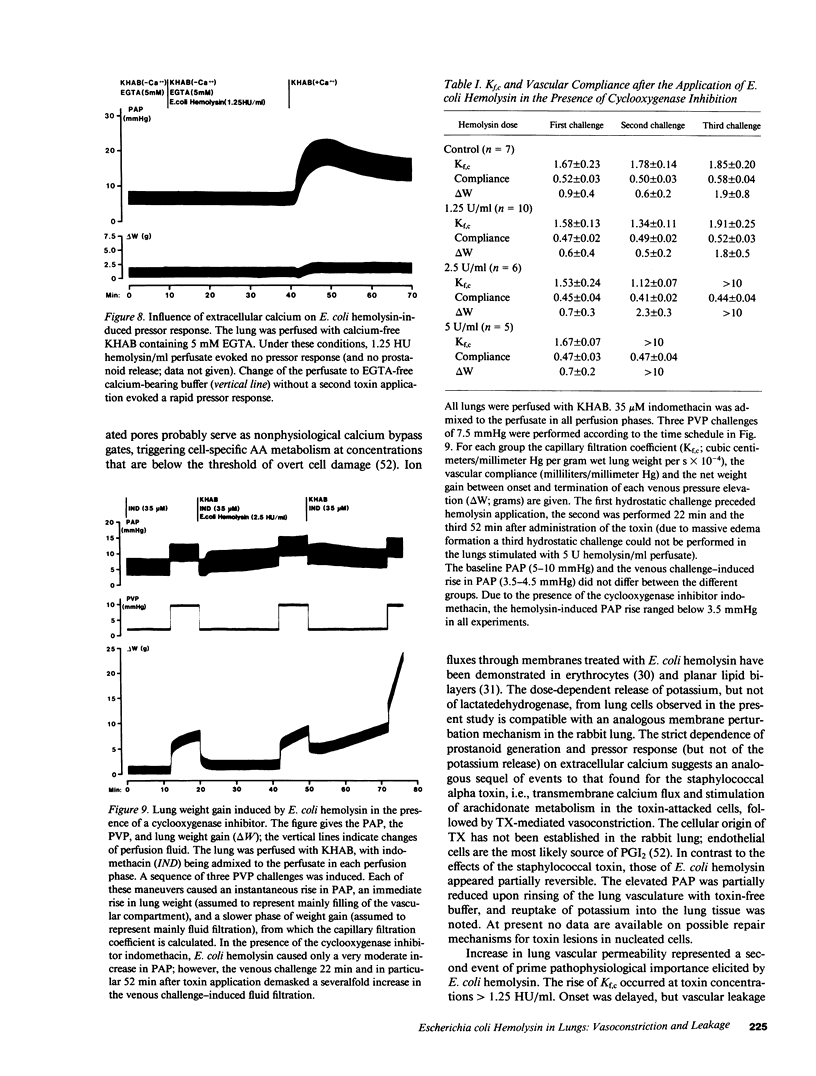
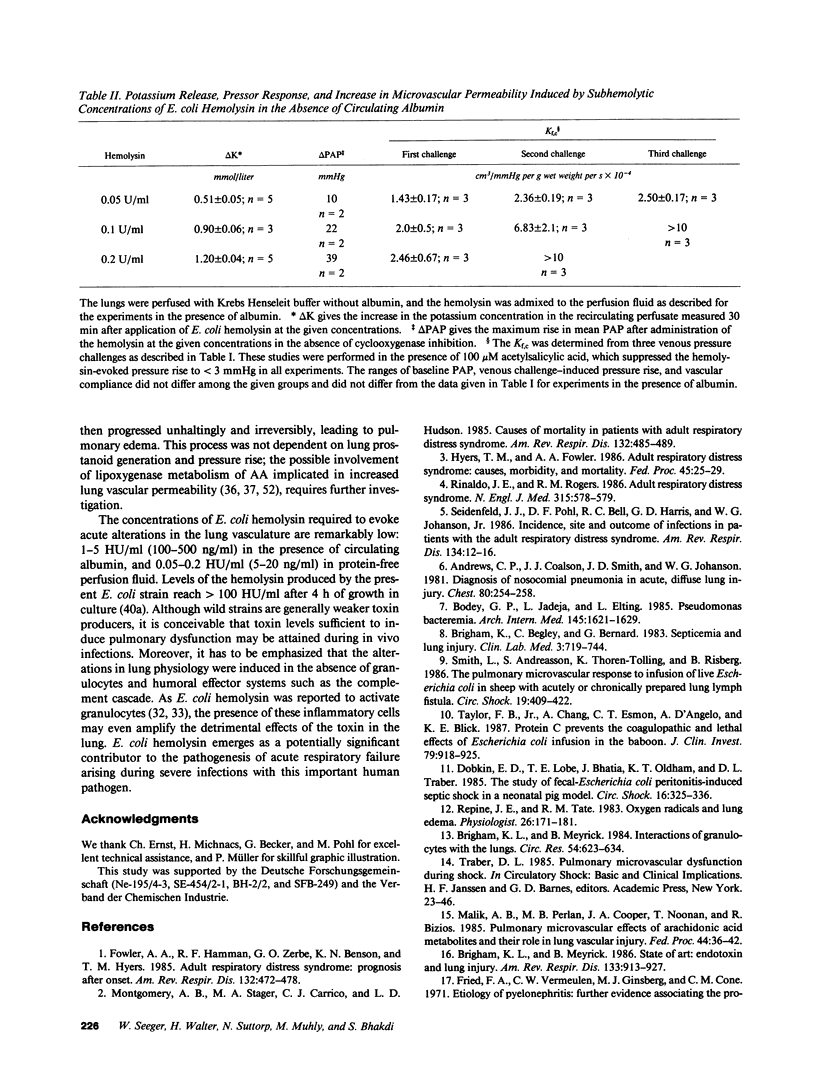
Abstract
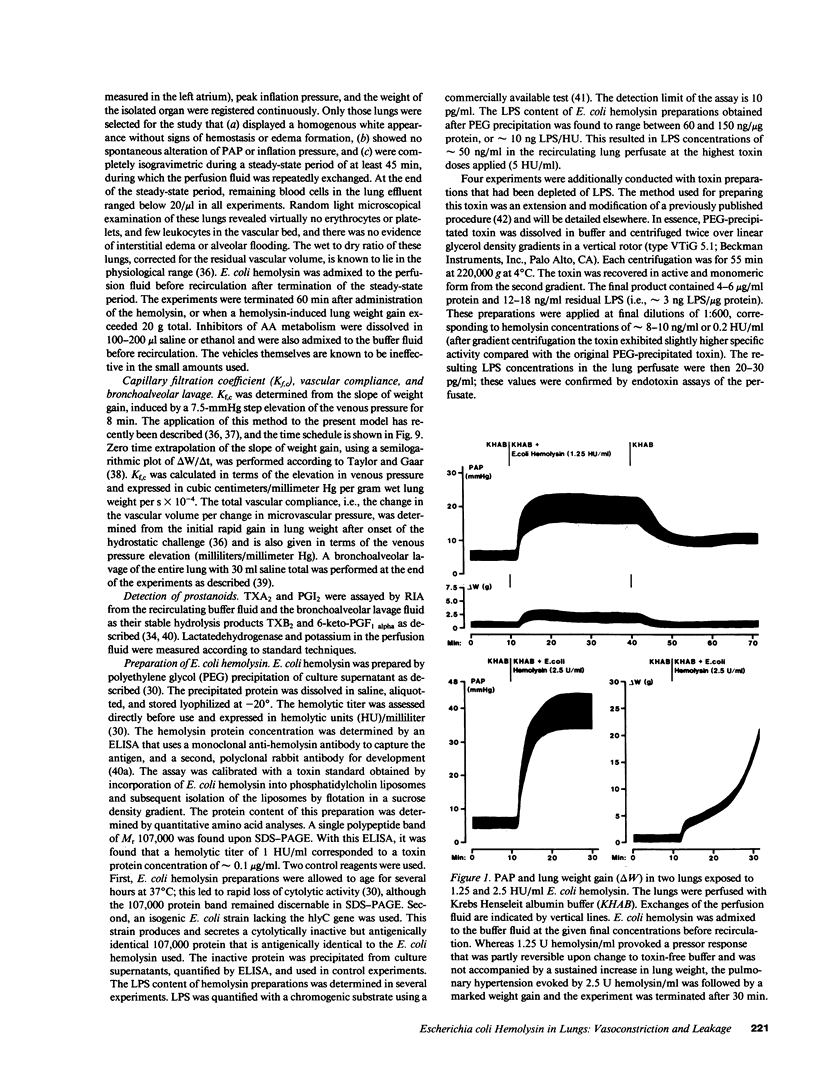
Escherichia coli hemolysin has been implicated as a pathogenicity factor in extraintestinal E. coli infections including sepsis. In the present study the effects of intravascular administration of hemolysin were investigated in isolated blood-free perfused rabbit lungs. Low concentrations of the toxin in the perfusate (0.05-5 hemolytic units/ml, corresponding to approximately 5-500 ng/ml), caused a dose- and time-dependent release of potassium, thromboxane A2, and prostaglandin I2, but not of lactate dehydrogenase, into the recirculating medium, as well as a dose-dependent liberation of the prostanoids into the bronchoalveolar space. These events were paralleled by a dose-dependent pulmonary hypertension, and studies with different inhibitors collectively indicated that the vasoconstrictor response was mediated predominantly by pulmonary thromboxane generation. In addition, E. coli hemolysin elicited a protracted, dose-dependent increase in the lung capillary filtration coefficient, which was independent of the prostanoid-mediated pressor response and resulted in severe pulmonary edema formation. We conclude that E. coli hemolysin can elicit thromboxane-mediated pulmonary hypertension combined with severe vascular leakage in isolated lungs in the absence of circulating inflammatory cells and humoral mediator systems, mimicking the key events in the development of acute respiratory failure in states of septicemia.
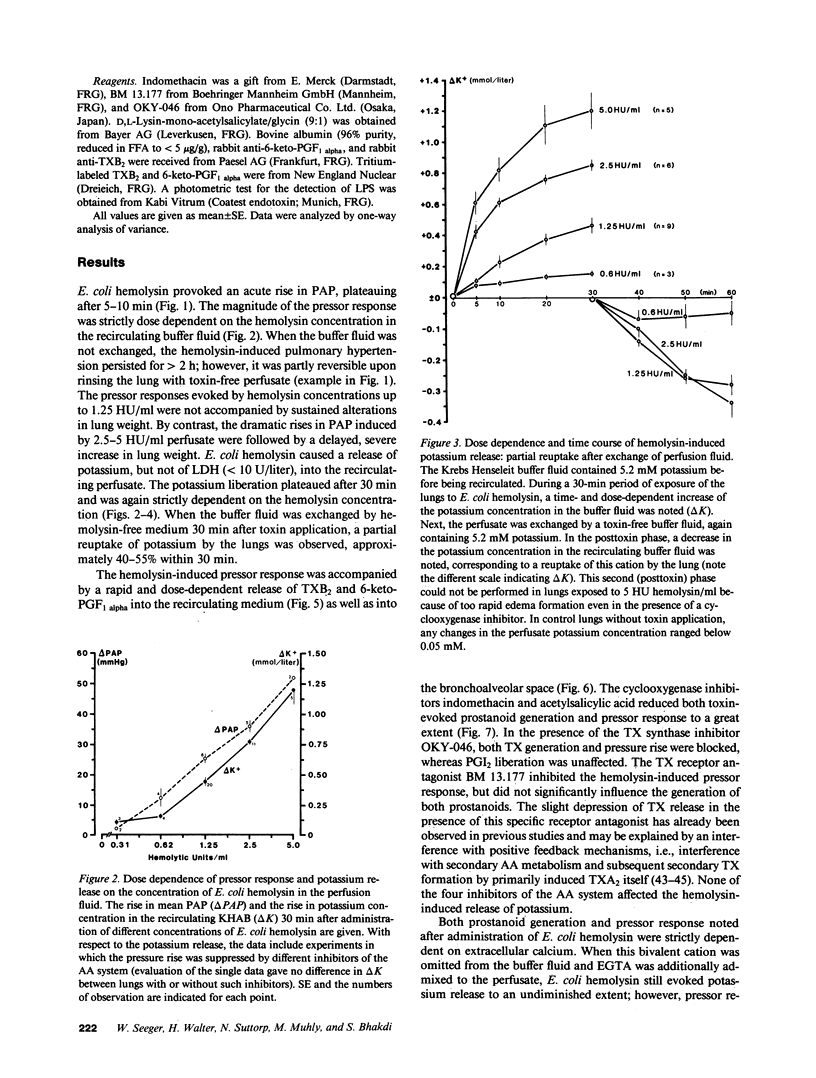
