Abstract
Bnip3 is a pro-apoptotic BH3-only protein which is associated with mitochondrial dysfunction and cell death. Bnip3 is also a potent inducer of autophagy in many cells. In this study, we have investigated the mechanism by which Bnip3 induces autophagy in adult cardiac myocytes. Overexpression of Bnip3 induced extensive autophagy in adult cardiac myocytes. Fluorescent microscopy studies and ultrastructural analysis revealed selective degradation of mitochondria by autophagy in myocytes overexpressing Bnip3. Oxidative stress and increased levels of intracellular Ca2+ have been reported by others to induce autophagy, but Bnip3-induced autophagy was not abolished by antioxidant treatment or the Ca2+ chelator BAPTA-AM. We also investigated the role of the mitochondrial permeability transition pore (mPTP) in Bnip3-induced autophagy. Although the mPTP has previously been implicated in the induction of autophagy and selective removal of damaged mitochondria by autophagosomes, mitochondria sequestered by autophagosomes in Bnip3-treated cardiac myocytes had not undergone permeability transition and treatment with the mPTP inhibitor cyclosporine A did not inhibit mitochondrial autophagy in cardiac myocytes. Moreover, cyclophilin D (cypD) is an essential component of the mPTP and Bnip3 induced autophagy to the same extent in embryonic fibroblasts isolated from wild-type and cypD-deficient mice. These results support a model where Bnip3 induces selective removal of the mitochondria in cardiac myocytes and that Bnip3 triggers induction of autophagy independent of Ca2+, ROS generation and mPTP opening.
Key words: Bnip3, autophagy, cardiac myocytes, mitochondria, permeability transition pore, cyclophilin D
Introduction
Autophagy is an evolutionarily conserved process involved in the degradation of long-lived proteins and organelles. Cytoplasmic material is sequestered by an autophagosome and subsequently delivered to the lysosome where it is degraded by lysosomal proteases.1 In the heart, autophagy is used to clear dysfunctional mitochondria and its deregulation has harmful consequences to the myocytes. This was recently demonstrated by Nakai et al. in which selective ablation of the essential autophagy gene atg5 in the heart resulted in accumulation of dysfunctional mitochondria and rapid decline in cardiac function.2 Autophagy is enhanced in the heart in response to ischemia/reperfusion (I/R) injury3–5 and pressure overload.6 However, the functional role of enhanced autophagy in the heart in response to stress is not clear and has been reported to be either protective or detrimental.2,3,6,7
Bcl-2 nineteen-kilodalton interacting protein 3 (Bnip3) is an atypical pro-apoptotic BH3-only protein and plays a key role in the pathogenesis of many diseases. Bnip3 has been reported to play a role in many cancers where alteration in Bnip3 expression and localization contributes to deregulation of cell death.8 In contrast, activation of Bnip3 has been shown to contribute to cell death in myocardial ischemia/reperfusion (I/R)-injury5,9,10 and post-infarct remodeling.11 Bnip3 localizes to the mitochondria where it induces mitochondrial dysfunction and subsequent cell death.10,12–14 Many studies have reported that Bnip3 also induces autophagy in cells5,15,16 but neither the reason nor the mechanism behind the upregulation of autophagy in response to Bnip3 is understood. In this study, we have investigated the mechanism by which Bnip3 induces autophagy in isolated adult cardiac myocytes.
Results
Bnip3 is a potent inducer of autophagy in adult rat cardiac myocytes.
