Abstract
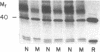
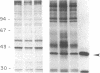
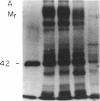
We investigated regulation of cardiac adenylate cyclase in 29-d-old BIO 14.6 Syrian hamsters, which inherit cardiomyopathy as an autosomal recessive trait. Pharmacologic stimulation of adenylate cyclase in cardiac membranes with isoproterenol, fluoride ion, guanine nucleotide, forskolin, and manganous ion indicated that there was defective coupling of the guanine nucleotide-binding protein that stimulates adenylate cyclase (Gs) to adenylate cyclase. Cyc complementation assays revealed congruent to 50% less Gs activity in cardiac and skeletal muscle from cardiomyopathic hamsters. Despite this decrease in functional Gs, there were no changes in immunologic levels of the alpha-subunit of Gs (alpha Gs) or in levels of mRNA encoding alpha Gs. The defect in Gs bioactivity was limited to cardiac and skeletal muscle, occurred only in animals homozygous for the dystrophic trait, and was demonstrable before any cardiac abnormalities were evident on light microscopy. By contrast, cardiac levels of beta-adrenergic receptors were not different in cardiac membranes from BIO 14.6 hamsters. We conclude that a functional defect in alpha Gs may contribute to a contractile abnormalities in the cardiomyopathic BIO 14.6 hamster. However, the etiology of the alpha Gs defect remains obscure.
Full text
PDF

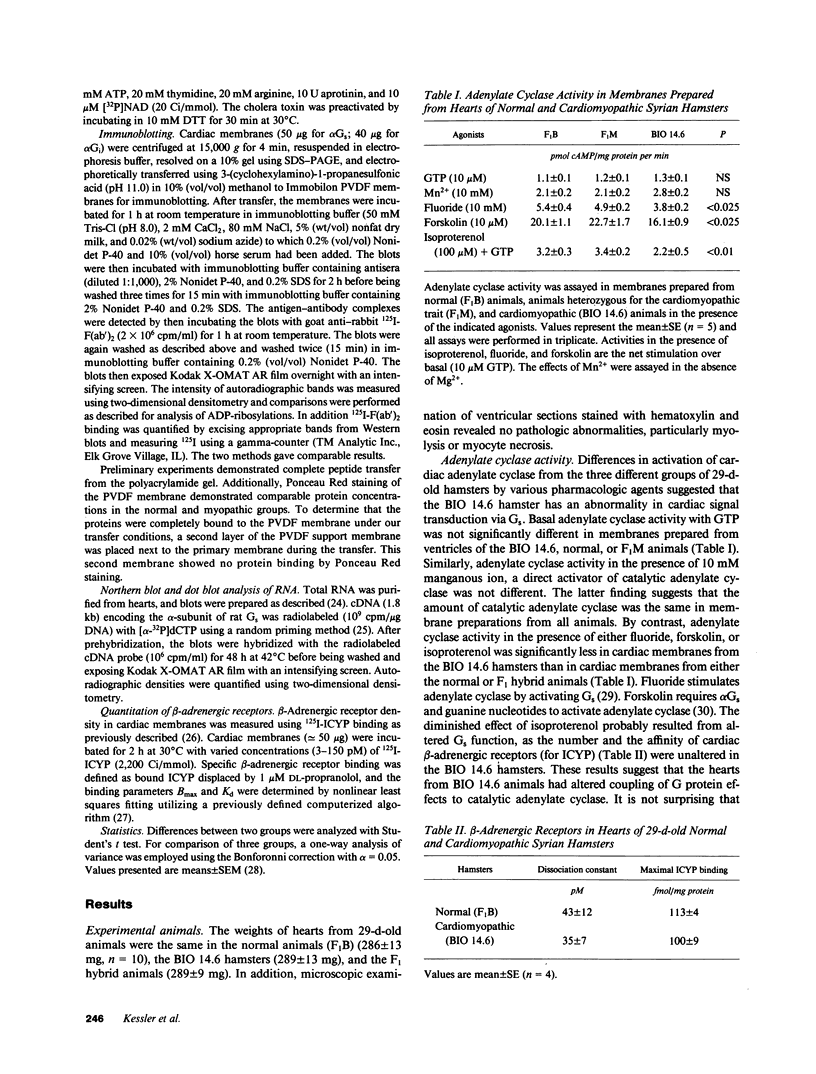
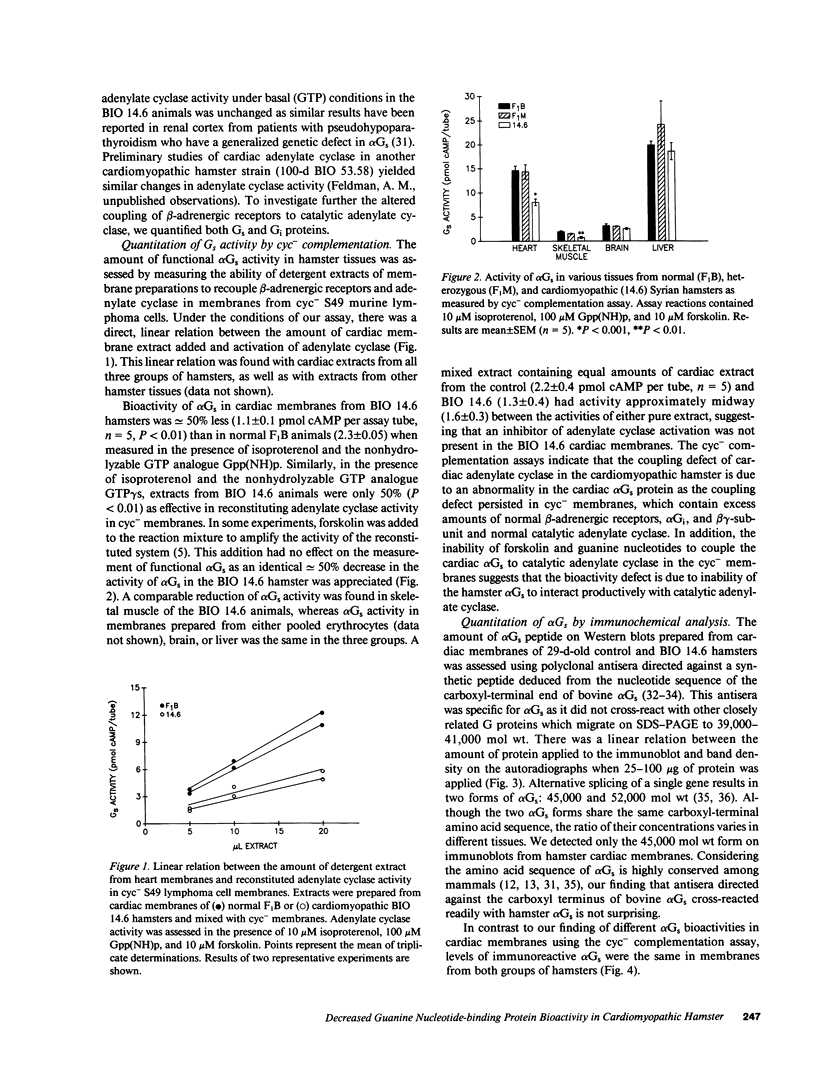


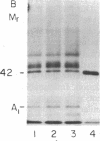
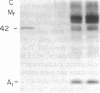
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajusz E. Hereditary cardiomyopathy: a new disease model. Am Heart J. 1969 May;77(5):686–696. doi: 10.1016/0002-8703(69)90556-0. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Garbers D. L., Domino S. E., Noland T. D., Van Dop C. Spermatozoa contain a guanine nucleotide-binding protein ADP-ribosylated by pertussis toxin. Biochem Biophys Res Commun. 1986 Jul 31;138(2):728–734. doi: 10.1016/s0006-291x(86)80557-5. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975 Feb 28;187(4178):750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- Bray P., Carter A., Simons C., Guo V., Puckett C., Kamholz J., Spiegel A., Nirenberg M. Human cDNA clones for four species of G alpha s signal transduction protein. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8893–8897. doi: 10.1073/pnas.83.23.