Abstract
The rewarding influence of drugs of abuse varies with time of day and appears to involve interactions between the circadian and the mesocorticolimbic dopamine systems. The circadian system is also intimately involved in measuring daylength. Thus, the present study examined the impact of changing daylength (photoperiod) on cocaine-seeking behaviors. Male Sprague Dawley rats were trained and tested on a 12L:12D light:dark schedule for cocaine-induced reinstatement of conditioned place preference (CPP) at three times of day (Zeitgeber time (ZT): 4, 12, and 20) to determine a preference score. Rats were then shifted to either shorter (6L:18D) or longer (18L:6D) photoperiods and then to constant conditions, re-tested for cocaine-induced reinstatement under each different condition, and then returned to their original photoperiod (12L:12D) and tested once more. Rats exhibited a circadian profile of preference score in constant darkness with a peak at 12h after lights-off. At both ZT4 and ZT20, but not at ZT12, shorter photoperiods profoundly suppressed cocaine reinstatement, which did not recover even after switching back to 12L:12D. In contrast, longer photoperiods did not alter reinstatement. Separate studies showed that the suppression of cocaine reinstatement was not due to repeated testing. In an additional experiment, we examined the photoperiodic regulation of tyrosine hydroxylase (TH) and dopamine transporter (DAT) proteins in drug-naive rats. These results revealed photoperiodic modulation of proteins in the prefrontal cortex and dorsal striatum, but not in the nucleus accumbens or ventral tegmental area. Together, these findings add further support to the circadian genesis of cocaine-seeking behaviors and demonstrate that drug-induced reinstatement is modulated by photoperiod. Furthermore, the results suggest that photoperiod partly contributes to the seasonal expression of certain drug-related behaviors in humans living at different latitudes and thus our findings may have implications for novel targeting of circadian rhythms in the treatment of addiction.
Keywords: circadian, cocaine, conditioned place preference, photoperiod, dopamine transporter, prefrontal cortex, tyrosine hydroxylase
INTRODUCTION
Biological clocks located in the brain are thought to provide a temporal organization of behavior (Pittendrigh, 1960, Aschoff and Wever, 1976, Reppert and Weaver, 2002, Hastings et al., 2008). Specifically, neurons located within the suprachiasmatic nucleus of the hypothalamus (SCN) comprise a so-called “master clock” – a hierarchy of molecular interactions responsible for generating a wide variety of daily rhythms (Davidson et al., 2003). Evidence supporting this role for the SCN includes: 1) loss of physiological rhythmicity following SCN destruction or clock gene disruption, 2) continued expression of behavioral rhythmicity in the absence of environmental light:dark cues, and 3) entrainment (i.e., resetting) of clock rhythms daily to the environmental light:dark cycle via inputs of the retinohypothalamic tract. It is also clear that the circadian system is important for the translation of seasonal time (Sumova et al., 1995, Schwartz et al., 2001). If these characteristics of the natural time-keeping system impact the addiction process in humans, then methods could be developed to take advantage of this temporal gating of behavior. Indeed, daily variation in drug overdoses (Morris, 1987, Raymond et al., 1992), seasonal variation in drug arrests (Langworthy and McKelvie, 2005) and a wide variety of other human behaviors have been reported (Bronson, 2004, Foster and Roenneberg, 2008). Additionally, a recent longitudinal study of cocaine and cocaine metabolites in wastewater reveal clear seasonal differences indicative of human seasonal cocaine use patterns (Mari et al., 2009). In addition, several groups have examined the seasonal expression of illicit behaviors in humans living at different latitudes (Sandyk and Kanofsky, 1992, Uitenbroek, 1996, Paschane, 1998, Sher, 2002, Rocchi et al., 2004, Veldhuizen et al., 2007) suggesting a link between season and behavior. Lastly, the frequent co-morbidity between drug abuse and depression (Quello et al., 2005), the positive effect of light treatment for seasonal affective disorder and its variation with latitude (Potkin et al., 1986), and the recent discovery of the role of clock genes in the etiology of bipolar disorder (McClung, 2007, Westrin and Lam, 2007), together suggest that a common mechanism may be involved.