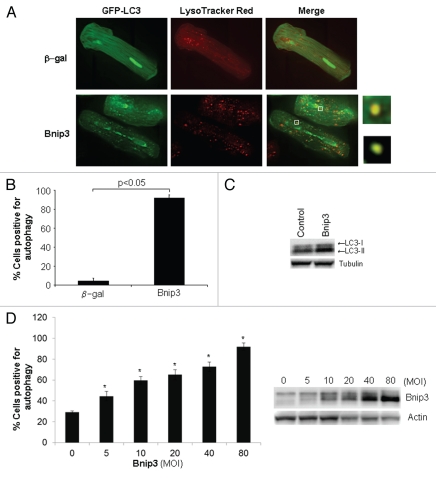
Autophagy is a prominent feature in I/R and since Bnip3 is activated during I/R, we investigated whether Bnip3 induced autophagy in adult cardiac myocytes isolated from the rat heart. To examine whether autophagy was enhanced in adult cardiac myocytes in response to Bnip3, cells were infected with adenoviruses encoding β-gal or Bnip3 plus GFP-LC3 as a marker for autophagosome formation.17 Live cell imaging of myocytes revealed that overexpression of Bnip3 induced significant autophagy in cardiac myocytes (Fig. 1A and B). Since the autophagosome delivers its content to the lysosome for degradation,1 we also monitored changes in lysosomal activity by staining live cells with Lysotracker Red (LTR), which labels highly acidic lysosomal vacuoles. We found low levels of basal lysosomal activity in β-gal overexpressing cells, whereas cells overexpressing Bnip3 had an increased number of lysosomes (Fig. 1A). We also found co-localization of GFP-LC3 and LTR, indicating fusion between autophagosomes and lysosomes. Upon induction of autophagy, LC3-I is recruited to the autophagosome where LC3-II is generated by proteolysis and lipidation.17 Thus, increased levels of LC3-II closely correlate to enhanced number of autophagosomes, serving as a good indicator of autophagy. Western blot analysis of endogenous LC3-I and -II levels confirmed an increase in LC3-II in cells overexpressing Bnip3 (Fig. 1C). In addition, Bnip3 caused a dose-dependent increase in autophagy in myocytes (Fig. 1D). Even at the lowest concentration of 5 MOI, Bnip3 induced autophagy in a significant number of cells. This suggests that Bnip3 is a potent activator of the autophagic-lysosomal pathway in adult cardiac myocytes.
Figure 1.
Bnip3 is a potent inducer of autophagy in adult cardiac myocytes. (A) Adult cardiac myocytes were infected with adenoviruses encoding β-gal or Bnip3 plus GFP-LC3 for 24 hours and then stained with LysoTracker Red. Live cells were examined by fluorescence microscopy. Merged image shows co-localization between autophagosomes and lysosomes (yellow). (B) Quantitation of autophagy (n = 3). (C) Western blot analysis of LC3-I and LC3-II levels in myocytes overexpressing Bnip3. (D) Bnip3 increased autophagy in a dose-dependent manner when overexpressed at increasing multiples of infection (MOI) (n = 3, *p < 0.05 compared to 0 MOI of Ad-Bnip3). Overexpression of Bnip3 was verified by western blotting.
Bnip3 overexpression causes substantial degradation of mitochondria by autophagy in adult cardiac myocytes.
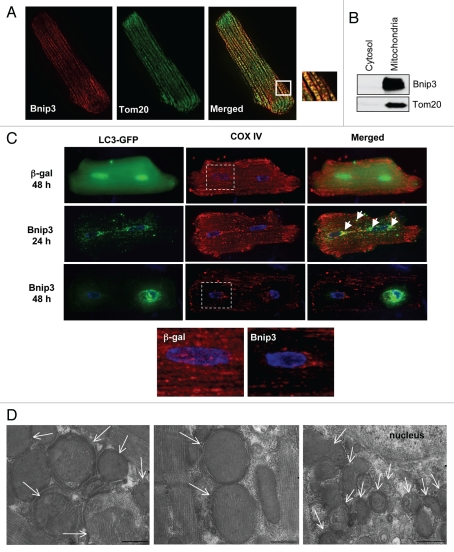
We have previously reported that endogenous Bnip3 localizes primarily to the mitochondria in rat hearts.5 In this study, we confirmed the mitochondrial localization of Bnip3 by staining myocytes overexpressing Bnip3 with antibodies against Bnip3 and Tom20, a mitochondrial outer membrane protein. As shown in Figure 2A, immunofluorescence analysis showed that Bnip3 co-localized with Tom20. Western blotting analysis of cytosolic and mitochondrial fractions confirmed that Bnip3 localized to mitochondria when overexpressed in cardiac myocytes (Fig. 2B). In cardiac myocytes, mitochondria constitute about 30% of cell volume to ensure efficient ATP supply for the contracting myocyte.18 As shown by staining for Cytochrome c Oxidase subunit IV (COX IV), mitochondria were very abundant in control infected myocytes (Fig. 2C). Since autophagy is important in removing dysfunctional organelles, we examined whether Bnip3-induced the removal of mitochondria by the autophagosomes. Cardiac myocytes overexpressing β-gal or Bnip3 plus GFP-LC3 were stained with an antibody against COX IV to label mitochondria. Analysis of cells by high-resolution imaging revealed extensive mitochondrial autophagy in Bnip3 overexpressing cells 24 h after infection where many of the GFP-LC3 labeled autophagosomes co-localized with mitochondria (Fig. 2C). Interestingly, we noted that the autophagosomes started to aggregate in the perinuclear region by 24 h and were exclusively found in that area