Full text
PDF
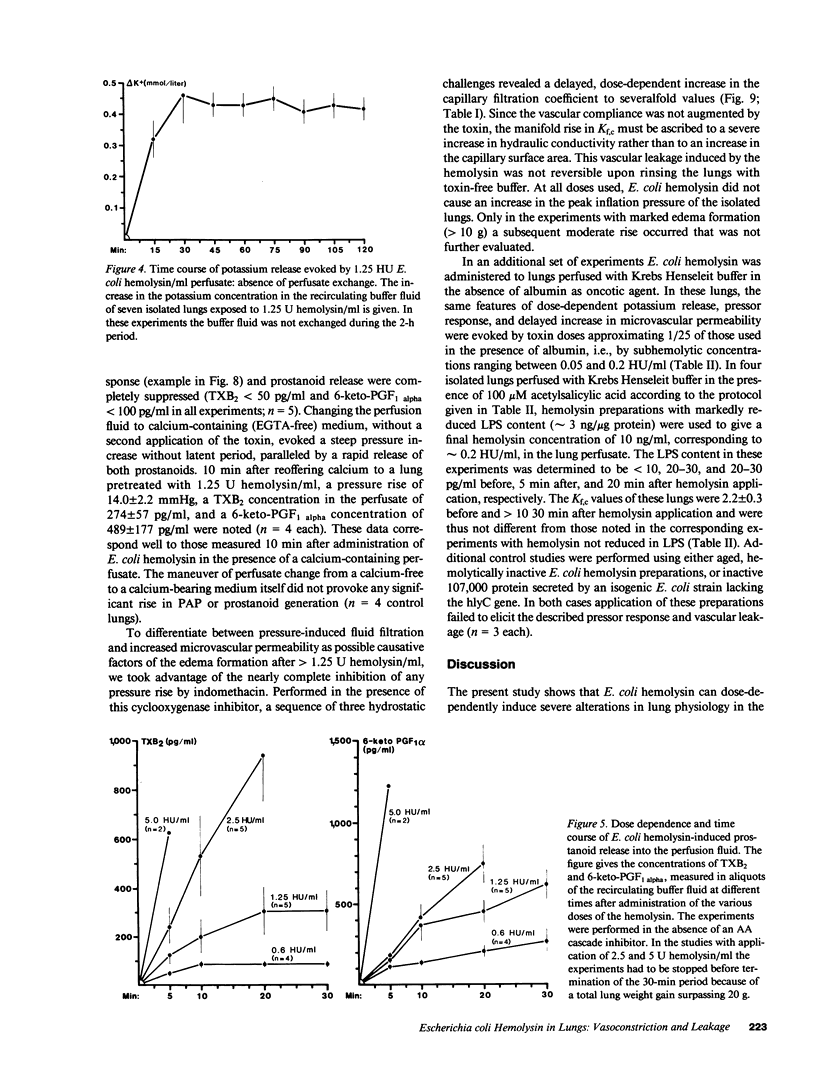

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
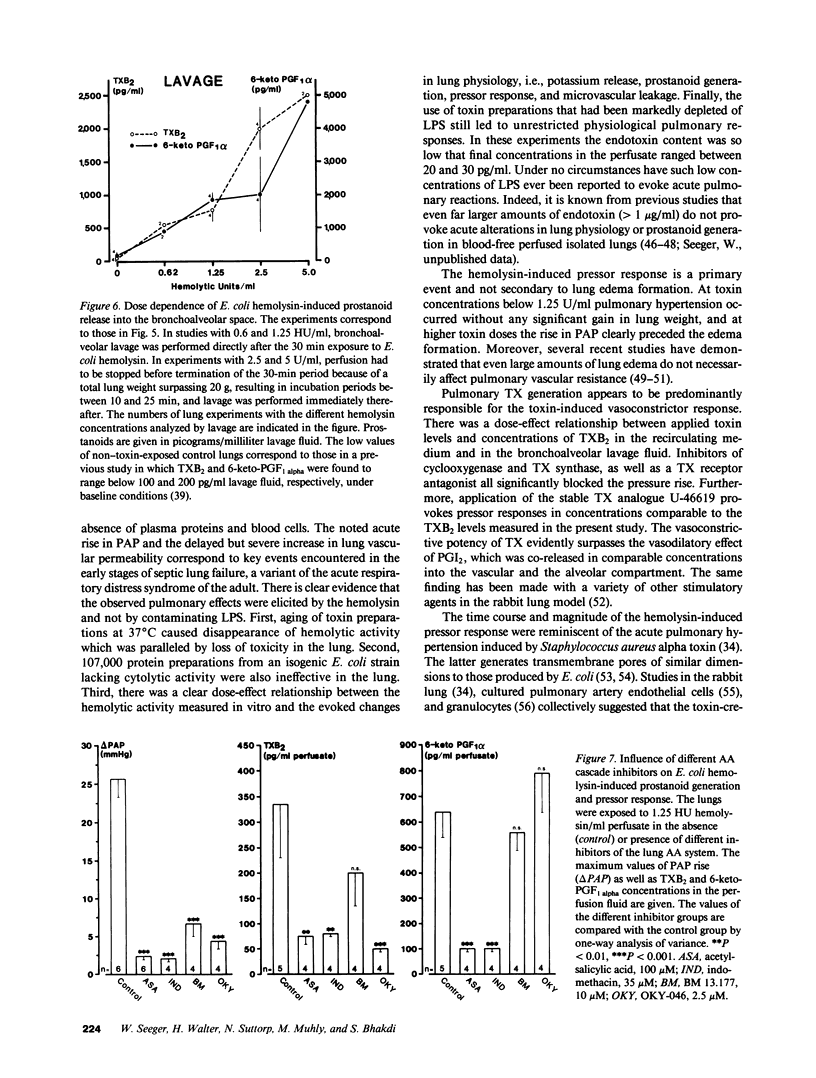
- Andrews C. P., Coalson J. J., Smith J. D., Johanson W. G., Jr Diagnosis of nosocomial bacterial pneumonia in acute, diffuse lung injury. Chest. 1981 Sep;80(3):254–258. doi: 10.1378/chest.80.3.254. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Greulich S., Muhly M., Eberspächer B., Becker H., Thiele A., Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989 Mar 1;169(3):737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Jadeja L., Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985 Sep;145(9):1621–1629. doi: 10.1001/archinte.145.9.1621. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Begley C. J., Bernard G. R., Hutchison A. A., Loyd J. E., Lucht W. D., Meyrick B., Newman J. H., Niedermeyer M. E., Ogletree M. L. Septicemia and lung injury. Clin Lab Med. 1983 Dec;3(4):719–744. [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Interactions of granulocytes with the lungs. Circ Res. 1984 Jun;54(6):623–635. doi: 10.1161/01.res.54.6.623. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982 Feb;15(1):11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- Cintora I., Bessa S., Goodale R. L., Jr, Motsay G. W., Borner J. W. Further studies of endotoxin and alveolocapillary permeability: effect of steroid pre-treatment and complement depletion. Ann Surg. 1974 Mar;179(3):372–375. doi: 10.1097/00000658-197403000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin E. D., Lobe T. E., Bhatia J., Oldham K. T., Traber D. L. The study of fecal-Escherichia coli peritonitis-induced septic shock in a neonatal pig model. Circ Shock. 1985;16(4):325–336. [PubMed] [Google Scholar]