The goal of the current series of experiments was to determine if daily and seasonal (i.e., photoperiodic) cues contribute to drug-seeking behavior in a rat model of drug-relapse: cocaine-induced reinstatement of conditioned place preference (CPP). To this end, we trained and tested rats at different times of day and examined their cocaine-induced reinstatement behavior before and after shifts to longer or shorter photoperiods.
We have previously shown that cocaine sensitization, cocaine-induced reinstatement of CPP, and the dopamine transporter (DAT) and tyrosine hydroxylase (TH) protein expression vary with time of day (Sleipness et al., 2005, 2007a, b). Much of this variation is due to an important role of the suprachiasmatic nucleus of the hypothalamus (SCN) (Sleipness et al., 2007a, b). In the context of the current study, we tested the hypothesis that these two proteins, important for regulating dopamine neurotransmission during cocaine treatment (Schmidt et al., 2001, Grimm et al., 2002, Thomsen et al., 2009, Ramamoorthy et al., 2010, Schmitt and Reith, 2010), are also under photoperiodic control as is the case for several other populations of DA neurons (Pozdeyev and Lavrikova, 2000, Thiery et al., 2002, Kang et al., 2007, Leclerc et al., 2010).
MATERIALS AND METHODS
Animals and Drugs
Male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA) weighing 280-300 g at the start of the experiment were used. Animals were pair-housed and kept on a 12h light:12h dark (12L:12D) photoperiod (actual time of lights-on and lights-off varied between rooms) with ad libitum access to food and water unless otherwise stated. The experiments conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised 1996. The Washington State University Institutional Animal Care and Use Committee approved all procedures. Cocaine hydrochloride was obtained from Sigma (St. Louis, MO), and doses are expressed as the weight of the salt.
Conditioned Place Preference
Rats were trained and tested at Zeitgeber times (ZT): 4, 12, or 20 (ZT0 = time of lights-on). These times were chosen based on our previous findings of low (ZT4) and high (ZT12) cocaine-induced sensitization (Sleipness et al., 2005); ZT20 was examined to obtain more complete time-of-day information on cocaine-induced CPP. To avoid the possible confound of Zeitgeber time as a contextual cue (Ralph et al., 2002), rats trained at one time of day were always tested for CPP or reinstated at the same time of day. Our studies employed a three-compartment CPP apparatus, and rats were trained and tested for CPP followed by extinction as described previously (Sleipness et al., 2007a) except that rats were counterbalanced for receiving cocaine in the preferred or non-preferred compartment, as determined from the Initial Preference day. The CPP chambers consisted of three compartments with ½” grid flooring and black walls in one chamber, solid flooring and gray walls in the middle chamber, and ½” bar flooring with white walls in the third chamber. All training and testing began under a 12L:12D photoperiod (Fig. 1) with rats trained and tested at ZT4 in the light as described (Sleipness et al., 2007a), and rats initially trained and tested for CPP at ZT12 or ZT20 in complete darkness.
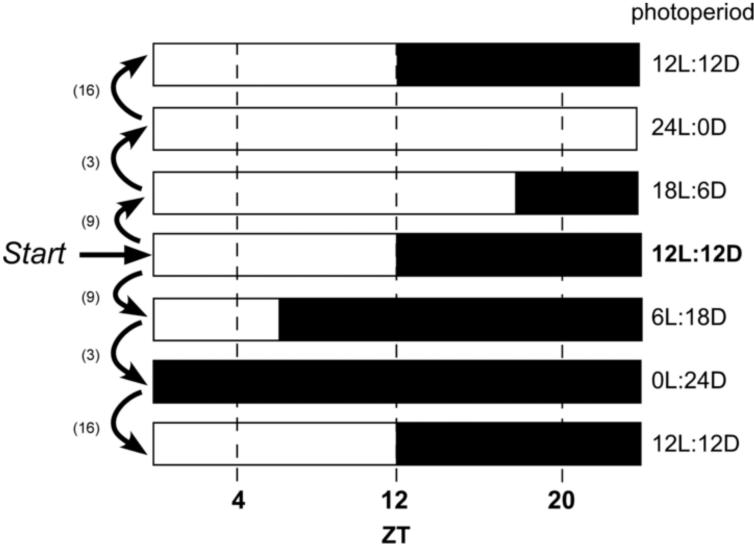
Figure 1.