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink A. J., Lochner A. Work performance of the isolated perfused beating heart in the hereditary myocardiopathy of the Syrian hamster. Circ Res. 1967 Sep;21(3):391–401. doi: 10.1161/01.res.21.3.391. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Strosberg A., Montgomery W., Minobe W. Pharmacology and inotropic potential of forskolin in the human heart. J Clin Invest. 1984 Jul;74(1):212–223. doi: 10.1172/JCI111404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Umans V., Fowler M., Minobe W., Rasmussen R., Zera P., Menlove R., Shah P., Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986 Sep;59(3):297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Drezner M. K., Burch W. M., Jr Altered activity of the nucleotide regulatory site in the parathyroid hormone-sensitive adenylate cyclase from the renal cortex of a patient with pseudohypoparathyroidism. J Clin Invest. 1978 Dec;62(6):1222–1227. doi: 10.1172/JCI109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. I., Severson D. L. Cyclic nucleotides and cardiac function. Circ Res. 1979 Feb;44(2):145–153. doi: 10.1161/01.res.44.2.145. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Cates A. E., Veazey W. B., Hershberger R. E., Bristow M. R., Baughman K. L., Baumgartner W. A., Van Dop C. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest. 1988 Jul;82(1):189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman A. M., Levine M. A., Baughman K. L., Van Dop C. NAD+-mediated stimulation of adenylate cyclase in cardiac membranes. Biochem Biophys Res Commun. 1987 Feb 13;142(3):631–637. doi: 10.1016/0006-291x(87)91461-6. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Levine M. A., Gerstenblith G., Kaufman K. D., Baughman K. L. Negative inotropic effects of furosemide in the isolated rabbit heart: a prostaglandin-mediated event. J Cardiovasc Pharmacol. 1987 Apr;9(4):493–499. doi: 10.1097/00005344-198704000-00015. [DOI] [PubMed] [Google Scholar]
- Franch H. A., Dixon R. A., Blaine E. H., Siegl P. K. Ventricular atrial natriuretic factor in the cardiomyopathic hamster model of congestive heart failure. Circ Res. 1988 Jan;62(1):31–36. doi: 10.1161/01.res.62.1.31. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Homburger F. Myopathy of hamster dystrophy: history and morphologic aspects. Ann N Y Acad Sci. 1979;317:1–17. [PubMed] [Google Scholar]
- Hoppel C. L., Tandler B., Parland W., Turkaly J. S., Albers L. D. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982 Feb 10;257(3):1540–1548. [PubMed] [Google Scholar]