after 48–72 h. The aggregation of autophagosomes in this region correlated with the almost complete loss of mitochondria in the same area. In fact, there were almost no detectable mitochondria left in the perinuclear region by 48 h in some of the cells (Fig. 2C). Ultrastructural analysis of myocytes overexpressing Bnip3 confirmed the presence of double-membrane autophagosomes containing mitochondria (Fig. 2D). Interestingly, almost all of the autophagosomes contained mitochondria, suggesting that Bnip3 specifically induces mitochondrial autophagy. The electron micrographs revealed the presence of many large autophagosomes comprising mitochondria throughout the cell, whereas smaller autophagosomes containing fragments of mitochondria were aggregated in the perinuclear region.
Figure 2.
Bnip3 localizes to the mitochondria where it induces mitochondrial autophagy. (A) A cardiac myocyte infected with Ad-Bnip3 was stained with antibodies against Bnip3 and Tom20. Fluorescence microscopy shows co-localization between Bnip3 (red) and Tom20 (green). (B) Western blotting of cytosolic and mitochondrial fractions confirms mitochondrial localization of Bnip3 when overexpressed in myocytes. Tom20 served as a mitochondrial marker. (C) Cells were stained with anti-COX IV to label mitochondria in µ-gal and Bnip3 overexpressing cells at 24 and 48 hrs post-infection. Co-localization between GFP -LC3 and COX IV at 24 hrs demonstrated extensive co-localization between autophagosomes and mitochondria (marked by arrows). After 48 hrs, there was substantial loss of mitochondria with accumulation of autophagosomes around the nucleus. (D) Electron micrographs revealed autophagosomes containing mitochondria (white arrows) in myocytes overexpressing Bnip3 (scale bar = 500 nm).
Bnip3 induces autophagy independent of Ca2+, ROS and mPTP opening.
Elevations in cytosolic Ca2+ levels,19,20 increased oxidative stress,21–23 and opening of the mPTP21,24 have all been implicated in the induction of autophagy in cells. We confirmed that starvation-induced autophagy was inhibited in the presence of the membrane-permeable Ca2+ chelator BAPTA-AM,25 the mPTP inhibitor cyclosporine A (CsA) or the antioxidant N-acetyl cysteine (NAC) in adult myocytes (Fig. 3A). Since the pro-apoptotic Bcl-2 proteins have been reported to increase intracellular Ca2+ levels by perturbing the ER,26,27 we investigated if Bnip3 induced autophagy by increasing cytosolic Ca2+ in cardiac myocytes. Cells were incubated with BAPTA-AM and autophagy was assessed. However, the presence of BAPTA-AM had no effect on autophagy induced by Bnip3 (Fig. 3B and C). Bnip3 has been reported to stimulate ROS production in cells,12 but we found that the presence of (NAC) did not have any effect on Bnip3-mediated autophagy (Fig. 3B and C). Bnip3 has been shown to induce mitochondrial dysfunction and cell death via opening of the mPTP,12,13,28 but treatment of cardiac myocytes with CsA did not affect the formation of autophagosomes in Bnip3 overexpressing cells (Fig. 3B and C). In addition, we found that CsA did not inhibit mitochondrial autophagy in cardiac myocytes as measured by fluorescence microscopy (Fig. 3D). This data is consistent with the ultrastructural analysis of Bnip3-mediated mitochondrial autophagy. Opening of the mPTP results in swelling of the inner mitochondrial membrane with disruption of the cristae and subsequent rupture of the outer membrane.29 However, electron micrographs revealed that the mitochondria inside the autophagosomes had intact cristae and preserved electron-dense matrix (Fig. 2D), suggesting that they had not undergone permeability transition. Cyclophilin D (CypD) is an essential component of the mPTP30 and to confirm that Bnip3-mediated autophagy was independent of the mPTP, we assessed autophagy in mouse embryonic fibroblasts (MEFs) isolated from wild-type (WT) and CypD-/- mice. Overexpression of Bnip3 and GFP-LC3 induced a significant increase in autophagy in both WT and CypD-/- MEFs (Fig. 3E and F), confirming that Bnip3-mediated autophagy does not require the mPTP.