Protocol for photoperiod shifts during CPP. Rats were initially housed on a 12L:12D schedule (arrow, Start) for CPP training, testing in the drug-free state, and for the first reinstatement. Separate groups of rats were then shifted to either shorter or longer days, followed by a shift to constant dark (0L:24D) or constant light (24L:0D), respectively, and then shifted back to the original 12L:12D photoperiod. Numbers in parentheses indicate the maximum number of days between photoperiods to allow for re-entrainment (range: 1-16) depending on the magnitude of the photoperiod shift (see Methods for details). Separate groups of rats were trained and tested at ZT4, ZT12, or ZT20. White bars represent the light period; black bars represent the dark period.
For the CPP Test day (i.e. acquisition of CPP), a Preference Score was derived by calculating the time (in seconds) spent on the cocaine-paired side during the Initial Preference test subtracted from the time spent on the cocaine-paired side during the CPP test. Rats whose preference score was not higher than Initial Preference were excluded from further testing and were not included in any of the analyses.
All rats then underwent extinction training and testing wherein the rats were allowed free access to all three chambers but did not receive drug. Rats were placed in the middle chamber and allowed to move freely between the chambers for 15 min. To determine when the rats had extinguished, the group mean was computed each day and compared to the initial preference day; extinction testing continued until no statistical difference was observed between the two preference scores.
Following extinction, reinstatement of cocaine-induced CPP behavior was examined. Reinstatement testing was performed under several different photoperiods and in constant conditions as shown in Figure 1. For cocaine-primed reinstatement, the Preference Score was defined as time spent on the cocaine-paired side during the Reinstatement Test day minus the time spent on the cocaine-paired side during the extinction session on the day immediately preceding the Reinstatement Test day. For the first reinstatement, rats were held under their 12L:12D light cycle and given a 15 mg/kg of body weight cocaine injection. Animals were allowed to freely explore all three compartments for 15 min, and their locomotor activity was monitored. At this point, their photoperiod was shifted to either a shorter photoperiod (6L:18D) or a longer photoperiod (18L:6D; Fig.1). After a 6-9 day period to allow for entrainment to the new lighting schedule (based on a conservative estimate of one day required for each hour changed), the rats were given 2-4 days of extinction training until they reached the criteria for extinction as described above. The next day, they were given a second reinstatement as described at ZT4, ZT12, or ZT20, depending on the group. Rats were then either switched from a 18L:6D photoperiod to constant light or from 6L:18D to constant darkness for 1-3 days and then given 1-3 additional days of extinction until they met criteria for extinction. The extinction duration was kept to a minimum to reduce the time that animals began to free-run. The free-running phenomenon is a normal aspect of circadian rhythmicity in the absence of a daily light:dark cycle and is characterized by a drift in the onset of a behavior over time (Pittendrigh, 1960). On the day following extinction, rats were given a third cocaine reinstatement at the circadian times corresponding to their initial training times (i.e., 4, 12, or 20). Lastly, rats were switched back to their original 12L:12D photoperiod and allowed to readjust to the new photoperiod for 5-16 days (Yamazaki et al., 2000, Reddy et al., 2002). At this time, 1-3 extinction days were given until rats reached criteria for extinction, and a final cocaine-primed reinstatement was given as described above. The photoperiod shifts used are not unlike those experienced by individuals living at high latitudes, albeit in a much more protracted manner.
