Abstract
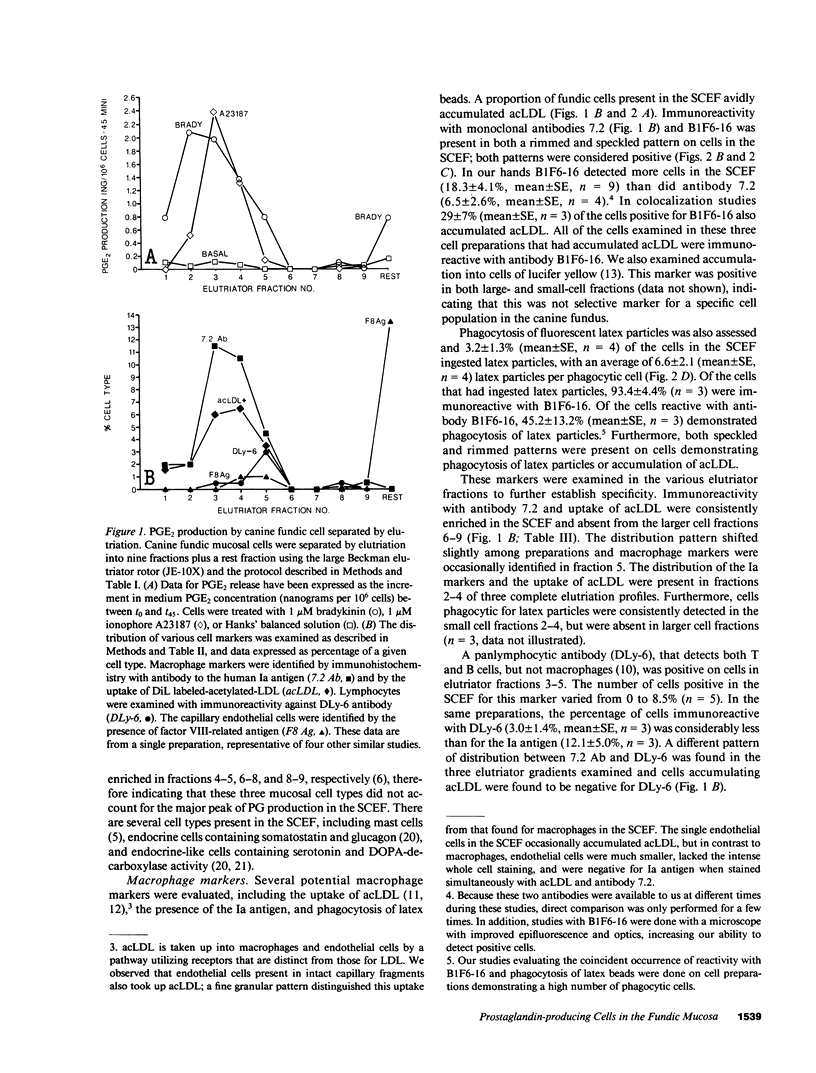
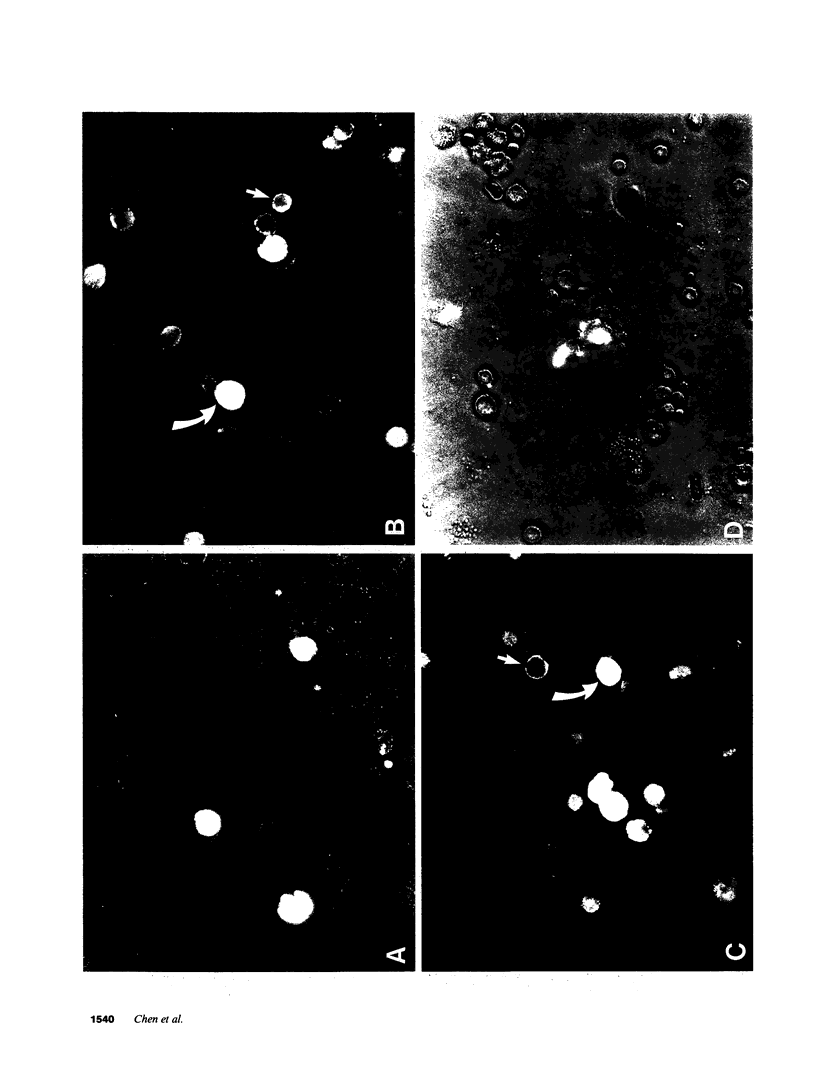
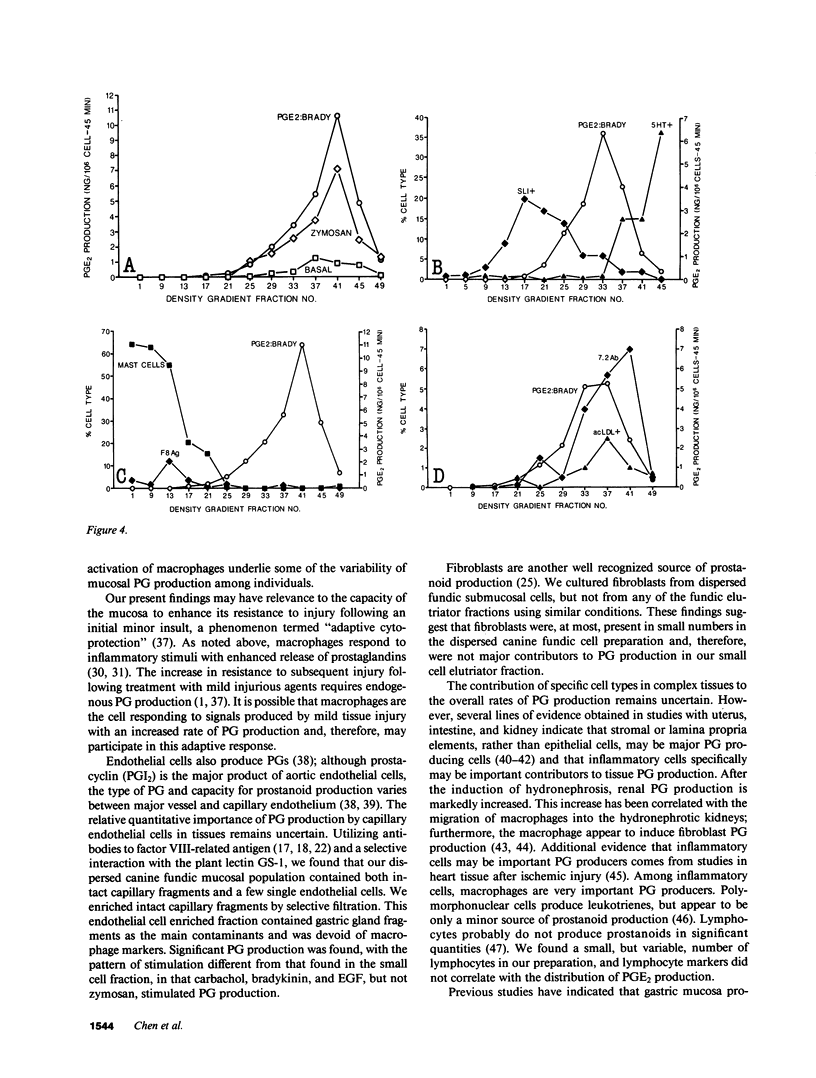
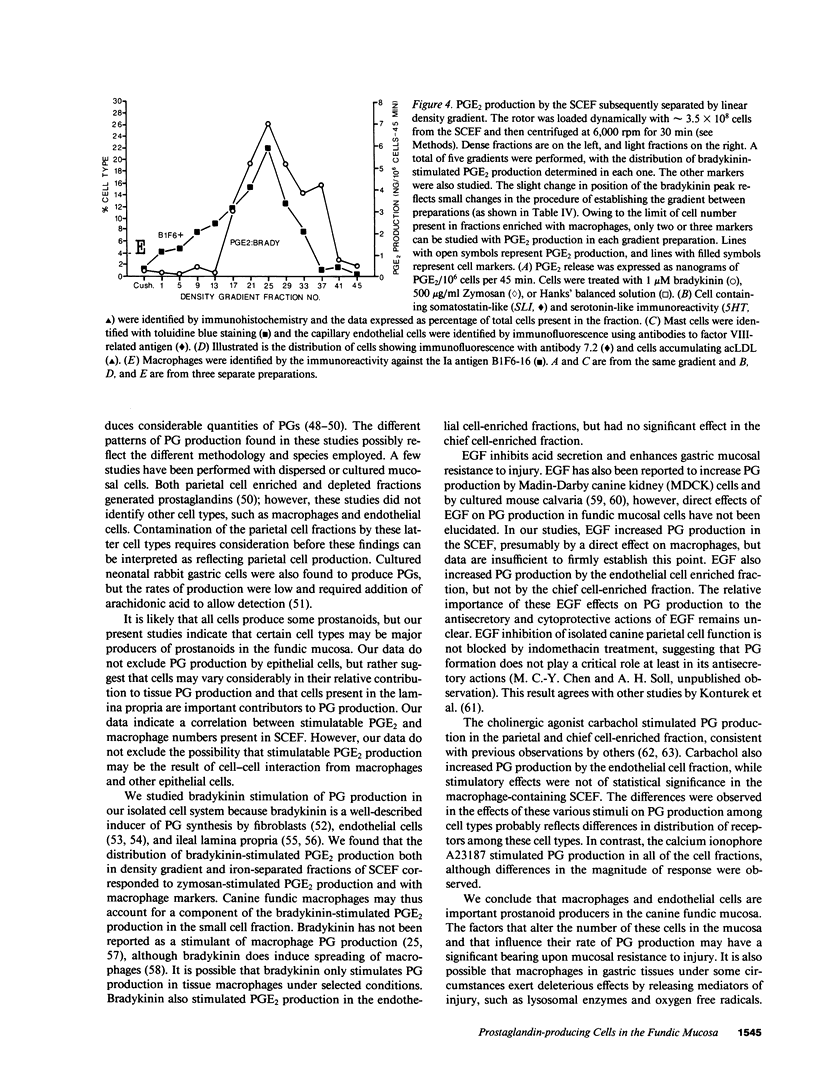
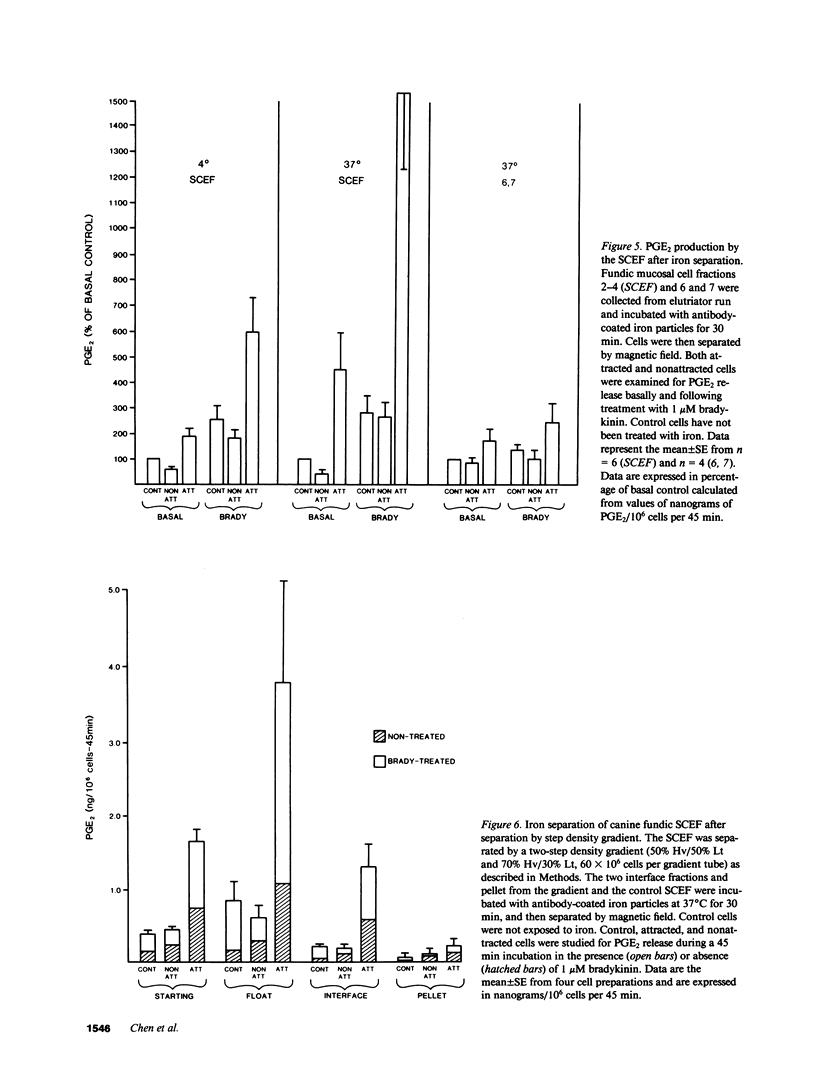
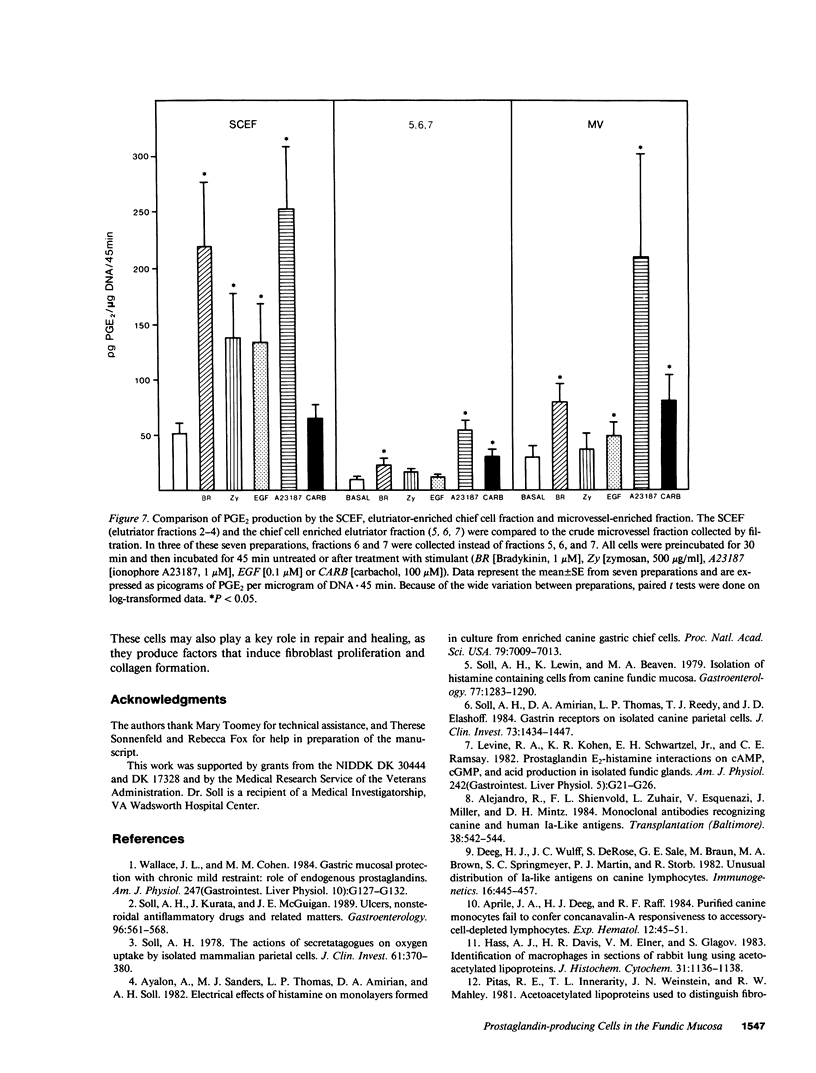
Endogenous prostaglandins (PGs) influence resistance of the gastric mucosa to injury, but the source of PGs is unknown. Using radioimmunoassay, we studied PG production by dispersed canine fundic mucosal cells. PGE2 production, stimulated by bradykinin, epidermal growth factor, zymosan, and calcium ionophore, was greater in the small-cell elutriator fraction (SCEF) than in the medium and large cell fractions, which contained mucous, chief, and parietal cells. Linear density gradients of SCEF cells revealed maximal PGE2 production in cells of light density. Mast, endocrine, and endothelial cells did not account for this PGE2 production. Macrophages, identified by uptake of acetylated-LDL, immunoreactivity with antibodies to the human Ia antigen, and phagocytosis of fluorescent latex particles, were enriched in the SCEF and correlated with PGE2 production in the density gradient. Magnetic separation of cells in the SCEF-ingesting iron particles enriched PGE2 production. Fractions enriched in endothelial cells present in intact capillary fragments, but depleted of macrophages, also produced PGE2. Regulation of PGE2 production differed among cell types. Fibroblasts were easily cultured from submucosa, but were not detected in the SCEF. We conclude that macrophages and capillary endothelial cells are major producers of PGE2 in the canine fundic mucosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Alejandro R., Shienvold F. L., Latif Z., Esquenazi V., Miller J., Mintz D. H. Monoclonal antibodies recognizing canine and human Ia-like antigens. Transplantation. 1984 Nov;38(5):542–544. doi: 10.1097/00007890-198411000-00021. [DOI] [PubMed] [Google Scholar]