- Eberspächer B., Hugo F., Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989 Mar;57(3):983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N., Ramwell P. W. In vivo and in vitro effects of endotoxin on prostaglandin release from rat lung. Br J Pharmacol. 1981 Jun;73(2):511–516. doi: 10.1111/j.1476-5381.1981.tb10450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. A., Hamman R. F., Zerbe G. O., Benson K. N., Hyers T. M. Adult respiratory distress syndrome. Prognosis after onset. Am Rev Respir Dis. 1985 Sep;132(3):472–478. doi: 10.1164/arrd.1985.132.3.472. [DOI] [PubMed] [Google Scholar]
- Friberger P., Knös M., Mellstam L. A quantitative endotoxin assay utilizing LAL and a chromogenic substrate. Prog Clin Biol Res. 1982;93:195–206. [PubMed] [Google Scholar]
- Fried F. A., Vermeulen C. W., Ginsburg M. J., Cone C. M. Etiology of pyelonephritis: further evidence associating the production of experimental pyelonephritis with hemolysis in Escherichia coli. J Urol. 1971 Sep;106(3):351–354. doi: 10.1016/s0022-5347(17)61286-2. [DOI] [PubMed] [Google Scholar]
- Fünfstück R., Tschäpe H., Stein G., Kunath H., Bergner M., Wessel G. Virulence properties of Escherichia coli strains in patients with chronic pyelonephritis. Infection. 1986 May-Jun;14(3):145–150. doi: 10.1007/BF01643482. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Carreró M. I., Zabala J. C., de la Cruz F., Ortiz J. M. Purification of alpha-hemolysin from an overproducing E. coli strain. Mol Gen Genet. 