- Jasmin G., Proschek L. Paradoxical effect of isoproterenol on hamster hereditary polymyopathy. Muscle Nerve. 1983 Jul-Aug;6(6):408–415. doi: 10.1002/mus.880060603. [DOI] [PubMed] [Google Scholar]
- Jasmin G., Solymoss B. Prevention of hereditary cardiomyopathy in the hamster by verapamil and other agents. Proc Soc Exp Biol Med. 1975 May;149(1):193–198. doi: 10.3181/00379727-149-38771. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Bourne H. R. Influence of cholera toxin on the regulation of adenylate cyclase by GTP. Biochem Biophys Res Commun. 1977 Sep 23;78(2):792–798. doi: 10.1016/0006-291x(77)90249-2. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984 May 25;259(10):6228–6234. [PubMed] [Google Scholar]
- Kaslow H. R., Johnson G. L., Brothers V. M., Bourne H. R. A regulatory component of adenylate cyclase from human erythrocyte membranes. J Biol Chem. 1980 Apr 25;255(8):3736–3741. [PubMed] [Google Scholar]
- Kozasa T., Itoh H., Tsukamoto T., Kaziro Y. Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Longabaugh J. P., Vatner D. E., Vatner S. F., Homcy C. J. Decreased stimulatory guanosine triphosphate binding protein in dogs with pressure-overload left ventricular failure. J Clin Invest. 1988 Feb;81(2):420–424. doi: 10.1172/JCI113335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossnitzer K., Janke J., Hein B., Stauch M., Fleckenstein A. Disturbed myocardial calcium metabolism: a possible pathogenetic factor in the hereditary cardiomyopathy of the Syrian hamster. Recent Adv Stud Cardiac Struct Metab. 1975;6:207–217. [PubMed] [Google Scholar]
- Malhotra A., Karell M., Scheuer J. Multiple cardiac contractile protein abnormalities in myopathic Syrian hamsters (BIO 53 : 58). J Mol Cell Cardiol. 1985 Feb;17(2):95–107. doi: 10.1016/s0022-2828(85)80013-4. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Isolation of an avian erythrocyte protein possessing ADP-ribosyltransferase activity and capable of activating adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3621–3624. doi: 10.1073/pnas.75.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S., Pang I. H., Gilman A. G., Sternweis P. C. Chromatographic resolution and immunologic identification of the alpha 40 and alpha 41 subunits of guanine nucleotide-binding regulatory proteins from bovine brain. J Biol Chem. 1988 Feb 5;263(4):2020–2026. [PubMed] [Google Scholar]
- Reichenbach D. D., Benditt E. P. Myofibrillar degeneration. A response of the myocardial cell to injury. Arch Pathol. 1968 Feb;85(2):189–199. [PubMed] [Google Scholar]