A separate control group of rats (n=8) was trained at ZT20 and tested repeatedly at intervals identical to those used for the main experimental rats, but in this case, no photoperiod shifts were included.
During CPP testing, locomotor activity was also measured by interruption of infrared photocell beam breaks.
Western blot analysis
Rats used for western blot analysis were treated exactly as described for CPP regarding photoperiod shifts. No handling or injections were administered prior to sacrifice. Tissue was collected and processed essentially as described previously (Sleipness et al., 2008) with some minor modifications as follows. For the mPFC, 50 μg of total protein was loaded; for the nucleus accumbens and dorsal striatum, 25 μg was loaded; and for the VTA, 16 μg was loaded onto 4-15% Tris-HCl gradient gels (Bio-Rad, Hercules, CA) and subjected to electrophoresis. The fluorescent secondary antibody for DAT was donkey anti-rat IgG (1:3,000 dilution; Jackson ImmunoResearch Laboratories, DyLight 649) and for TH was donkey anti-rabbit IgG (1:10,000 dilution; Li-Cor Biosciences, IRDye 800CW).
Western blot images were obtained using the Odyssey infrared imaging system (Li-Cor Bioscience). The optical density of bands was quantified using Odyssey software (v. 2.0.5), and each band was normalized to the optical density of samples pooled from the same brain regions of naive rats held under 12L:12D. Final values reported are expressed as a percentage of the optical density from rats held under the 12L:12D cycle prior to any photoperiod shifts.
Studies employing western blotting techniques typically include normalization to housekeeping proteins such as actin or tubulin. However, as both of these are differentially expressed in several brain regions depending on the time of day (Bredow et al., 1997, Taishi et al., 1997), we quantified the amount of protein loaded using the Coomassie method as stated above, which has a sensitivity of 1 μg/mL.
Data analysis
All CPP data from each group of rats that underwent a switch in photoperiod were analyzed using a one-way analysis of variance (ANOVA) with one repeated measure over days. For analysis of CPP data among the different testing times, a one-way ANOVA was conducted. In the case of a significant difference across days in the ANOVA, a protected least significant difference (Fisher’s PLSD) analysis was performed. For western blot analysis, a one-way ANOVA was conducted separately for DAT and TH for each brain area followed by a Fisher’s PLSD test. In the case of comparison between two different groups across days, a two-way ANOVA with one repeated measure over days was conducted. Differences were considered statistically significant if p < 0.05.
RESULTS
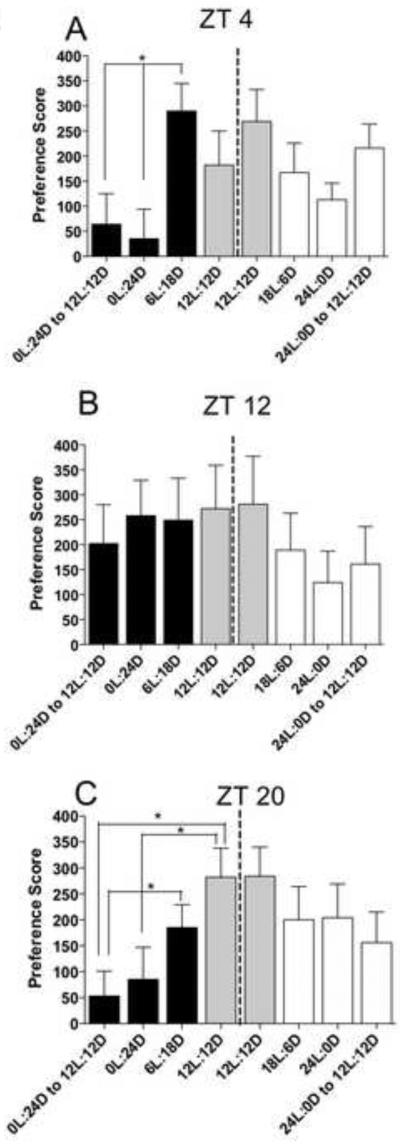
The main goal of this study was to reveal the photoperiodic and circadian contributions to cocaine-seeking behavior. Figure 2A-C shows the effect of different photoperiods on cocaine-induced reinstatement of CPP in rats trained and tested at ZT4, ZT12 or ZT20. At ZT4 (Figure 2A), when rats were switched from 12L:12D to shorter photoperiod and then returned to 12L:12D (6L:18D to 24D and back to 12L:12D), there was a significant effect of photoperiod (F11,43 = 5.12, p < 0.005). Post-hoc analysis revealed a reduction in cocaine-primed reinstatement in rats exposed to constant darkness (0L:24D) compared with rats held under a short photoperiod (6L:18D). In addition, reinstatement remained suppressed even when rats were switched from constant darkness back to a 12L:12D photoperiod. In contrast, no significant effects on cocaine-primed reinstatement were observed in animals that were switched from a 12L:12D photoperiod to longer photoperiod and then returned to 12L:12D (18L:6D to 24L and back to 12L:12D).