Figure 3.
Bnip3 induces autophagy independent of Ca2+, ROS and mPTP opening. (A) Starvation-induced autophagy is inhibited in the presence of 0.2 µM BAPTA-AM, 1 µM CsA or 1 mM NAC in adult myocytes (n = 3, *p < 0.05 compared with non-starved cells). (B) Bnip3-mediated autophagy is not inhibited in the presence of 0.2 µM BAPTA-AM, 1 mM NAC or 1 µM CsA. (C) Quantification of Bnip3-mediated autophagy in the presence of DMSO or inhibitors (*, **, *** and ****p ≤ 0.05 compared with control, n = 3). (D) The presence of CsA failed to inhibit Bnip3-mediated mitochondrial autophagy. Representative image of an adult myocyte infected with Bnip3 for 24 h in the presence of 1 µm CsA. Maximum intensity projection of a cardiac myocyte observed along the XY (center), XZ (top) and YZ (right) planes. Colocalization scatter plot of mitochondria (quadrant 1) and LC3-GFP (quadrant 2). Quadrant 3 represents colocalization of mitochondria and LC3GFP. (E) Mouse embryonic fibroblasts isolated from WT and CypD-/- mice were infected with adenoviruses encoding µ-gal or Bnip3 plus GFP-LC3 for 24 hours and then examined by fluorescence microscopy. Shown are representative images. (F) Quantitation of autophagy (*p ≤ 0.05 compared with control, n = 3).
Discussion
Cardiac myocytes are very active cells and require large amounts of energy, which is provided by mitochondria through oxidative phosphorylation. Due to this large demand for ATP, cardiac myocytes have the highest volume density of mitochondria of all the cells in the body. This ubiquitous presence ensures efficient ATP supply and delivery to the continuously contracting myocyte. In the heart, autophagy is used to clear excess and dysfunctional mitochondria and its deregulation is harmful to the myocytes. Autophagy-deficient cardiac myocytes rapidly accumulate dysfunctional mitochondria which is accompanied by a decline in cardiac function and subsequent heart failure.2 Defective mitochondria can serve as a source of reactive oxygen species which can cause further damage to nearby mitochondria. For instance, inhibition of autophagy in yeast resulted in higher levels of intracellular ROS and reduced mitochondrial oxygen consumption.31 Bnip3 is well known to induce mitochondrial dysfunction10,12,13 and in this study we found that Bnip3 overexpression also resulted in substantial removal of mitochondria by autophagosomes in adult cardiac myocytes. It is still controversial whether autophagy is protective or detrimental to the cell. We have previously found that upregulation of autophagy protects against Bnip3-mediated cell death in HL-1 cells presumably by removing damaged mitochondria.5,7 Although the myocytes with extensive mitochondrial autophagy looked healthy as characterized by their rod-shaped morphology, it is important to keep in mind that these are non-contracting myocytes. Thus, it is very likely that loss of this large amount of mitochondria will be detrimental to myocytes in the beating heart due to insufficient energy supply.
