Abstract
While polar organelles hold the key to understanding the fundamentals of cell polarity and cell biological principles in general, they have served in the past merely for taxonomical purposes. Here, we highlight recent efforts in unraveling the molecular basis of polar organelle positioning in bacterial cells. Specifically, we detail the role of members of the Ras-like GTPase superfamily and coiled-coil-rich scaffolding proteins in modulating bacterial cell polarity and in recruiting effector proteins to polar sites. Such roles are well established for eukaryotic cells, but not for bacterial cells that are generally considered diffusion-limited. Studies on spatial regulation of protein positioning in bacterial cells, though still in their infancy, will undoubtedly experience a surge of interest, as comprehensive localization screens have yielded an extensive list of (polarly) localized proteins, potentially reflecting subcellular sites of functional specialization predicted for organelles.
Ras-like GTPases, scaffold proteins, and the actin relative MreB control bacterial cell polarity and organelle positioning by targeting proteins to specific subcellular locations.
Since the first electron micrographs that revealed flagella at the cell poles of bacteria, we have known that bacterial cells are polarized and that they are able to decode the underlying positional information to confine the assembly of an extracellular organelle to a polar cellular site (Fig. 1). Foraging into this unknown territory has been challenging, but recent efforts that exploit the power of bacterial genetics along with modern imaging methods to visualize proteins in the minute bacterial cells has yielded several enticing entry points to dissect polarity-based mechanisms and explore potentially contributing subdiffusive characteristics (Golding and Cox 2006).
Figure 1.
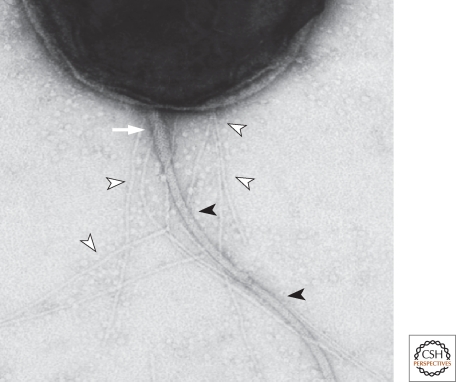
Transmission electron micrograph (taken by Jeff Skerker) of a Caulobacter crescentus swarmer cell showing the polar pili (empty arrowheads), the polar flagellum with the flagellar filament (filled arrowheads), and the hook (white arrow) (see Fig. 2A).
While polar organelles are a visual manifestation of polarity, it is important to point out that polarity can also be inherent to cells, at least in molecular terms, even in the absence of discernible polar structures. In other words, molecular anatomy can reveal that a bacterial cell, such as an Escherichia coli cell, features specialized protein complexes at or near the poles, despite a perfectly symmetrical morphology (Maddock and Shapiro 1993; Lindner et al. 2008). Such systemic polarization in bacteria, likely stemming from the distinctive division history of each pole, has the potential to be widespread and to be exploited for positioning of polar organelles and protein complexes. As excellent reviews have been published detailing the interplay between cell polarity and protein localization (Dworkin 2009; Shapiro et al. 2009; Kaiser et al. 2010; Rudner and Losick 2010), here we focus on recent progress in understanding the function and localization of spatial regulators of polar organelles. Considering that the ever-growing list of polar protein complexes emerging from systematic and comprehensive localization studies (Kitagawa et al. 2005; Russell and Keiler 2008; Werner et al. 2009; Hughes et al. 2010) is suggestive of multiple polarly confined (organelle-like) functions, understanding their spatial regulation is also of critical relevance in the realm of medical bacteriology, as many virulence determinants also underlie polarity (Goldberg et al. 1993; Scott et al. 2001; Judd et al. 2005; Jain et al. 2006; Jaumouille et al. 2008; Carlsson et al. 2009). Below, we highlight a few prominent examples of overtly polar organelles and the proteins known to date that regulate their polar positioning.
THE POLARITY OF MOTILITY ORGANELLES
Flagellar Motility: FlhFG
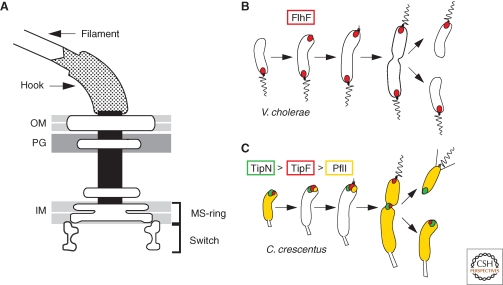
The Ras-like GTPase FlhF is required for proper assembly and placement of the polar flagellum in several bacterial lineages, including Vibrio, Pseudomonas, Helicobacter, and Campylobacter species (Pandza et al. 2000; Niehus et al. 2004; Murray and Kazmierczak 2006; Kusumoto et al. 2008; Balaban et al. 2009; Green et al. 2009). Flagellum biogenesis occurs through an “inside-out” process by which components of the early structure first assemble a platform on the cytoplasmic membrane (the MS-ring) that will facilitate the subsequent polymerization and growth of the trans-envelope structures through the periplasm (the flagellar rod), the peptidoglycan (P-ring), and outer membrane (L-ring), ultimately to enable assembly of the extracellular hook and flagellar filament (Macnab 2003) (Fig. 2A). FlhF mutants often exhibit a reduced ability to assemble flagella and in the case of Vibrio cholerae this seems to be attributable to a requirement of FlhF for the polar recruitment of the flagellar baseplate (MS-ring) protein FliF (Green et al. 2009). In the absence of FlhF, FliF is dispersed from the poles and flagella are formed at a reduced frequency and at aberrant (nonpolar) locations. Conversely, functional FlhF-GFP is localized to the flagellated pole in V. cholerae and Campylobacter jejuni (Murray and Kazmierczak 2006; Ewing et al. 2009; Green et al. 2009) independently of flagellar structural proteins (Ewing et al. 2009; Green et al. 2009) (Fig. 2B). Thus, FlhF plays a role in both flagellum positioning and assembly, and the latter function may in part be mediated at the level of gene expression (Niehus et al. 2004; Correa et al. 2005). A member of the (SIMIBI) Ras-like GTPase superfamily, FlhF associates peripherally with the cytoplasmic membrane (Green et al. 2009) and exhibits extensive sequence homology to the signal recognition particle (SRP) component Ffh, a GTPase that mediates the co-translational insertion of membrane proteins (Driessen and Nouwen 2008). This event requires a heteromeric interaction of Ffh with its receptor, the FtsY GTPase. A truncated fragment of Bacillus subitilis FlhF has been crystallized as a dimer, forming a composite active site in which the two GTP molecules are arranged in a head-to-tail configuration (Bange et al. 2007). While this same configuration in the composite active site of the Ffh-FtsY heterodimer induces GTP hydrolysis, this is not the case for B. subtilis FlhF. However, recent in vitro assays with recombinant full-length FlhF from C. jejuni harboring an N-terminal His6-tag exhibited GTPase and comparatively low ATPase activity (Balaban et al. 2009). Point mutations that cripple the GTPase activity of C. jejuni FlhF result in flagellar placement defects that resemble the phenotypes observed for the