- Aprile J. A., Deeg H. J., Wulff J. C., Raff R. F. Purified canine monocytes fail to confer concanavalin-A responsiveness to accessory-cell-depleted lymphocytes. Exp Hematol. 1984 Jan;12(1):45–51. [PubMed] [Google Scholar]
- Ayalon A., Sanders M. J., Thomas L. P., Amirian D. A., Soll A. H. Electrical effects of histamine on monolayers formed in culture from enriched canine gastric chief cells. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7009–7013. doi: 10.1073/pnas.79.22.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Hastain E., Goodwin J. S., Peake G. T. The nature of the prostaglandin-producing mononuclear cell in human peripheral blood. J Lab Clin Med. 1981 Feb;97(2):179–186. [PubMed] [Google Scholar]
- Beaven M. A., Soll A. H., Lewin K. J. Histamine synthesis by intact mast cells from canine fundic mucosa and liver. Gastroenterology. 1982 Feb;82(2):254–262. [PubMed] [Google Scholar]
- Beelen R. H., Walker W. S. Dynamics of cytochemically distinct subpopulations of macrophages in elicited rat peritoneal exudates. Cell Immunol. 1983 Dec;82(2):246–257. doi: 10.1016/0008-8749(83)90159-4. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. C., Amirian D. A., Toomey M., Sanders M. J., Soll A. H. Prostanoid inhibition of canine parietal cells: mediation by the inhibitory guanosine triphosphate-binding protein of adenylate cyclase. Gastroenterology. 1988 May;94(5 Pt 1):1121–1129. doi: 10.1016/0016-5085(88)90002-9. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Conway T. M., Bennett C. F., Crooke S. T., Stadel J. M. Islet-activating protein inhibits leukotriene D4- and leukotriene C4- but not bradykinin- or calcium ionophore-induced prostacyclin synthesis in bovine endothelial cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7320–7324. doi: 10.1073/pnas.83.19.