Several studies have reported roles for increased ROS and mPTP opening as triggers of autophagy. For instance, hydrogen peroxide or 2-methoxyestradiol treatment of cells caused significant upregulation of autophagy.21 Rodriguez-Hernandez et al. recently reported that autophagy was upregulated in Coenzyme Q10 deficient fibroblasts that was abolished by antioxidants or CsA treatment.32 Opening of the mPTP has been implicated in the selective removal of damaged mitochondria.24 A functional mPTP is also required for starvation-induced mitochondrial autophagy.33 Although Bnip3 can induce opening of the mPTP,12 we found that overexpression of Bnip3 in the presence of CsA or in MEFs lacking a functional pore (cypD-/- MEFs) had no effect on the levels of autophagy. We also found that CsA did not inhibit Bnip3-mediated mitochondrial autophagy in adult cardiac myocytes. Recently, we reported that Bnip3-mediated mitochondrial membrane permeabilization and cell death still occurred in the presence of CsA and in cypD-/- MEFs.29 Clearly, Bnip3 can induce both mitochondrial autophagy and cell death independent of the mPTP.
Beclin 1 is a Bcl-2 interacting protein and essential for the initiation of autophagy.34 Bcl-2 can inhibit autophagy by sequestering Beclin 1, whereas BH3 mimetics can induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2.35,36 Recently, Bellot et al. reported that Bnip3's BH3 domain was able to disrupt the interaction between Beclin 1 and Bcl-2 in vitro.15 When we investigated the possibility that Bnip3 disrupted the interaction between Beclin 1 and Bcl-2, we found that very little Beclin 1 was associated with Bcl-2 in the rat heart (data not shown). Thus, it is unlikely that Bnip3 induces autophagy by disrupting an interaction between Beclin 1 and Bcl-2 in cardiac myocytes. Mammalian target of rapamycin (mTOR) is a negative regulator of autophagy which exists in two functionally distinct protein complexes, TOR complex 1 (TORC1) and TOR complex 2 (TORC2).37 Rheb is a potent upstream activator of TORC1 and Bnip3 was recently identified to directly bind to and inhibit Rheb-mediated activation of TORC1.38 Thus, it is possible that Bnip3 induces autophagy in myocytes via regulation of the Rheb-TORC1 pathway. The Bnip3 homologue Nix/Bnip3L is also an inducer of mitochondrial autophagy and is essential for mitochondrial clearance in maturing erythrocytes.39 Recently, it was reported that Nix/Bnip3L can directly interact with LC3, suggesting that binding of Nix to LC3 on the autophagosome tethers the mitochondrion to the autophagosome.40 It is still unknown how Bnip3 targets mitochondria to the autophagosomes and if Bnip3 can also act as an autophagy receptor.
Together, our results suggest that Bnip3 is a potent inducer of mitochondrial autophagy in cardiac myocytes. The upregulation of autophagy occurs independently of ROS production, increased intracellular Ca2+ and mPTP opening. Thus, further studies are required to delineate the mechanisms by which Bnip3 induces autophagy in cells and whether Bnip3 is a specific mitochondrial receptor for autophagosomes. Since Bnip3 is significantly upregulated in failing hearts,9,28 it will also be important to investigate the functional consequences of extensive mitochondrial autophagy in vivo. The autophagic pathway may represent a potential therapeutic target to treat or prevent heart failure. Therefore, it is important to gain better understanding of the relationship between Bnip3 and autophagy.
Materials and Methods
Isolation of cardiac myocytes.
Adult cardiac myocytes were prepared from 200 to 250 g male Sprague Dawley rats as previously described.41 All animal procedures were in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee. Briefly, the rat was anesthetized with sodium pentobarbital, the heart excised and rapidly cannulated via the aorta. The heart was perfused with a Ca2+-free buffer followed by perfusion with 0.6 mg/mL collagenase 2 (Worthington Biochemical Corporation, 25004177) and 8.3 µM CaCl2 in perfusion buffer. After perfusion with collagenase solution for 15 min, the heart was minced and the myocytes were filtered through fine gauze. A stopping buffer containing 5% newborn calf serum (Invitrogen, 16010-159) and 12.5 µM CaCl2 was added to the cells, followed by stepwise reintroduction of calcium up to a concentration of 1 mM. The cells were centrifuged at 100x g for 1 min and the pellet was washed in M199 medium (Invitrogen, 11043-023), containing 10 mM HEPES (Sigma, H4034), 5 mM taurine (Sigma, T8691), 5 mM creatine (Sigma, C0780), 2 mM carnitine (Sigma, 544361), 0.5% fatty acid free BSA (Sigma, A6003) and 100 U/mL penicillin-streptomycin. Myocytes were plated on laminin (Roche, 11-243-217-001) (20 µg/mL laminin for glass or 10 µg/mL for plastic dishes) at 5 × 104 cells per dish. Cell viability based on rod-shaped morphology at the outset of the experiment was routinely >90%.