flhF deletion mutant of V. cholerae, Pseudomonas putida, and Pseudomonas aeruginosa (Pandza et al. 2000; Murray and Kazmierczak 2006; Balaban et al. 2009; Green et al. 2009). Moreover, FlhF with a mutation in the conserved nucleotide-binding P-loop (K295A) has a reduced ability to bind GTP and is unable to support motility in V. cholerae (Green et al. 2009). These findings indicate a link between flagellar positioning and the ability to bind and/or hydrolyze GTP. The FlhF polypetide is sequentially subdivided into the B-N-G regions. The conserved GTPase domain of FlhF resides in the C-terminal G-region. The G-region is preceded by the central N-region that is rich in α-helices, while the basic and unstructured (protease-sensitive) B-region is located at the N-terminus of FlhF (Bange et al. 2007). Unlike the G-region, the B- and N-regions are quite variable in length among FlhF orthologs, raising the possibility that these regions account for the structural and functional differences observed in vitro and in vivo. In fact, the N-region included in the crystal structure of B. subtilis FlhF is significantly shorter than that from FlhF encoded in the genomes of polarly flagellated bacteria. Deletion analysis in V. cholerae pointed to a key role for the N-region in polar localization of FlhF (Green et al. 2009). FlhF-GFP deleted for the N-region was not localized to the pole and unable to confer motility. The N-region could support polar localization, but not motility, when fused to either the B region (i.e., as BN-GFP fusion) or the G-region (i.e., as NG-GFP fusion). However, the N-region alone (i.e., as N-GFP construct) was unable to direct GFP to the pole (Green et al. 2009). It is conceivable that an association with the membrane is a prerequisite for FlhF to recognize localization determinant(s) that may reside in the envelope, as is the case for other polarly localized proteins (Jacobs et al. 1999; Boyd 2000). If so, the N-region might be required for this membrane association, while the B- and G-regions recognize the localization determinant(s). Perhaps one region is used for polar targeting, while the other region functions as polar retention domain. This would imply that the mechanism and targets for the polar localization for BN-GFP and NG-GFP is different. In support of the findings with truncated FlhF derivatives that are localized but not functional, full length FlhF-GFP with the GTP-binding K295A mutation did not support motility either, while still allowing polar recruitment, indicating that bound GTP is not a prerequisite for polar localization (Green et al. 2009). The identity of the polar determinant(s) recognized by FlhF is not known, but it is not a structural component of the flagellum as FlhF-GFP is still at the pole in Vibrio mutants that do not express any flagellar subunits (Kusumoto et al. 2008; Green et al. 2009). It would be interesting to examine whether the BN-GFP and NG-GFP constructs are still polar in the absence of the flagellar components to determine whether each of the FlhF subdomains relies on flagellar parts for polar recruitment and/or retention. The principal determinant for FlhF localization appears to be widely conserved across bacterial species, since FlhF-GFP from V. cholerae is also polar in E. coli (Green et al. 2009). Thus, even a peritrichously-flagellated bacterium such as E. coli encodes a positional signal that is recognized by the B- and/or G-region of FlhF. Interestingly, FlhF is localized to both poles in E. coli, but in the native host FlhF-GFP was also seen in a predominantly unipolar localization pattern (Murray and Kazmierczak 2006; Kusumoto et al. 2008; Green et al. 2009), suggesting that a specific mechanism exists to impart polar specificity on FlhF localization and, thus, the new flagellum assembly site.
Figure 2.
Spatial regulation of the polar flagellum (A) by the FlhF GTPase in Vibrio cholerae (B) and by the TipF c-di-GMP receptor protein, the TipN birth scar protein, and the PflI positioning factor in Caulobacter crescentus (C). In Panel A, the outer membrane, peptidoglycan layer, and inner membrane are abbreviated by OM, PG, and IM, respectively. In Panels B and C, the wavy and straight lines denote the flagellum and the pili, respectively.
Although FlhF may regulate the correct positioning of the core flagellum assembly platform in the cytoplasmic membrane through FliF localization, recent work in C. jejuni has unearthed flagellar glycosylation pathway components that are localized to the cell pole independently of FlhF (Ewing et al. 2009). In many polarly flagellated bacteria, the flagellar filament is polymerized from glycosylated flagellins. Glycosylation seems to precede the secretion of the flagellin (Ewing et al. 2009), yet its general function is not understood. Many components of the glycosylation pathway are dispensable for motility. This may be attributable to a certain redundancy in glycosylation components, as several polarly flagellated bacteria of the genera Campylobacter, Helicobacter, and Caulobacter are unable to polymerize a flagellar filament and unable to secrete unglycosylated flagellins when other glycosylation proteins are inactivated (Leclerc et al. 1998; Goon et al. 2003; Schirm et al. 2003). Thus, glycosylation seems to fulfill an important and mysterious role in the assembly of polar flagella. Pertinent to the tentative link between polar flagellation and glycosylation, the components of the glycosylation machinery PseC and PseE are localized to the pole in C. jejuni (Ewing et al. 2009). Although FlhF is also at the cell pole in C. jejuni, it is not required for the polar positioning of PseC and PseE. In support of this, PseC and PseE are also still at the poles in the absence of the flagellar export apparatus. Thus, a FlhF-independent flagellar localization pathway exists that remains to be uncovered (Ewing et al. 2009).
FlhF is also found in peritrichously flagellated bacteria and an investigation on FlhF in Bacillus cereus determined that inactivation of FlhF reduced flagellation and resulted in a bias toward polar flagella from a normal peritrichous flagellar arrangement (Salvetti et al. 2007). While the function of FlhF in flagellum biogenesis does not appear to be specialized for polar flagellation, certain bacteria may have appropriated FlhF to recruit flagellar components to the pole and, therefore, to confine the assembly of the flagellar structure to a polar site, simply by directing FlhF to the pole. In other cases, such as B. cereus, FlhF may be localized to nonpolar sites to obtain a peritrichous flagellar disposition, implying an underlying default pathway for polar flagellation in B. cereus that FlhF acts upon to guide flagellar assembly factors to lateral sites.