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Conway T. M., Mong S., Steiner S., Crooke S. T. Leukotriene D4 treatment of bovine aortic endothelial cells and murine smooth muscle cells in culture results in an increase in phospholipase A2 activity. J Biol Chem. 1986 Aug 15;261(23):10713–10718. [PubMed] [Google Scholar]
- Davis B. B., Thomasson D., Zenser T. V. Renal disease profoundly alters cortical interstitial cell function. Kidney Int. 1983 Mar;23(3):458–464. doi: 10.1038/ki.1983.42. [DOI] [PubMed] [Google Scholar]
- Deeg H. J., Wulff J. C., DeRose S., Sale G. E., Braun M., Brown M. A., Springmeyer S. C., Martin P. J., Storb R. Unusual distribution of Ia-like antigens on canine lymphocytes. Immunogenetics. 1982;16(5):445–457. doi: 10.1007/BF00372103. [DOI] [PubMed] [Google Scholar]
- Dilley R., Herring M., Boxer L., Gardner A., Glover J. Immunofluorescent staining for factor VIII-related antigen: a tool for study of healing in vascular prostheses. J Surg Res. 1979 Sep;27(3):149–155. doi: 10.1016/0022-4804(79)90124-0. [DOI] [PubMed] [Google Scholar]
- Eanes R. Z. Effects of fructose on human fibroblast metabolism: the application of DNA measurements as a basis for interpretation. In Vitro Cell Dev Biol. 1985 Jun;21(6):328–332. doi: 10.1007/BF02691580. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Evers A. S., Murphree S., Saffitz J. E., Jakschik B. A., Needleman P. Effects of endogenously produced leukotrienes, thromboxane, and prostaglandins on coronary vascular resistance in rabbit myocardial infarction. J Clin Invest. 