1985;199(1):106–110. doi: 10.1007/BF00327518. [DOI] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Hacker J., Roberts A., Goebel W. Hemolysin production as a virulence marker in symptomatic and asymptomatic urinary tract infections caused by Escherichia coli. Infect Immun. 1983 Feb;39(2):546–551. doi: 10.1128/iai.39.2.546-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyers T. M., Fowler A. A. Adult respiratory distress syndrome: causes, morbidity, and mortality. Fed Proc. 1986 Jan;45(1):25–29. [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Holland I. B. Secretion of a 107 K dalton polypeptide into the medium from a haemolytic E. coli K12 strain. Mol Gen Genet. 1984;193(2):312–315. doi: 10.1007/BF00330686. [DOI] [PubMed] [Google Scholar]
- Malik A. B., Perlman M. B., Cooper J. A., Noonan T., Bizios R. Pulmonary microvascular effects of arachidonic acid metabolites and their role in lung vascular injury. Fed Proc. 1985 Jan;44(1 Pt 1):36–42. [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Michel R. P., Zocchi L., Rossi A., Cardinal G. A., Ploy-Song-Sang Y., Poulsen R. S., Milic-Emili J., Staub N. C. Does interstitial lung edema compress airways and arteries? A morphometric study. J Appl Physiol (1985) 1987 Jan;62(1):108–115. doi: 10.1152/jappl.1987.62.1.108. [DOI] [PubMed] [Google Scholar]
- Montgomery A. B., Stager M. A., Carrico C. J., Hudson L. D. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1985 Sep;132(3):485–489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- Patscheke H., Stegmeier K. Investigation on a selective non-prostanoic thromboxane antagonist, BM 13.177, in human platelets. Thromb Res. 1984 Feb 1;33(3):277–288. doi: 10.1016/0049-3848(84)90163-4. [DOI] [PubMed] [Google Scholar]