The mechanism(s) of spatial regulation of FlhF likely holds the key to understanding the FlhF-dependent polar flagellation in Vibrios, Pseudomads, and others. Circumstantial evidence has implicated FlhG (also known as FleN in Pseudomonas spp.), which resembles the ParA/MinD superfamily of ATPases (cd01983) that feature a deviant Walker A-type nucleotide-binding pocket (the P-loop), in the regulation of the polar localization of FlhF (Kusumoto et al. 2008). MinD and ParA adopt key roles as part of positioning systems that direct the proper placement of the cytokinetic organelle or (chromosomal or plasmid) DNA, respectively, within the prokaryotic cell or the eukaryotic chloroplasts (de Boer et al. 1991; Margolin 2005; Gerdes et al. 2010; Thanbichler 2010). In the absence of FlhG, the fluorescent cluster of FlhF-GFP at the cell pole was brighter than when FlhG was present (Kusumoto et al. 2008). While this result suggested that the polar localization of FlhF is improved in FlhG-deficient cells, they also had higher steady-state levels of FlhF. Nevertheless, the diffuse background fluorescence of FlhF-GFP in the cytoplasm was also reduced in cells lacking FlhG, pointing toward a role of FlhG in interference with the polar sequestration of FlhF (Kusumoto et al. 2008). Interestingly, mutant forms of FlhF have been identified that show the opposite phenotype of the FlhG-deficient cells (i.e., strong dependency on the presence of FlhG for polar localization), and GFP-tagged FlhG is also polar (Kusumoto et al. 2009). These observations are consistent with the proposed role of the ParA/MinD ATPases acting as binary regulatory switches that control the localization of cellular constituents, a recurring theme throughout this article. Below we outline the role of other ParA/MinD-homologs in the localization of organellar components, including that of a chemosensory complex (Thompson et al. 2006), a pilus biogenesis apparatus (Viollier et al. 2002a), a DNA transfer machine (Atmakuri et al. 2007), and a polysaccharide export system (Le Quere and Ghigo 2009).
Flagellar Motility: TipNF-PflI
While FlhG may be a key element for the spatial regulation of FlhF, many polarly flagellated bacterial lineages, primarily from the α-proteobacteria, do not encode FlhF in their genomes. Thus, nature has devised another path to polar flagellation, one that seems to involve a conserved positioning protein, PflI. PflI was identified using a bioinformatic approach and then characterized in the vibroid-shaped bacterium Caulobacter crescentus (Obuchowski and Jacobs-Wagner 2007). In C. crescentus, the flagellum is always built at the new pole (i.e., the pole that emerged from the most recent division event). PflI, a bitopic membrane protein with a cytoplasmic coiled-coil domain followed by a proline-rich stretch of 102 residues, is localized to the future flagellated pole well before the flagellar proteins are expressed. Moreover, PflI is still localized to the new pole in the absence of the MS-ring protein FliF, indicating that, akin to FlhF, PflI does not depend on an intact flagellar structure for polar localization. Importantly, inactivation or overexpression of PflI results in a high proportion of cells with misplaced flagella, while not affecting the localization of polar marker proteins; for example, that of the pilus assembly machinery (see below) or signaling proteins that normally colocalize with the flagellum. Thus, PflI seems to function as a positioning factor specifically for the flagellum. How PflI influences the flagellum positioning is unknown, but it is likely that additional localization pathways work in concert with PflI. This conjecture is based on the finding that in PflI-deficient or -overproducing cells, the flagellum misplacement is not as pervasive, with 30–60% of the flagellated cells still having a polar flagellum (Obuchowski and Jacobs-Wagner 2007). Progress has recently been made in understanding how PflI localization is regulated (Davis et al. 2010, in prep). As hinted by the similar localization pattern during the cell cycle (Fig. 2C) and in flagellar mutants, the recruitment of PflI to the future flagellated pole depends on the EAL-domain protein TipF (Huitema et al. 2006; Obuchowski and Jacobs-Wagner 2007). A polytopic membrane protein previously known to regulate flagellar assembly and implicated in flagellar positioning, TipF is also localized to the new pole even in the absence of early flagellar structural components such as the FliF MS-ring protein (Huitema et al. 2006) (Davis et al. 2010, in prep). The cytoplasmic EAL-domain is responsible for TipF function in flagellum assembly and the recruitment of PflI to the pole (Huitema et al. 2006). In related proteins, the EAL-domain is responsible for the binding and subsequent hydrolysis of cyclic-di-GMP, a signaling molecule that drives the switch from motility to sessility in eubacteria. (Jenal and Malone 2006; Hengge 2009). The EAL domain can also act as c-di-GMP binding domain or protein–protein interaction domain in the absence of hydrolytic activity (Newell et al. 2009; Tschowri et al. 2009). The TipF EAL-domain also lacks c-di-GMP hydrolytic activity, but has retained the ability to bind c-di-GMP (Davis et al. 2010, in prep). When TipF is removed or when c-di-GMP-binding is prevented either by point mutation or by depletion of cellular c-di-GMP levels, flagella are not assembled and PflI is not localized (Davis et al. 2010, in prep). TipF is also required for the localization of the components of the flagellar switch complex (Fig. 2A), but conversely the switch complex is not necessary for the polar positioning of TipF. Intriguingly, in the absence of an association with c-di-GMP, TipF remains dispersed in the cell envelope and does not cluster at the new cell pole. Moreover, under these conditions, flagella are also not assembled and PflI also remains dispersed (Davis et al. 2010, in prep). Thus, binding of c-di-GMP induces the polar localization of TipF to enable the recruitment of a placement factor and structural proteins for flagellum assembly at the new pole. TipF localization was previously shown to be determined by the TipN birth scar protein, a polytopic membrane protein with an extensive coiled-coil-rich domain facing the cytoplasm, that is required for proper localization of the flagellum at the new pole (Huitema et al. 2006; Lam et al. 2006). When TipN was inactivated, flagella and TipF (but not pili) were frequently mispositioned at seemingly identical aberrant locations (Huitema et al. 2006), hinting that akin to FlhF, TipF regulates flagellar placement and assembly. In dividing cells, TipN is localized at the division plane in a manner that requires the FtsZ cytokinetic tubulin homolog and then retained at the newborn pole that emerges from division (Huitema et al. 2006; Lam et al. 2006), the site of flagellum assembly and TipF/PflI localization. Thus, the protein localization and ensuing flagellum positioning pathway seems to follow the order: FtsZ > TipN > TipF > PflI. The key element of this polarity pathway for flagellar assembly and positioning is that protein localization relies on a cytokinetic mark or remnant that is inherited by the newborn pole of each daughter cell, thereby preselecting these poles as sites of flagellum biogenesis that attract the downstream assembly factors (Huitema et al. 2006; Lam et al. 2006). Intriguingly, TipN was recently shown to serve as the spatial cue that provides the correct directionality to the new pole in bacterial chromosome segregation (Ptacin et al. 2010; Schofield et al. 2010), showing that other polarized cellular functions also rely on the TipN-dependent polarity axis.
In summary, two mechanisms are known, FlhFG and TipNF-PflI, that both use a guanine nucleotide derivative to regulate the polar placement of the flagellum. The physiological significance of the guanine nucleotide-dependent input is unclear, but may be related to the variation in cellular concentration of the nucleotides over time. C-di-GMP levels fluctuate during the cell cycle (Paul et al. 2008; Christen et al. 2010b) and GTP levels change as cells transition from logarithmic growth into stationary phase (Freese et al. 1979; Buckstein et al. 2008; Paul et al. 2008; Christen et al. 2010b). Exploiting such fluctuation(s) would allow cells to tie the assembly of flagellar structures with the cell cycle or with the growth phase when the energetic cost for the assembly of such a complex nanomachine can be afforded.