1985 Mar;75(3):992–999. doi: 10.1172/JCI111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal D., Casey M. L., Johnston J. M., MacDonald P. C. Mesenchyme-epithelial interactions in human endometrium. Prostaglandin synthesis in separated cell types. J Clin Invest. 1982 Oct;70(4):798–805. doi: 10.1172/JCI110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen M. E., Cheli C. D. Arachidonic acid and prostaglandin endoperoxide metabolism in isolated rabbit and coronary microvessels and isolated and cultivated coronary microvessel endothelial cells. J Clin Invest. 1983 Nov;72(5):1658–1671. doi: 10.1172/JCI111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder J. P., Doe W. F. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983 Apr;84(4):795–802. [PubMed] [Google Scholar]
- Goldstein R. H., Miller K., Glassroth J., Linscott R., Snider G. L., Franzblau C., Polgar P. Influence of asbestos fibers on collagen and prostaglandin production in fibroblast and macrophage co-cultures. J Lab Clin Med. 1982 Nov;100(5):778–785. [PubMed] [Google Scholar]
- Goldyne M. E., Burrish G. F., Poubelle P., Borgeat P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J Biol Chem. 1984 Jul 25;259(14):8815–8819. [PubMed] [Google Scholar]
- Hass A. J., Davis H. R., Elner V. M., Glagov S. Identification of macrophages in sections of rabbit lung using acetoacetylated lipoproteins. J Histochem Cytochem. 1983 Sep;31(9):1136–1138. doi: 10.1177/31.9.6688438. [DOI] [PubMed] [Google Scholar]
- Hojvat S. A., Musch M. W., Miller R. J. Stimulation of prostaglandin production in rabbit ileal mucosa by bradykinin. J Pharmacol Exp Ther. 1983 Sep;226(3):749–755. [PubMed] [Google Scholar]
- Hopper K. E., Cahill J. M. Immunoregulation by macrophages II. Separation of mouse peritoneal macrophages having tumoricidal and bactericidal activities and those secreting PGE and interleukin I. J Reticuloendothel Soc. 1983 Jun;33(6):443–456. [PubMed] [Google Scholar]
