Abstract
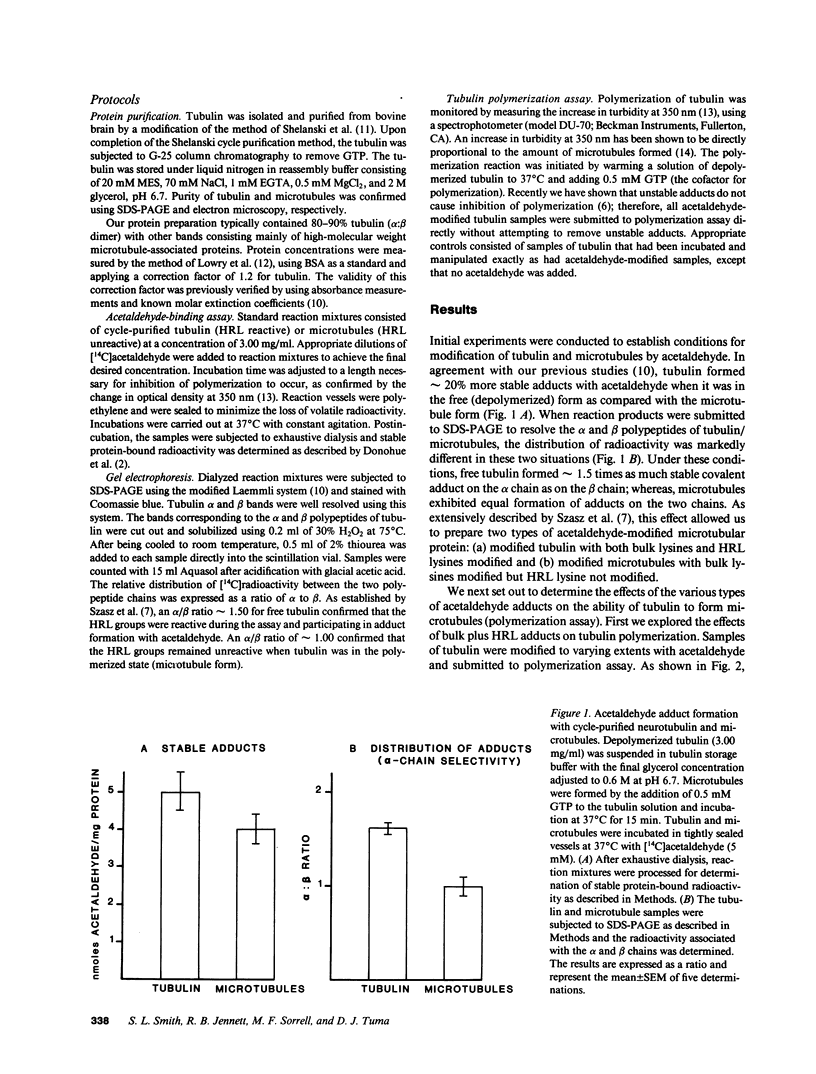
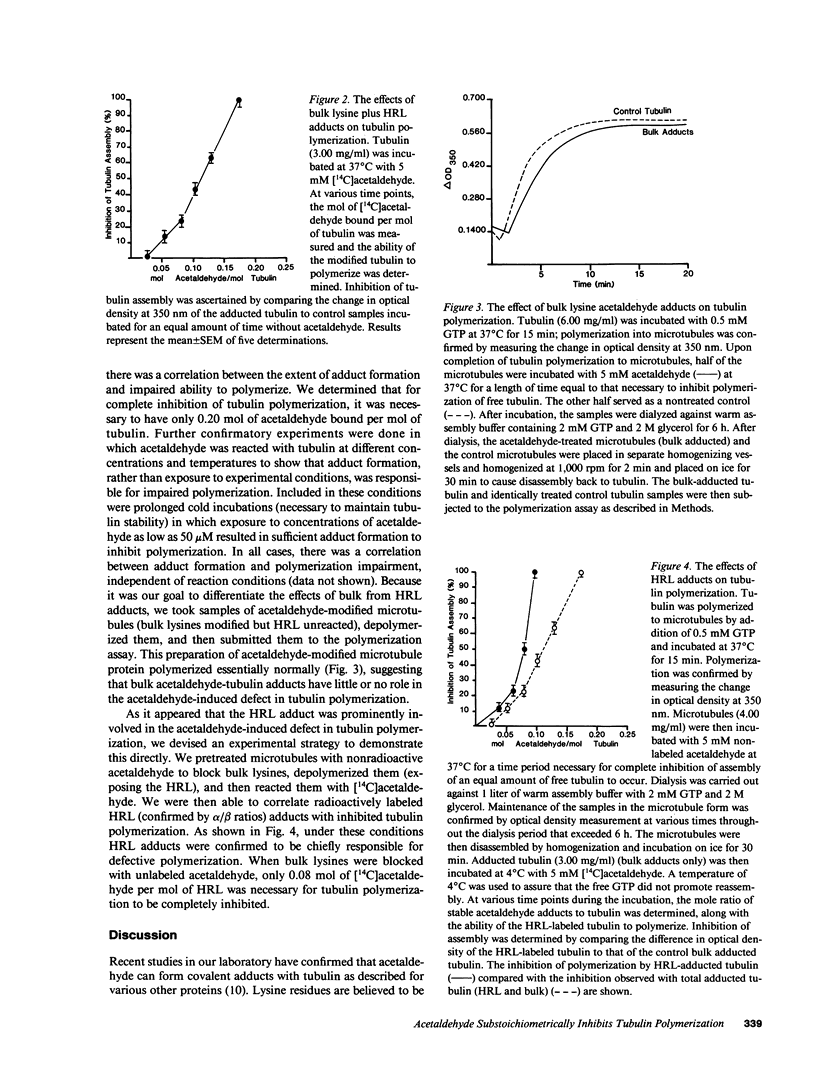
Acetaldehyde is known to form covalent adducts with tubulin and to inhibit microtubule formation. Available evidence indicates that lysine residues are prominently involved in adduct formation. Previous work has shown that lysines on tubulin can be divided into two general classes based upon their reactivity toward acetaldehyde; those of normal reactivity ("bulk" lysines) and a highly reactive lysine (HRL) located on the alpha-polypeptide subunit. We took advantage of the fact that the HRL is unreactive when tubulin is in the microtubule form to differentiate the effects of bulk from HRL adducts on tubulin polymerization. Under conditions where both bulk lysines and HRL formed adducts, 0.2 mol acetaldehyde/mol tubulin caused complete inhibition of polymerization. When we modified bulk lysines, but not HRL, tubulin polymerized essentially normally. Finally, when we first blocked bulk lysines on microtubules (HRL unreactive) using unlabeled acetaldehyde and then measured the amount of [14C]acetaldehyde adduct formed with tubulin after depolymerization (HRL reactive), 0.08 mol acetaldehyde/mol tubulin resulted in completely impaired polymerization. These data show that microtubule formation is very sensitive to even small mole fractions of acetaldehyde-modified tubulin (especially with HRL) and further suggest that small amounts of acetaldehyde adduct could be damaging to cytoskeleton function in the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donohue T. M., Jr, Tuma D. J., Sorrell M. F. Acetaldehyde adducts with proteins: binding of [14C]acetaldehyde to serum albumin. Arch Biochem Biophys. 1983 Jan;220(1):239–246. doi: 10.1016/0003-9861(83)90406-x. [DOI] [PubMed] [Google Scholar]