Gliding Motility: MglAB
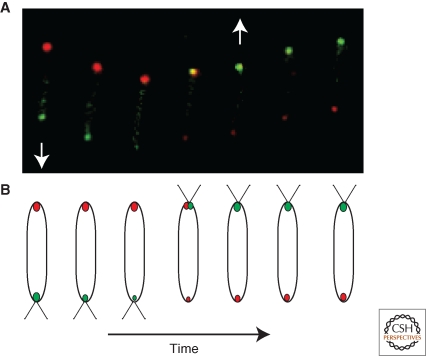
In a striking parallel to the role of the FlhF GTPase in determining the polarity of the flagellum-dependent motility system, recent research illustrates the role of the Ras-like GTPase MglA in regulating the dynamic polarity switch of (flagellum-independent) gliding motility of Myxcoccus xanthus cells on surfaces (Leonardy et al. 2010; Patryn et al. 2010; Zhang et al. 2010). M. xanthus cells move by way of cooperative, but distinct, motility systems, termed S (conferred by pilus motor proteins) and A (unidentified motor proteins), whose activity are modulated by polarized effectors (see Kaiser et al. 2010). A hallmark of motile M. xanthus cells is that they exhibit periodic reversals in the direction of movement and in a fashion that, at the subcellular level, is exquisitely tuned with the dynamic and often anticyclical polar localization of the A-motility effectors RomR and AglZ (Leonardy et al. 2007; Mignot et al. 2007) and the S-motility effectors PilT and FrzS (Mignot et al. 2005; Bulyha et al. 2009; Mauriello et al. 2010). The dynamic pole-to-pole oscillation of several of these effectors shows a strong dependency on the active, presumably GTP-bound, form of MglA (Leonardy et al. 2010; Zhang et al. 2010). Mutations in MglA that impair hydrolysis but not GTP binding 1) alter the cellular reversal frequency, 2) block polar oscillations of RomR, AglZ, and PilT and 3) prevent motility. Moreover, these MglA point mutant strains phenocopy those with a deletion in the gene encoding MglB, which is encoded by the open reading frame immediately upstream of that for MglA. MglA is localized to the leading pole (Mauriello et al. 2010; Patryn et al. 2010) while MglB clusters at the lagging pole (Leonardy et al. 2010; Zhang et al. 2010). Recent biochemical studies demonstrated that MglB essentially functions as a GTPase activating protein (GAP) to MglA to regulate its polar localization dynamics and activity (Leonardy et al. 2010; Zhang et al. 2010). In moving cells, MglA and MglB oscillate from pole to pole with a seemingly identical period that is out-of-phase with one another and coordinated with cell reversals (Fig. 3). The anticyclical polar localization of MglA is ablated when GTP hydrolysis is crippled by inactivation of MglB or by GTPase mutations in MglA (Leonardy et al. 2010; Zhang et al. 2010). Moreover, MglA also modulates the dynamics of MglB localization (Leonardy et al. 2010). Based on the reciprocal regulation of MglAB localization dynamics and the dependency of downstream motility effectors on MglAB (Mauriello et al. 2010), it stands to reason that the MglAB system is a core constituent of the molecular oscillator that regulates the dynamic polarity of cell reversals in M. xanthus. Fine-tuning of the MglAB localization and activity is accomplished through the Frz chemosensory system and the actin-like MreB cytosleketon, albeit in an unknown way (Leonardy et al. 2010; Mauriello et al. 2010; Zhang et al. 2010). Additional mechanisms in polarity control remain to be identified, as the motility effectors are still recruited to the pole in the absence of MglA, albeit often to the erroneous one.
Figure 3.
Polarity switching by the Ras-like GTPase MglA (green oval) and its GTPase-activating protein (GAP) MglB (red oval) in gliding Myxococcus xanthus cells. (A) A time series of gliding M. xanthus cells expressing MglA-YFP and MglB-mCherry that were imaged every 20 seconds by fluorescence microscopy (by Yong Zhang and Tâm Mignot). The yellow coloring indicates the colocalized MglA and MglB. The white arrow indicates the direction of movement that reverses during the course of the experiment. (B) A schematic of the cells and polarly localized proteins from panel A. The straight lines denote the pili that confer S-motility.
POLARITY CONTROL OF PILI AND OTHER ORGANELLAR PROTEINS BY ACTIN HOMOLOGS, PARA-LIKE ATPASES, AND A POLARITY FACTOR
Polar Pili
The pili assembled by P. aeruginosa are very similar with respect to structure, function, and localization to the pili that confer gliding motility in M. xanthus (see the schematic of the pilus in Fig. 3 of Kaiser et al. 2010). They are built from homologous components into polar trans-envelope structures that confer a form of flagellar-independent motility. In P. aeruginosa, this second form of motility is called (pilus-mediated) twitching motility. Akin to the aforementioned gliding motility, twitching motility involves the regular and coordinated extension/retraction cycles of the pilus filament from the cell surface (Mattick 2002). Consistent with the role of the actin-like MreB cytoskeleton in maintaining the polar localization of the S-motility regulator FrzS in M. xanthus (Mauriello et al. 2010), MreB seems to play a crucial role in the localization of pili in P. aeruginosa (Cowles and Gitai 2010). When P. aeruginosa cells were pretreated with the small molecule MreB-inhibitor A22 before being placed on medium that induces pilus production, cells accumulated pili at lateral sites instead of the pole. Also, unlike untreated cells, those pre-exposed to A22 mislocalized the PilT retraction ATPase to nonpolar sites. Thus, MreB maintains the polar disposition of pili and pilus regulators in an unknown way. A likely model would hold that pilus structural and regulatory proteins such as PilT and FrzS travel to the pole along the helical tracks of MreB filaments lining the cytoplasmic face of the inner membrane (Cowles and Gitai 2010; Mauriello et al. 2010). Remarkably, the PilM pilus assembly protein that is encoded in the pil clusters of both P. aeruginosa and M. xanthus is a member of the actin superfamily of proteins (Martin et al. 1995). As MreB isoforms are thought to inter-digitate into heteromeric cytoskeletal filaments (Carballido-Lopez et al. 2006), it is conceivable that PilM is also integrated into a cytoskeletal structure to connect the localization of the pilus structure and its constituents with the cytoskeleton. It will be interesting to determine the subcellular distribution of PilM and whether it is affected by treatment with A22. MreB has also been shown, directly or indirectly, to influence the polar localization of the virulence determinant IcsA of Shigella spp., the E. coli chemoreceptor Tar, several developmental kinases, and the origin of replication (Cori) in C. crescentus (Goldberg et al. 1993; Gitai et al. 2004; Shih et al. 2005). Actin-like proteins also determine the subcellular distribution of plasmids in E. coli (Gerdes et al. 2010), organelles like magnetosomes in Magnetospirrillum magneticum (Komeili et al. 2006; Murat et al. 2010), and cell wall biosynthetic enzymes (Dye et al. 2005).