- Hsueh W., Kuhn C., 3rd, Needleman P. Relationship of prostaglandin secretion by rabbit alveolar macrophages to phagocytosis and lysosomal enzyme release. Biochem J. 1979 Nov 15;184(2):345–354. doi: 10.1042/bj1840345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P. E., DeSchryver K., Okegawa T., Leahy K., Needleman P. Presence of inflammatory cells associated with exaggerated arachidonic acid metabolism in renal injury. Adv Prostaglandin Thromboxane Leukot Res. 1983;12:65–73. [PubMed] [Google Scholar]
- Jonas P. E., Leahy K. M., DeSchryver-Kecskemeti K., Needleman P. Cellular interactions and exaggerated arachidonic acid metabolism in rabbit renal injury. J Leukoc Biol. 1984 Jan;35(1):55–64. doi: 10.1002/jlb.35.1.55. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Piastucki I., Dembinski A., Radecki T., Dembinska-Kiec A., Zmuda A., Gregory H. Role of mucosal prostaglandins and DNA synthesis in gastric cytoprotection by luminal epidermal growth factor. Gut. 1981 Nov;22(11):927–932. doi: 10.1136/gut.22.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafranconi W. M., Spall R. D., Sipes I. G., Duhamel R. C., Meezan E., Brendel K. Rapid isolation of type II pneumocytes with magnetic removal of macrophages. Exp Lung Res. 1983 Apr;4(3):191–204. doi: 10.3109/01902148309046060. [DOI] [PubMed] [Google Scholar]
- Lawson L. D., Powell D. W. Bradykinin-stimulated eicosanoid synthesis and secretion by rabbit ileal components. Am J Physiol. 1987 Jun;252(6 Pt 1):G783–G790. doi: 10.1152/ajpgi.1987.252.6.G783. [DOI] [PubMed] [Google Scholar]