Isolation of mouse embryonic fibroblasts.
WT and CypD-/- mouse embryonic fibroblasts (MEFs) were isolated from day 15.5–16.5 embryos.30 MEFs were maintained in DMEM (Invitrogen, 10569-044) supplemented with 10% FBS (Invitrogen, 16000-044), 100 U/ml penicillin and 100 U/ml streptomycin.
Starvation experiment.
Isolated cardiac myocytes infected with GFP-LC3 were incubated in Medium 199 (Invitrogen, 11043-023) (nonstarved) or starvation media (in mM: 20 HEPES, 110 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.25 MgSO4, 1.2 CaCl2, 25 NaHCO3) at 37°C in an atmosphere of 5% CO2, in the presence of 1 µm CsA (Sigma, C3662), 0.2 µm BAPTA-AM (EMD Biosciences, 196419) or 1 mM N-acetylcysteine (NAC) (Sigma, A9165). After 24 hrs, cells were analyzed by fluorescence microscopy for the presence of autophagosomes.
Immunofluorescence analysis.
Freshly isolated adult cardiac myocytes were infected with adenoviruses encoding µ-Gal or Bnip3 plus LC3-GFP. Cells were examined by immunofluorescence 24 h and 48 h later. For Bnip3 co-localization experiments, cells infected with Ad-Bnip3 for 24 h were fixed in 4% formaldehyde (Ted Pella Inc., 18505) in PBS (pH 7.4), permeabilized with 0.2% Triton X-100 in PBS and then blocked in 5% goat serum. The cells were first incubated with anti-Bnip3 (1:100, Sigma, B7931) and anti-Tom20 (1:50, Santa Cruz Biotech., sc-11415) and then with goat anti-rabbit Alexa-488 (Invitrogen, 11034) and goat anti-mouse Alexa-594 (Invitrogen, 11032) secondary antibodies. After infecting WT and CypD-/- MEFs with adenoviruses encoding LC3-GFP plus β-Gal or Bnip3, the cells were fixed and then analyzed by fluorescent microscopy. For experiments aimed at assessing autophagy, cells were inspected at 60x magnification and classified as: (a) cells with diffuse LC3-GFP fluorescence; or as (b) cells with numerous LC3-GFP puncta (>30 dots/cell), representing autophagosomes 24 h and 48 h after infection. At least 150 cells were scored from two replicate dishes in three independent experiments. For experiments aimed at determining lysosomal activity, acidic organelles were labeled with acidotropic dye 50 nM LysoTracker Red (Molecular Probes, L-7528) diluted in culture medium for 5 min according to manufacturer's instructions. To label mitochondria, cells were fixed with 4% formaldehyde in PBS (pH 7.4), then stained with an antibody to COX IV (Invitrogen, A21348). Cells were observed through a Nikon TE300 fluorescence microscope equipped with a cooled CCD camera (Orca-ER, Hamamatsu) or a Carl Zeiss AxioObserver Z1 fitted with a motorized Z-stage and an Apotome for optical sectioning. For high-resolution microscopy, Z-stacks were acquired at ×60 magnification with 0.3 µm increments and deconvolved using 10 iterations of a three-dimensional blind deconvolution algorithm (AutoQuant). Alternatively, Z-stacks were acquired at 63X magnification with 0.6 µm increments in ApoTome mode using a high-resolution AxioCam MRm digital camera, a 63X Plan-Apochromat (Oil-immersion) objective and Zeiss AxioVision 4.8 software (Carl Zeiss).
Western blotting.