Pilus machineries, evolutionarily less-related to that of P. aeruguinosa and M. xanthus, are also polarized. The pili of C. crescentus are assembled from a prototypical pilus/protein secretion apparatus encoded by a widely-conserved locus (the cpa or tad locus) at the new (flagellated) pole (Skerker and Shapiro 2000; Tomich et al. 2007). The CpaE component of the C. crescentus pilus system (also known as TadZ in Aggregatibacter actinomycetemcomitans) has extensive sequence homology to the ParA/MinD-like ATPases and is localized to the piliated pole, where it induces the formation of the polar pilus secretion channel (CpaC) in the outer membrane (Skerker and Shapiro 2000; Viollier et al. 2002a). How this occurs is unknown, but it could involve the spatial regulation of the CpaF secretion ATPase or the CpaD lipopotein that is presumably anchored to the inner leaflet of the outer membrane. Both CpaF and CpaD are polarly localized in C. crescentus (Werner et al. 2009), and a likely functional analog to CpaD (Tgl) is required for the formation of the pilus channel at both M. xanthus poles (Nudleman et al. 2006). In support of the notion that the ParA/MinD-like CpaE protein regulates the localization of the CpaF secretion ATPase, the polarly localized TadZ protein (the CpaE ortholog) was recently found to recruit the hexameric TadA secretion ATPase (the CpaF ortholog) (Bhattacharjee et al. 2001) to the poles of A. actinomycetemcomitans (B. Perez and D. Figurski, pers. comm.; Perez 2007). Thus, the common theme that emerges from the CpaEF and FlhGF (or FleN-FlhF) regulatory pairs is that ParA/MinD-like regulator influences the correct localization of the adjacently encoded NTPase.
Polar Polysaccharide Clusters
The central function of ParA/MinD-homologs in coordinating subcellular organization, potentially from within a cytoskeletal structure (Shih et al. 2003), is evident from several studies investigating how DNA and/or proteins are precisely positioned at the cell center or the cell extremities (reviewed in Gerdes et al. 2010). RpfA and MipZ determine the medial localization of TlpT chemoreceptor complex in Rhodobacter sphaeroides and the cytokinetic FtsZ-ring in C. crescentus, respectively (Thanbichler and Shapiro 2006; Thompson et al. 2006). The ParA/MinD-homologs VirC1 and BcsQ are polarly localized and dictate the recruitment of T-DNA nucleoprotein complexes or cellulose fibers to the cell pole, respectively (Atmakuri et al. 2007; Le Quere and Ghigo 2009). The activity of VirC1 and BcsQ, but not their polar localization, is impeded by mutations in the deviant Walker A motif that are thought to be required for ATP binding and hydrolysis (Leonard et al. 2005; Atmakuri et al. 2007; Le Quere and Ghigo 2009). For example, VirC1 bearing a mutation (K15Q) of lysine at the corresponding position 6 in the Walker-A motif (Fig. 4) is still localized to the cell poles. Interestingly, the corresponding mutation is naturally present in CpaE and BcsQ, which are also polarly localized. It is unknown whether CpaE, BcsQ, VirC1, and the K15Q derivative can bind or hydrolyze ATP on their own in vitro. If this is not the case, CpaE and BcsQ might rely on ancillary factors to augment ATP binding/hydrolysis in vivo. Alternatively, the modified P-loop might accommodate a nucleotide (derivative) other than ATP, possibly even the signaling molecule c-di-GMP, which is known to regulate cellulose production at the post-translational level (Jenal and Malone 2006; Le Quere and Ghigo 2009). It has been hypothesized that BcsQ-orthologs of Pseudomonas fluorescens, WssA, and WssJ, control the localization of the cellulose synthase complex (Spiers et al. 2002), raising the intriguing possibility that this regulation is mediated by c-di-GMP via BcsQ/WssA/J.
Figure 4.
Alignment of conserved residues of deviant Walker-A boxes found in polarly localized members of the ParA/MinD family of ATPases. Shown are Escherichia coli MinD and BcsQ, Caulobacter crescentus ParA, MipZ and CpaE, Rhodobacter sphaeroides PpfA, Vibrio cholerae FlhG, Agrobacterium tumefaciens VirC1, Aggregatibacter actinomycetemcomitans TadZ, and Pseudomonas fluorescens WssJ. Residues that are not identical, but similar, to the consensus sequence in red are shown in blue. Black color codes for variable residues.
The adhesive holdfast of C. crescentus, the UPP (unipolar polysaccharide) of its cousins A. tumefaciens and Rhizobium leguminosarum, reflect other polar sites where exopolysaccharides are concentrated (Merker and Smit 1988; Laus et al. 2006; Tomlinson and Fuqua 2009; Hardy et al. 2010). While studies on the localization of UPP are still in their infancy, a recent study revealed that the polar localization of the C. crescentus holdfast attachment proteins (Hfàs) requires the PodJ polarity factor that is localized to the new cell pole (Crymes et al. 1999; Viollier et al. 2002b; Hinz et al. 2003; Hardy et al. 2010). PodJ is a bitopic membrane protein that features N-terminal coiled-coil rich domain facing the cytoplasm and a C-terminal putative peptidoglycan-binding domain. The Hfa proteins are thought to tether the holdfast polysaccharide to the flagellated cell pole (Cole et al. 2003; Smith et al. 2003; Levi and Jenal 2006). PodJ is sequestered to the pole well before the Hfàs are expressed (Janakiraman and Brun 1999; Viollier et al. 2002b; Hinz et al. 2003). In the absence of PodJ, HfaA/B/D were no longer polar (Hardy et al. 2010), indicating that PodJ provides positional information to enable the polar sequestration of these holdfast proteins. Interestingly, PodJ is also required for the polar clustering of the ParA/MinD-paralog and pilus assembly regulator CpaE (see above) and the cell fate histidine kinase/phosphatase PleC (Viollier et al. 2002b; Hinz et al. 2003). These multifunctional activities are reflected in the pleiotropic phenotype of the PodJ mutant that includes a pilus and holdfast assembly defect and aberrant expression of developmental genes. It is currently unknown whether PodJ regulates CpaE, PleC, and/or the Hfàs directly or not. However, since the polar localization of the Hfàs also depends on the presence of the holdfast synthesis (hfs) genes, it is possible that the effect of PodJ on Hfàs is indirect and is mediated through hfs-encoded proteins (Hardy et al. 2010). Recent evidence also suggests that CpaE contributes to the polar recruitment of PleC (Christen et al. 2010a), arguing that at least one PodJ-dependent mechanism indirectly regulates the localization of PleC to the new (flagellated) pole. How PodJ itself is attracted to the new cell pole is currently a mystery. It is possible that PodJ localization is cued by an analogous mechanism, as that of TipN (see above). If so, then PodJ must also recognize a cytokinetic signal left behind at the newly formed cell poles in the wake of division. To be recognized by PodJ, this cytokinetic signal must remain at the newborn poles until new PodJ protein is synthesized after the daughter cells separate. At the time of division, only a proteolytic fragment of PodJ is present, while the synthesis of new (full-length) PodJ occurs during the inter-division period (Viollier et al. 2002b; Hinz et al. 2003; Chen et al. 2005; Chen et al. 2006). In support of the idea that localization of PodJ to the new pole is cued by a cytokinetic signal, PodJ can be seen at the division septum when it is constitutively synthesized (P Viollier, unpubl.). Moreover, overexpression of C-terminally truncated form of PodJ interferes with division and is lethal (Crymes et al. 1999). These results are consistent with the view that PodJ, akin to TipN, interacts with the division apparatus or an ancillary division protein. PodJ is still polar in the absence of TipN, indicating that the signal at the newborn pole is not dependent on TipN.