- Raj J. U., Bland R. D., Lai-Fook S. J. Microvascular pressures measured by micropipettes in isolated edematous rabbit lungs. J Appl Physiol (1985) 1986 Feb;60(2):539–545. doi: 10.1152/jappl.1986.60.2.539. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Tate R. M. Oxygen radicals and lung edema. Physiologist. 1983 Jun;26(3):177–181. [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory distress syndrome. N Engl J Med. 1986 Aug 28;315(9):578–580. doi: 10.1056/NEJM198608283150909. [DOI] [PubMed] [Google Scholar]
- Schulz R., Seeger W. Release of leukotrienes into the perfusate of calcium-ionophore stimulated rabbit lungs. Influence of 5-lipoxygenase inhibitors. Biochem Pharmacol. 1986 Jan 15;35(2):183–193. doi: 10.1016/0006-2952(86)90512-5. [DOI] [PubMed] [Google Scholar]
- Seeger W., Bauer M., Bhakdi S. Staphylococcal alpha-toxin elicits hypertension in isolated rabbit lungs. Evidence for thromboxane formation and the role of extracellular calcium. J Clin Invest. 1984 Sep;74(3):849–858. doi: 10.1172/JCI111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger W., Ernst C., Walmrath D., Neuhof H., Roka L. Influence of the thromboxane antagonist BM 13.177 on the arachidonic acid-induced increase in pulmonary vascular resistance and permeability in rabbit lungs. Thromb Res. 1985 Dec 15;40(6):793–805. doi: 10.1016/0049-3848(85)90316-0. [DOI] [PubMed] [Google Scholar]