- LeDuc L. E., Needleman P. Regional localization of prostacyclin and thromboxane synthesis in dog stomach and intestinal tract. J Pharmacol Exp Ther. 1979 Oct;211(1):181–188. [PubMed] [Google Scholar]
- Levine L., Hassid A. Epidermal growth factor stimulates prostaglandin biosynthesis by canine kidney (MDCK) cells. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1181–1187. doi: 10.1016/0006-291x(77)90980-9. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Tanaka M., Mitsui Y., Murota S. I. Cultured rabbit gastric epithelial cells producing prostaglandin I2. Gastroenterology. 1983 Mar;84(3):498–505. [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Payne N. A., Gerber J. G. Prostaglandin E2 and [14C]arachidonic acid release by carbachol in the isolated canine parietal cell. J Pharmacol Exp Ther. 1987 Nov;243(2):511–516. [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981 May-Jun;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Kempe J., Cohn Z. A. Prostaglandin synthesis by macrophages requires a specific receptor-ligand interaction. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4279–4282. doi: 10.1073/pnas.77.7.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepp W., Steffen B., Schusdziarra V., Classen M. Calcium, calmodulin, and cyclic adenosine monophosphate modulate prostaglandin E2 release from isolated human gastric mucosal cells. J Clin Endocrinol Metab. 1986 Oct;63(4):886–891. doi: 10.1210/jcem-63-4-886. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Pawlowski N. A., Murray H. W., Andreach M., Zrike J., Cohn Z. A. Regulation of arachidonic acid metabolism by macrophage activation. J Exp Med. 1982 Apr 1;155(4):1148–1160. doi: 10.1084/jem.155.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. E., Anderson N. V. Isolation and functional analysis of normal canine blood monocytes and resident alveolar macrophages. Am J Vet Res. 1984 Jan;45(1):87–90. [PubMed] [Google Scholar]