WHAT WE KNOW, WE DON'T KNOW
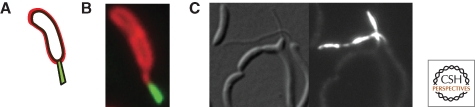
For a large number of polar organelles or protein complexes, we do not yet have the foot in the door to the secret of their positioning mechanism(s). One such case is that of the polar stalk of C. crescentus (Fig. 5A,B) that is elaborated by the cylindrical polar outgrowth of the cell envelope. Attempts to identify structural genes for the stalk have been futile, perhaps because of the essential nature of proteins required for stalk synthesis (Wagner et al. 2005). Alternatively, the imposed genetic selections or bioinformatic criteria might have been suboptimal. Comprehensive and genome-wide protein localization efforts may provide an alternative to such recalcitrant cases. Indeed, they hold the potential to directly identify the players involved with a cell biological rather than a genetic bias and, at the very least, can yield new screening tools that represent entry points for the dissection of the organelle positioning and other localization mechanisms (Werner et al. 2009; Hughes et al. 2010; Ingerson-Mahar et al. 2010) (Fig. 5C).
Figure 5.
Discovery of stalked protein X (StpX) of Caulobacter crescentus from a genome-wide localization screen (Hughes et al. 2010). (A) A schematic of the C. crescentus cell in panel B with a fluorescent polar stalk. (B) Fluorescence micrograph from cells expressing StpX-GFP and a red-fluorescent membrane stain (picture taken by P. Viollier). (C) A mutant with a mispositioned stalk containing StpX-GFP. The left image is a DIC (differential interference contrast) micrograph, and the image on the right represents the corresponding (green) fluorescent image.
To put the underlying mechanisms addressed above into perspective, one must ask why cells have developed such extensive regulatory pathways to ensure the polar placement of organelles and protein complexes. An appealing model posits that polar localization serves simply to ensure the proper partitioning of functions into the corresponding daughter cell that inherits a particular pole. This is of course widely accepted in the case of sister chromosomes that must be segregated into both daughter cells, but the same principle should apply to polar organelles or protein complexes that are present in too low a copy number to rely on stochastic distribution into daughters (see Fig. 2B). Perhaps not coincidentally, relatives of ParA, a protein that localizes chromosomal regions and plasmids to polar regions to ensure their segregation (Gerdes et al. 2010), have been appropriated by bacteria to fulfill key roles in the polar positioning of organelles and, by inference, their partitioning to progeny at division.
ACKNOWLEDGMENTS
We thank Tâm Mignot, Yong Zhang, and Jeff Skerker for providing micrographs. Funding support is from the U.S. Department of Energy, Office of Science (Biological and Environmental Research, Grant # DE-FG02-05ER64136), the Swiss National Science Foundation (Grant # 31003A_127287), and the Human Frontiers Science Program (Program Grant # RGP0051/2010).
Footnotes
Editors: Lucy Shapiro and Richard M. Losick
Additional Perspectives on Cell Biology of Bacteria available at www.cshperspectives.org
REFERENCES
- Atmakuri K, Cascales E, Burton OT, Banta LM, Christie PJ 2007. Agrobacterium ParA/MinD-like VirC1 spatially coordinates early conjugative DNA transfer reactions. EMBO J 26: 2540–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban M, Joslin SN, Hendrixson DR 2009. FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. J Bacteriol 191: 6602–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange G, Petzold G, Wild K, Parlitz RO, Sinning I 2007. The crystal structure of the third signal-recognition particle GTPase FlhF reveals a homodimer with bound GTP. Proc Natl Acad Sci U S A 104: 13621–13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee MK, Kachlany SC, Fine DH, Figurski DH 2001. Nonspecific adherence and fibril biogenesis by Actinobacillus actinomycetemcomitans: TadA protein is an ATPase. J Bacteriol 183: 5927–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JM 2000. Localization of the histidine kinase PilS to the poles of Pseudomonas aeruginosa and identification of a localization domain. Mol Microbiol 36: 153–162 [DOI] [PubMed] [Google Scholar]
- Buckstein MH, He J, Rubin H 2008. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J Bacteriol 190: 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulyha I, Schmidt C, Lenz P, Jakovljevic V, Hone A, Maier B, Hoppert M, Sogaard-Andersen L 2009. Regulation of the type IV pili molecular machine by dynamic localization of two motor proteins. Mol Microbiol 74: 691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballido-Lopez R, Formstone A, Li Y, Ehrlich SD, Noirot P, Errington J 2006. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev Cell 11: 399–409 [DOI] [PubMed] [Google Scholar]
- Carlsson F, Joshi SA, Rangell L, Brown EJ 2009. Polar localization of virulence-related Esx-1 secretion in mycobacteria. PLoS Pathog 5: e1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Hottes AK, McAdams HH, McGrath PT, Viollier PH, Shapiro L 2006. Cytokinesis signals truncation of the PodJ polarity factor by a cell cycle-regulated protease. EMBO J 25: 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Viollier PH, Shapiro L 2005. A membrane metalloprotease participates in the sequential degradation of a Caulobacter polarity determinant. Mol Microbiol 55: 1085–1103 [DOI] [PubMed] [Google Scholar]
- Christen B, Fero MJ, Hillson NJ, Bowman G, Hong SH, Shapiro L, McAdams HH 2010a. High-throughput identification of protein localization dependency networks. Proc Natl Acad Sci U S A 107: 4681–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M, Kulasekara HD, Christen B, Kulasekara BR, Hoffman LR, Miller SI 2010b. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science 328: 1295–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JL, Hardy GG, Bodenmiller D, Toh E, Hinz A, Brun YV 2003. The HfaB and HfaD adhesion proteins of Caulobacter crescentus are localized in the stalk. Mol Microbiol 49: 1671–1683 [DOI] [PubMed] [Google Scholar]
- Correa NE, Peng F, Klose KE 2005. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol 187: 6324–6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles KN, Gitai Z 2010. Surface association and the MreB cytoskeleton regulate pilus production, localization and function in Pseudomonas aeruginosa. Mol Microbiol 76: 1411–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crymes WB Jr, Zhang D, Ely B 1999. Regulation of podJ expression during the Caulobacter crescentus cell cycle. J Bacteriol 181: 3967–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer PA, Crossley RE, Hand AR, Rothfield LI 1991. The MinD protein is a membrane ATPase required for the correct placement of the Escherichia coli division site. Embo J 10: 4371–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AJ, Nouwen N 2008. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem 77: 643–667 [DOI] [PubMed] [Google Scholar]