- Robishaw J. D., Russell D. W., Harris B. A., Smigel M. D., Gilman A. G. Deduced primary structure of the alpha subunit of the GTP-binding stimulatory protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1251–1255. doi: 10.1073/pnas.83.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]
- Rouleau J. L., Chuck L. H., Hollosi G., Kidd P., Sievers R. E., Wikman-Coffelt J., Parmley W. W. Verapamil preserves myocardial contractility in the hereditary cardiomyopathy of the Syrian hamster. Circ Res. 1982 Mar;50(3):405–412. doi: 10.1161/01.res.50.3.405. [DOI] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobeck J. E., Factor S. M., Bhan A., Sole M., Liew C. C., Fein F., Sonnenblick E. H. Hereditary and acquired cardiomyopathies in experimental animals: mechanical, biochemical, and structural features. Ann N Y Acad Sci. 1979;317:59–88. doi: 10.1111/j.1749-6632.1979.tb56511.x. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dop C., Bourne H. R. Pseudohypoparathyroidism. Annu Rev Med. 1983;34:259–266. doi: 10.1146/annurev.me.34.020183.001355. [DOI] [PubMed] [Google Scholar]
- Van Dop C., Tsubokawa M., Bourne H. R., Ramachandran J. Amino acid sequence of retinal transducin at the site ADP-ribosylated by cholera toxin. J Biol Chem. 1984 Jan 25;259(2):696–698. [PubMed] [Google Scholar]
- Van Vleet J. F., Ferrans V. J. Myocardial diseases of animals. Am J Pathol. 1986 Jul;124(1):98–178. [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., Reynolds I. J., Weisman H. F., Dudeck P., Weisfeldt M. L., Snyder S. H. Calcium antagonist receptors in cardiomyopathic hamster: selective increases in heart, muscle, brain. Science. 1986 Apr 25;232(4749):515–518. doi: 10.1126/science.3008330. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Whitmer J. T., Kumar P., Solaro R. J. Calcium transport properties of cardiac sarcoplasmic reticulum from cardiomyopathic Syrian hamsters (BIO 53.58 and 14.6): evidence for a quantitative defect in dilated myopathic hearts not evident in hypertrophic hearts. Circ Res. 1988 Jan;62(1):81–85. doi: 10.1161/01.res.62.1.81. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Van Dop C., Neer E. J., Snyder S. H. Go, a guanine nucleotide-binding protein: immunohistochemical localization in rat brain resembles distribution of second messenger systems. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4561–4565. doi: 10.1073/pnas.83.12.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Codina J., Hamilton S. L., Brown A. M., Birnbaumer L. The stimulatory G protein of adenylyl cyclase, Gs, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem. 1988 Jul 15;263(20):9887–9895. [PubMed] [Google Scholar]