Figure 2.
Cocaine-induced reinstatement of CPP is suppressed at ZT4 and ZT20 by step-wise photoperiod shifts to shorter days. Data are mean (± SEM) of the preference score at ZT4 (A), ZT12 (B), and ZT20 (C). Gray bars on either side of the center dotted line represent the group of rats given their first reinstatement under the12L:12D photoperiod prior to their subsequent step-wise photoperiod shifts. The groups trained and tested at ZT4 and ZT20 and shifted to shorter days (black bars) showed a suppressed reinstatement, while the group trained and tested at ZT12 did not suppress reinstatement. None of the groups shifted to longer days (white bars) suppressed reinstatement. For all ZTs, N = 11-13/group. Significant differences between groups are indicated by an asterisk.
At ZT12 (Figure 2B), there were no significant effects of photoperiod switches on cocaine-primed reinstatement.
At ZT20, the effect of photoperiod on reinstatement was somewhat similar to that found at ZT4. Figure 2C shows that when rats were switched from a 12L:12D photoperiod to shorter photoperiod, there was a significant effect of photoperiod (F12,51 = 3.53, p < 0.003). Post-hoc analysis revealed a decrease in cocaine-primed reinstatement under constant darkness (24D) compared with rats at 12L:12D, and this suppression persisted even after switching back to 12L:12D. In addition, rats first taken through shorter photoperiod and then returned to 12L:12D were also suppressed compared with rats held at 6L:18D. Similar to what was found at ZT4 and ZT12, animals that were switched from a 12L:12D photoperiod to longer photoperiod did not demonstrate changes in cocaine-primed reinstatement.
To confirm that the changes in preference scores were not simply a reflection of changes in locomotor activity, we assessed the number of total photocell beam breaks over the course of the 15 min reinstatement periods. A two-way ANOVA revealed no significant differences between ZTs or across the multiple reinstatement tests (Table 1). Thus, the suppression of reinstatement is not simply attributed to a suppression of locomotor activity.
Table 1.