- Dworkin J 2009. Cellular polarity in prokaryotic organisms. Cold Spring Harb Perspect Biol 1: a003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye NA, Pincus Z, Theriot JA, Shapiro L, Gitai Z 2005. Two independent spiral structures control cell shape in Caulobacter. Proc Natl Acad Sci U S A 102: 18608–18613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing CP, Andreishcheva E, Guerry P 2009. Functional characterization of flagellin glycosylation in Campylobacter jejuni 81–176. J Bacteriol 191: 7086–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E, Heinze JE, Galliers EM 1979. Partial purine deprivation causes sporulation of Bacillus subtilis in the presence of excess ammonia, glucose and phosphate. J Gen Microbiol 115: 193–205 [DOI] [PubMed] [Google Scholar]
- Gerdes K, Howard M, Szardenings F 2010. Pushing and pulling in prokaryotic DNA segregation. Cell 141: 927–942 [DOI] [PubMed] [Google Scholar]
- Gitai Z, Dye N, Shapiro L 2004. An actin-like gene can determine cell polarity in bacteria. Proc Natl Acad Sci U S A 101: 8643–8648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MB, Barzu O, Parsot C, Sansonetti PJ 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. Infect Agents Dis 2: 210–211 [PubMed] [Google Scholar]
- Golding I, Cox EC 2006. Physical nature of bacterial cytoplasm. Phys Rev Lett 96: 098102. [DOI] [PubMed] [Google Scholar]
- Goon S, Kelly JF, Logan SM, Ewing CP, Guerry P 2003. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol Microbiol 50: 659–671 [DOI] [PubMed] [Google Scholar]
- Green JC, Kahramanoglou C, Rahman A, Pender AM, Charbonnel N, Fraser GM 2009. Recruitment of the earliest component of the bacterial flagellum to the old cell division pole by a membrane-associated signal recognition particle family GTP-binding protein. J Mol Biol 391: 679–690 [DOI] [PubMed] [Google Scholar]
- Hardy GG, Allen RC, Toh E, Long M, Brown PJ, Cole-Tobian JL, Brun YV 2010. A localized multimeric anchor attaches the Caulobacter holdfast to the cell pole. Mol Microbiol 76: 409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7: 263–273 [DOI] [PubMed] [Google Scholar]
- Hinz AJ, Larson DE, Smith CS, Brun YV 2003. The Caulobacter crescentus polar organelle development protein PodJ is differentially localized and is required for polar targeting of the PleC development regulator. Mol Microbiol 47: 929–941 [DOI] [PubMed] [Google Scholar]
- Hughes HV, Huitema E, Pritchard S, Keiler KC, Brun YV, Viollier PH 2010. Protein localization and dynamics within a bacterial organelle. Proc Natl Acad Sci U S A 107: 5599–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema E, Pritchard S, Matteson D, Radhakrishnan SK, Viollier PH 2006. Bacterial birth scar proteins mark future flagellum assembly site. Cell 124: 1025–1037 [DOI] [PubMed] [Google Scholar]
- Ingerson-Mahar M, Briegel A, Werner JN, Jensen GJ, Gitai Z 2010. The metabolic enzyme CTP synthase forms cytoskeletal filaments. Nat Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C, Domian IJ, Maddock JR, Shapiro L 1999. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell 97: 111–120 [DOI] [PubMed] [Google Scholar]
- Jain S, van Ulsen P, Benz I, Schmidt MA, Fernandez R, Tommassen J, Goldberg MB 2006. Polar localization of the autotransporter family of large bacterial virulence proteins. J Bacteriol 188: 4841–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman RS, Brun YV 1999. Cell cycle control of a holdfast attachment gene in Caulobacter crescentus. J Bacteriol 181: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaumouille V, Francetic O, Sansonetti PJ, Tran Van Nhieu G 2008. Cytoplasmic targeting of IpaC to the bacterial pole directs polar type III secretion in Shigella. EMBO J 27: 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U, Malone J 2006. Mechanisms of Cyclic-di-GMP Signaling in Bacteria. Annu Rev Genet 40: 385–407 [DOI] [PubMed] [Google Scholar]
- Judd PK, Kumar RB, Das A 2005. Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc Natl Acad Sci U S A 102: 11498–11503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D, Robinson M, Kroos L 2010. Myxobacteria, Polarity, and Multicellular Morphogenesis. Cold Spring Harb Perspect Biol 2: a000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12: 291–299 [DOI] [PubMed] [Google Scholar]
- Komeili A, Li Z, Newman DK, Jensen GJ 2006. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311: 242–245 [DOI] [PubMed] [Google Scholar]
- Kusumoto A, Nishioka N, Kojima S, Homma M 2009. Mutational analysis of the GTP-binding motif of FlhF which regulates the number and placement of the polar flagellum in Vibrio alginolyticus. J Biochem 146: 643–650 [DOI] [PubMed] [Google Scholar]
- Kusumoto A, Shinohara A, Terashima H, Kojima S, Yakushi T, Homma M 2008. Collaboration of FlhF and FlhG to regulate polar-flagella number and localization in Vibrio alginolyticus. Microbiology 154: 1390–1399 [DOI] [PubMed] [Google Scholar]
- Lam H, Schofield WB, Jacobs-Wagner C 2006. A landmark protein essential for establishing and perpetuating the polarity of a bacterial cell. Cell 124: 1011–1023 [DOI] [PubMed] [Google Scholar]
- Laus MC, Logman TJ, Lamers GE, Van Brussel AA, Carlson RW, Kijne JW 2006. A novel polar surface polysaccharide from Rhizobium leguminosarum binds host plant lectin. Mol Microbiol 59: 1704–1713 [DOI] [PubMed] [Google Scholar]
- Le Quere B, Ghigo JM 2009. BcsQ is an essential component of the Escherichia coli cellulose biosynthesis apparatus that localizes at the bacterial cell pole. Mol Microbiol 72: 724–740 [DOI] [PubMed] [Google Scholar]
- Leclerc G, Wang SP, Ely B 1998. A new class of Caulobacter crescentus flagellar genes. J Bacteriol 180: 5010–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard TA, Butler PJ, Lowe J 2005. Bacterial chromosome segregation: structure and DNA binding of the Soj dimer–a conserved biological switch. EMBO J 24: 270–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardy S, Freymark G, Hebener S, Ellehauge E, Sogaard-Andersen L 2007. Coupling of protein localization and cell movements by a dynamically localized response regulator in Myxococcus xanthus. EMBO J 26: 4433–4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardy S, Miertzschke M, Bulyha I, Sperling E, Wittinghofer A, Sogaard-Andersen L 2010. Regulation of dynamic polarity switching in bacteria by a Ras-like G-protein and its cognate GAP. EMBO J 29: 2276–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A, Jenal U 2006. Holdfast formation in motile swarmer cells optimizes surface attachment during Caulobacter crescentus development. J Bacteriol 188: 5315–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner AB, Madden R, Demarez A, Stewart EJ, Taddei F 2008. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc Natl Acad Sci U S A 105: 3076–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM 2003. How bacteria assemble flagella. Annu Rev Microbiol 57: 77–100 [DOI] [PubMed] [Google Scholar]
- Maddock JR, Shapiro L 1993. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259: 1717–1723 [DOI] [PubMed] [Google Scholar]
- Margolin W 2005. FtsZ and the division of prokaryotic cells and organelles. Nat Rev Mol Cell Biol 6: 862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Watson AA, McCaul TF, Mattick JS 1995. Characterization of a five-gene cluster required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol 16: 497–508 [DOI] [PubMed] [Google Scholar]