- Seeger W., Menger M., Walmrath D., Becker G., Grimminger F., Neuhof H. Arachidonic acid lipoxygenase pathways and increased vascular permeability in isolated rabbit lungs. Am Rev Respir Dis. 1987 Oct;136(4):964–972. doi: 10.1164/ajrccm/136.4.964. [DOI] [PubMed] [Google Scholar]
- Seeger W., Stöhr G., Wolf H. R., Neuhof H. Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J Appl Physiol (1985) 1985 Feb;58(2):326–338. doi: 10.1152/jappl.1985.58.2.326. [DOI] [PubMed] [Google Scholar]
- Seeger W., Suttorp N. Role of membrane lipids in the pulmonary vascular abnormalities caused by bacterial toxins. Am Rev Respir Dis. 1987 Aug;136(2):462–466. doi: 10.1164/ajrccm/136.2.462. [DOI] [PubMed] [Google Scholar]
- Seeger W., Walmrath D., Menger M., Neuhof H. Increased lung vascular permeability after arachidonic acid and hydrostatic challenge. J Appl Physiol (1985) 1986 Nov;61(5):1781–1789. doi: 10.1152/jappl.1986.61.5.1781. [DOI] [PubMed] [Google Scholar]
- Seeger W., Walmrath D., Neuhof H., Lutz F. Pulmonary microvascular injury induced by Pseudomonas aeruginosa cytotoxin in isolated rabbit lungs. Infect Immun. 1986 Jun;52(3):846–852. doi: 10.1128/iai.52.3.846-852.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenfeld J. J., Pohl D. F., Bell R. C., Harris G. D., Johanson W. G., Jr Incidence, site, and outcome of infections in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1986 Jul;134(1):12–16. doi: 10.1164/arrd.1986.134.1.12. [DOI] [PubMed] [Google Scholar]
- Smith L., Andreasson S., Thorén-Tolling K., Risberg B. The pulmonary microvascular response to infusion of live Escherichia coli in sheep with acutely or chronically prepared lung lymph fistula. Circ Shock. 1986;19(4):409–422. [PubMed] [Google Scholar]