To prepare whole cell lysates, myocytes were lysed in ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100 and complete protease inhibitor cocktail (Roche, 11873580001) and cleared by centrifugation at 20,000x g for 20 min. To isolate mitochondria and cytosol, we employed a protocol routinely used in our laboratory.42 Briefly, myocytes were disrupted by sonication on ice in isolation buffer (10 mM MOPS pH 7.4, 250 mM sucrose, 5 mM KH2PO4, 2 mM MgCl2, 1 mM EGTA and complete protease inhibitor cocktail). Homogenates were centrifuged for 10 min at 600x g to remove unbroken cells and nuclei and the supernatants were centrifuged for 10 min at 3,000x g to pellet mitochondria. The mitochondrial pellet was washed once in isolation buffer and then resuspended in 1X NuPAGE sample buffer (Invitrogen, NP0007). The supernatant was further centrifuged at 20,000x g to obtain the cytosolic fraction. Proteins were separated by SDS-PAGE, transferred to nitrocellulose and immunoblotted with an antibodies against Bnip3 (1:1,000, Sigma B7931), LC3 (1:1,000, Cell Signaling Tech., 4108), Tubulin (1:1,000, Sigma T6074) or Actin (1:1,000, Sigma A4700).
Transmission electron microscopy.
Adult cardiac myocytes were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, post fixed in 1% osmium tetroxide and then treated with 0.5% tannic acid, 1% sodium sulfate, cleared in 2-hydroxypropyl methacrylate and embedded in LX112 (Ladd Research, Williston, VT). Thin sections were cut with a diamond knife (Diatome, Hatfield, PA), mounted on copper slot grids coated with parlodion and stained with uranyl acetate and lead citrate for examination on a Philips CM100 electron microscope (FEI, Hillsbrough, OR). Images were documented using a Megaview III CCD camera (Olympus Soft Imaging Solutions, Lakewood, CO) and then handled in Adobe Photoshop.
Statistical analysis.
All values are expressed as means ± Standard Deviation (S.D). ANOVA was performed to identify statistical significance in multiple-group comparisons and Student's t-test was used to evaluate significance between two experimental conditions. A p < 0.05 was considered to be statistically significant.
Acknowledgements
This work was supported by NIH HL087023 to Å.B.G. and a Post-Baccalaureate Research Supplement from NHLBI to M.N.Q.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13005
References
- 1.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 2.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 3.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 4.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29487. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 5.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a Protective Response to Bnip3-Mediated Apoptotic Signaling in the Heart. Autophagy. 2006;2:307–309. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- 8.Burton TR, Gibson SB. The role of Bcl-2 family member BNIP3 in cell death and disease: NIPping at the heels of cell death. Cell Death Differ. 2009;16:515–523. doi: 10.1038/cdd.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of bnip3 death pathways by calcium, phosphorylation and hypoxia-reoxygenation. Antioxid Redox Signal. 2007;9:1309–1316. doi: 10.1089/ars.2007.1726. [DOI] [PubMed] [Google Scholar]
- 10.Kubli DA, Ycaza JE, Gustafsson AB. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007;405:407–415. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vande VC, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. MolCell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 14.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295:H2025–H2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–548. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 19.Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. Febs J. 2007;274:3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 22.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aucello M, Dobrowolny G, Musaro A. Localized accumulation of oxidative stress causes muscle atrophy through activation of an autophagic pathway. Autophagy. 2009;5:527–529. doi: 10.4161/auto.5.4.7962. [DOI] [PubMed] [Google Scholar]
- 24.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 25.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 26.Oakes SA, Lin SS, Bassik MC. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr Mol Med. 2006;6:99–109. doi: 10.2174/156652406775574587. [DOI] [PubMed] [Google Scholar]
- 27.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinsay MN, Lee Y, Rikka S, Sayen MR, Molkentin JD, Gottlieb RA, Gustafsson AB. Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J Mol Cell Cardiol. 2010;48:1146–1156. doi: 10.1016/j.yjmcc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Qi H, Taylor R, Xu W, Liu LF, Jin S. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–346. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Hernandez A, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P, et al. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 33.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6:462–472. doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 36.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, et al. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 39.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, Jr, Gottlieb RA. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104:157–167. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustafsson AB, Tsai JG, Logue SE, Crow MT, Gottlieb RA. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J Biol Chem. 2004;279:21233–21238. doi: 10.1074/jbc.M400695200. [DOI] [PubMed] [Google Scholar]