- Mattick JS 2002. Type IV pili and twitching motility. Annu Rev Microbiol 56: 289–314 [DOI] [PubMed] [Google Scholar]
- Mauriello EM, Mouhamar F, Nan B, Ducret A, Dai D, Zusman DR, Mignot T 2010. Bacterial motility complexes require the actin-like protein, MreB and the Ras homologue, MglA. EMBO J 29: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker RI, Smit J 1988. Characterization of the adhesive holdfast of marine and freshwater caulobacters. Appl Environ Microbiol 54: 2078–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot T, Merlie JP Jr, Zusman DR 2005. Regulated pole-to-pole oscillations of a bacterial gliding motility protein. Science 310: 855–857 [DOI] [PubMed] [Google Scholar]
- Mignot T, Shaevitz JW, Hartzell PL, Zusman DR 2007. Evidence that focal adhesion complexes power bacterial gliding motility. Science 315: 853–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat D, Quinlan A, Vali H, Komeili A 2010. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc Natl Acad Sci U S A 107: 5593–5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TS, Kazmierczak BI 2006. FlhF is required for swimming and swarming in Pseudomonas aeruginosa. J Bacteriol 188: 6995–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell PD, Monds RD, O'Toole GA 2009. LapD is a bis-(3’,5’)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0–1. Proc Natl Acad Sci U S A 106: 3461–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehus E, Gressmann H, Ye F, Schlapbach R, Dehio M, Dehio C, Stack A, Meyer TF, Suerbaum S, Josenhans C 2004. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol Microbiol 52: 947–961 [DOI] [PubMed] [Google Scholar]
- Nudleman E, Wall D, Kaiser D 2006. Polar assembly of the type IV pilus secretin in Myxococcus xanthus. Mol Microbiol 60: 16–29 [DOI] [PubMed] [Google Scholar]
- Obuchowski PL, Jacobs-Wagner C 2007. PflI, a protein involved in flagellar positioning in Caulobacter crescentus. J Bacteriol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandza S, Baetens M, Park CH, Au T, Keyhan M, Matin A 2000. The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol Microbiol 36: 414–423 [DOI] [PubMed] [Google Scholar]
- Patryn J, Allen K, Dziewanowska K, Otto R, Hartzell PL 2010. Localization of MglA, an essential gliding motility protein in Myxococcus xanthus. Cytoskeleton (Hoboken) 67: 322–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Jaeger T, Abel S, Wiederkehr I, Folcher M, Biondi EG, Laub MT, Jenal U 2008. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133: 452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez BA 2007. The Actinobacillus actinomycetemcomitans tad locus: Defining the Genetic Requirements for Biofilm Formation and Characterization of the TadZ Protein. in Department of Microbiology & Immunology. Columbia University, New York [Google Scholar]
- Ptacin JL, Lee SF, Garner EC, Toro E, Eckart M, Comolli LR, Moerner WE, Shapiro L 2010. A spindle-like apparatus guides bacterial chromosome segregation. Nat Cell Biol 12: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner DZ, Losick R 2010. Protein subcellular localization in bacteria. Cold Spring Harb Perspect Biol 2: a000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JH, Keiler KC 2008. Screen for localized proteins in Caulobacter crescentus. PLoS One 3: e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti S, Ghelardi E, Celandroni F, Ceragioli M, Giannessi F, Senesi S 2007. FlhF, a signal recognition particle-like GTPase, is involved in the regulation of flagellar arrangement, motility behaviour and protein secretion in Bacillus cereus. Microbiology 153: 2541–2552 [DOI] [PubMed] [Google Scholar]
- Schirm M, Soo EC, Aubry AJ, Austin J, Thibault P, Logan SM 2003. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol Microbiol 48: 1579–1592 [DOI] [PubMed] [Google Scholar]
- Schofield WE, Lim HC, Jacobs-Wagner C 2010. Cell cycle coordination and regulation of bacterial chromosome segregation dynamics by polarly localized proteins. Embo J doi:10.1038/emboj.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott ME, Dossani ZY, Sandkvist M 2001. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc Natl Acad Sci U S A 98: 13978–13983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, McAdams HH, Losick R 2009. Why and how bacteria localize proteins. Science 326: 1225–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YL, Kawagishi I, Rothfield L 2005. The MreB and Min cytoskeletal-like systems play independent roles in prokaryotic polar differentiation. Mol Microbiol 58: 917–928 [DOI] [PubMed] [Google Scholar]
- Shih YL, Le T, Rothfield L 2003. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc Natl Acad Sci U S A 100: 7865–7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Shapiro L 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. Embo J 19: 3223–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Hinz A, Bodenmiller D, Larson DE, Brun YV 2003. Identification of Genes Required for Synthesis of the Adhesive Holdfast in Caulobacter crescentus. J Bacteriol 185: 1432–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers AJ, Kahn SG, Bohannon J, Travisano M, Rainey PB 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M 2010. Synchronization of chromosome dynamics and cell division in bacteria. Cold Spring Harb Perspect Biol 2: a000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M, Shapiro L 2006. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126: 147–162 [DOI] [PubMed] [Google Scholar]
- Thompson SR, Wadhams GH, Armitage JP 2006. The positioning of cytoplasmic protein clusters in bacteria. Proc Natl Acad Sci U S A 103: 8209–8214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich M, Planet PJ, Figurski DH 2007. The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol 5: 363–375 [DOI] [PubMed] [Google Scholar]
- Tomlinson AD, Fuqua C 2009. Mechanisms and regulation of polar surface attachment in Agrobacterium tumefaciens. Curr Opin Microbiol 12: 708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschowri N, Busse S, Hengge R 2009. The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev 23: 522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollier PH, Sternheim N, Shapiro L 2002a. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. Embo J 21: 4420–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollier PH, Sternheim N, Shapiro L 2002b. Identification of a localization factor for the polar positioning of bacterial structural and regulatory proteins. Proc Natl Acad Sci U S A 99: 13831–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JK, Galvani CD, Brun YV 2005. Caulobacter crescentus requires RodA and MreB for stalk synthesis and prevention of ectopic pole formation. J Bacteriol 187: 544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JN, Chen EY, Guberman JM, Zippilli AR, Irgon JJ, Gitai Z 2009. Quantitative genome-scale analysis of protein localization in an asymmetric bacterium. Proc Natl Acad Sci U S A 106: 7858–7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Franco M, Ducret A, Mignot T 2010. A Bacterial Ras-Like Small GTP-Binding Protein and Its Cognate GAP Establish a Dynamic Spatial Polarity Axis to Control Directed Motility. PLoS Biol 8: e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]