- Stegmeier K., Pill J., Müller-Beckmann B., Schmidt F. H., Witte E. C., Wolff H. P., Patscheke H. The pharmacological profile of the thromboxane A2 antagonist BM 13.177. A new anti-platelet and anti-thrombotic drug. Thromb Res. 1984 Aug 15;35(4):379–395. doi: 10.1016/0049-3848(84)90230-5. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: role of calcium. Am J Physiol. 1985 Jan;248(1 Pt 1):C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Zucker-Reimann J., Roka L., Bhakdi S. Mechanism of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect Immun. 1987 Jan;55(1):104–110. doi: 10.1128/iai.55.1.104-110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Esmon C. T., D'Angelo A., Vigano-D'Angelo S., Blick K. E. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987 Mar;79(3):918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. G., Hakim T. S., Michel R. P., Chang H. K. Segmental pulmonary vascular resistance in progressive hydrostatic and permeability edema. J Appl Physiol (1985) 1985 Jul;59(1):242–247. doi: 10.1152/jappl.1985.59.1.242. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984 Jan;43(1):156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch J. F., Postma P., Koopman P. A., de Graaff J., MacLaren D. M., van Brenk D. G., Guinée P. A. Virulence of urinary and faecal Escherichia coli in relation to serotype, haemolysis and haemagglutination. J Hyg (Lond) 1982 Jun;88(3):567–577. doi: 10.1017/s002217240007042x. [DOI] [PMC free article] [PubMed] [Google Scholar]