- Skoglund M. L., Gerber J. G., Murphy R. C., Nies A. S. Prostaglandin production by intact isolated gastric parietal cells. Eur J Pharmacol. 1980 Aug 22;66(1):145–148. doi: 10.1016/0014-2999(80)90309-x. [DOI] [PubMed] [Google Scholar]
- Smith G. S., Warhurst G., Turnberg L. A. Synthesis and degradation of prostaglandin E2 in the epithelial and sub-epithelial layers of the rat intestine. Biochim Biophys Acta. 1982 Dec 13;713(3):684–687. doi: 10.1016/0005-2760(82)90331-9. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Kurata J., McGuigan J. E. Ulcers, nonsteroidal antiinflammatory drugs, and related matters. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):561–568. doi: 10.1016/s0016-5085(89)80051-4. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Lewin K., Beaven M. A. Isolation of histamine-containing cells from canine fundic mucosa. Gastroenterology. 1979 Dec;77(6):1283–1290. [PubMed] [Google Scholar]
- Soll A. H. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Wollin A. Histamine and cyclic AMP in isolated canine parietal cells. Am J Physiol. 1979 Nov;237(5):E444–E450. doi: 10.1152/ajpendo.1979.237.5.E444. [DOI] [PubMed] [Google Scholar]
- Stahl K. W., Roch-Arveiller M., Regoli D., Giroud J. P. Receptors for bradykinin in murine peritoneal macrophages: modulation of short-term spreading. Agents Actions. 1981 Dec;11(6-7):624–627. doi: 10.1007/BF01978768. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Yamada T., Soll A. H., Park J., Elashoff J. Autonomic regulation of somatostatin release: studies with primary cultures of canine fundic mucosal cells. Am J Physiol. 1984 Nov;247(5 Pt 1):G567–G573. doi: 10.1152/ajpgi.1984.247.5.G567. [DOI] [PubMed] [Google Scholar]