- Donohue T. M., Jr, Tuma D. J., Sorrell M. F. Binding of metabolically derived acetaldehyde to hepatic proteins in vitro. Lab Invest. 1983 Aug;49(2):226–229. [PubMed] [Google Scholar]
- Jennett R. B., Sorrell M. F., Johnson E. L., Tuma D. J. Covalent binding of acetaldehyde to tubulin: evidence for preferential binding to the alpha-chain. Arch Biochem Biophys. 1987 Jul;256(1):10–18. doi: 10.1016/0003-9861(87)90420-6. [DOI] [PubMed] [Google Scholar]
- Jennett R. B., Sorrell M. F., Saffari-Fard A., Ockner J. L., Tuma D. J. Preferential covalent binding of acetaldehyde to the alpha-chain of purified rat liver tubulin. Hepatology. 1989 Jan;9(1):57–62. doi: 10.1002/hep.1840090109. [DOI] [PubMed] [Google Scholar]
- Jennett R. B., Tuma D. J., Sorrell M. F. Effect of ethanol and its metabolites on microtubule formation. Pharmacology. 1980;21(5):363–368. doi: 10.1159/000137453. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. C., Samson F. E., Jr, Houston L. L., Himes R. H. The in vitro polymerization of tubulin from beef brain. J Neurobiol. 1974;5(4):317–330. doi: 10.1002/neu.480050404. [DOI] [PubMed] [Google Scholar]
- Lin R. C., Smith R. S., Lumeng L. Detection of a protein-acetaldehyde adduct in the liver of rats fed alcohol chronically. J Clin Invest. 1988 Feb;81(2):615–619. doi: 10.1172/JCI113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon G., de Jersey J., Shanley B., Ward L. The reaction of acetaldehyde with brain microtubular proteins: formation of stable adducts and inhibition of polymerization. Neurosci Lett. 1987 Aug 18;79(1-2):163–168. doi: 10.1016/0304-3940(87)90690-2. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G., Rosenberry T. L., Sternlicht H. Identification of lysine residues essential for microtubule assembly. Demonstration of enhanced reactivity during reductive methylation. J Biol Chem. 1983 Feb 25;258(4):2148–2156. [PubMed] [Google Scholar]
- Sorrell M. F., Tuma D. J. Hypothesis: alcoholic liver injury and the covalent binding of acetaldehyde. Alcohol Clin Exp Res. 1985 Jul-Aug;9(4):306–309. doi: 10.1111/j.1530-0277.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Fantl W. J., Newman C. B., Sims R. V., Cerami A., Peterson C. M. Acetaldehyde adducts with hemoglobin. J Clin Invest. 1981 Feb;67(2):361–369. doi: 10.1172/JCI110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz J., Burns R., Sternlicht H. Effects of reductive methylation on microtubule assembly. Evidence for an essential amino group in the alpha-chain. J Biol Chem. 1982 Apr 10;257(7):3697–3704. [PubMed] [Google Scholar]
- Szasz J., Yaffe M. B., Elzinga M., Blank G. S., Sternlicht H. Microtubule assembly is dependent on a cluster of basic residues in alpha-tubulin. Biochemistry. 1986 Aug 12;25(16):4572–4582. doi: 10.1021/bi00364a018. [DOI] [PubMed] [Google Scholar]
- Tuma D. J., Jennett R. B., Sorrell M. F. The interaction of acetaldehyde with tubulin. Ann N Y Acad Sci. 1987;492:277–286. doi: 10.1111/j.1749-6632.1987.tb48681.x. [DOI] [PubMed] [Google Scholar]
- Tuma D. J., Newman M. R., Donohue T. M., Jr, Sorrell M. F. Covalent binding of acetaldehyde to proteins: participation of lysine residues. Alcohol Clin Exp Res. 1987 Dec;11(6):579–584. doi: 10.1111/j.1530-0277.1987.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Tuma D. J., Sorrell M. F. Effects of ethanol on protein trafficking in the liver. Semin Liver Dis. 1988 Feb;8(1):69–80. doi: 10.1055/s-2008-1040529. [DOI] [PubMed] [Google Scholar]