Locomotor activity during cocaine-primed reinstatement
| Original Photoperiod |
Shorter Photoperiods |
Constant Darkness |
Return to Original Photoperiod |
|
|---|---|---|---|---|
| ZT (N) | Reinstate 12L:12D |
Reinstate 6L:18D |
Reinstate 0L:24D |
Reinstate 12L:12D |
| 4 (12) | 2025 ± 152 | 1857 ± 115 | 1973 ± 115 | 1773 ± 85 |
| 12 (11) | 1465 ± 217 | 1799 ± 215 | 2267 ± 235 | 2065 ± 230 |
| 20 (13) | 1396 ± 97 | 2365 ± 704 | 1582 ± 142 | 1581 ± 112 |
| Original Photoperiod |
Longer Photoperiods |
Constant Light |
Return to Original Photoperiod |
|
|---|---|---|---|---|
| ZT (N) | Reinstate 12L:12D |
Reinstate 18L:6D |
Reinstate 24L:0D |
Reinstate 12L:12D |
| 4 (12) | 2163 ± 162 | 2513 ± 190 | 2014 ± 224 | 2266 ± 209 |
| 12 (12) | 1810 ± 261 | 1931 ± 173 | 2095 ± 155 | 1651 ± 183 |
| 20 (12) | 1708 ± 142 | 2042 ± 169 | 2016 ± 130 | 1995 ± 190 |
A second goal of this study was to determine if daily variation in cocaine-seeking behavior was generated endogenously (i.e., in the absence of light:dark cues) to signify its circadian genesis as was recently described (Bass et al., 2010). Comparing panels A, B and C in Figure 2 under conditions of complete darkness (0L:24D) it is evident that reinstatement varied between test times - peaking at 12hr after lights off and lower at both 4hr and 20hr after lights-off (Time - F2,32 = 2.82, p < 0.024). In contrast, rats placed in constant light (24L:0D) did not exhibit a significant daily variation in reinstatement.
There were no differences in place preference behavior among ZTs for the drug-free CPP test (p = 0.603) or for the first cocaine-primed reinstatement under 12L:12D (p = 0.732, not shown). This finding is similar to what we observed previously (Sleipness et al., 2007a).
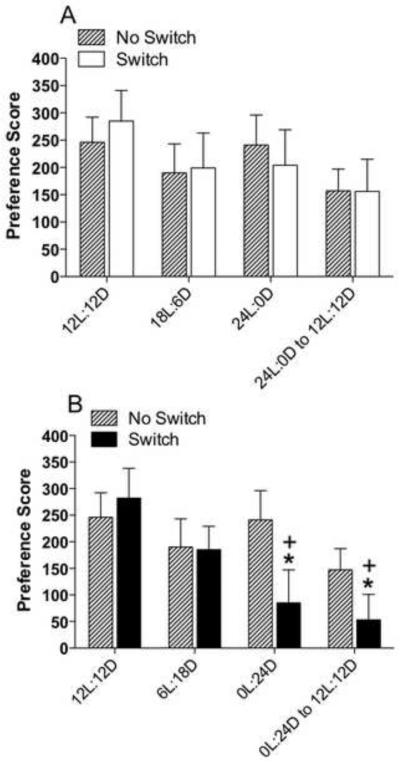
One possibility is that repeated testing for cocaine-primed reinstatement diminished the extent of reinstatement over sessions and that the effects attributed to changes in photoperiod were due instead to the phenomenon of extinction during repeated testing. To test for this possibility, a separate group of rats was trained and tested at ZT20 and given CPP training and extinction as before and then re-tested for reinstatement multiple times while maintained under a 12L:12D photoperiod. Figure 3 shows cocaine-primed reinstatement results in animals held under the 12L:12D photoperiod in comparison with reinstatement responses under longer or shorter photoperiods at ZT20 (taken from Figure 2C). Rats maintained under the 12L:12D schedule did not significantly alter their reinstatement response over repeated testing (p > 0.05). When reinstatement responses at 12L:12D were compared with those of animals given longer photoperiod or switched back to 12L:12D after longer daylengths (Figure 3A), again no differences were found. However, when these reinstatement responses were compared with those from animals given shorter photoperiod or switched back to 12L:12D after housing under shorter photoperiod (Figure 3B), there was a significant effect of time (F3,75 = 6.15, p < 0.0009) and a significant interaction (F3,75 = 2.85, p < 0.043). Thus, the suppression of cocaine-induced reinstatement observed when animals were placed in constant darkness and also when they were returned to a 12L:12D photoperiod shown in Figure 2C was a result of the photoperiod, not repeated reinstatement testing.
Figure 3.
Repeated testing does not suppress cocaine reinstatement in rats maintained on a 12L:12D photoperiod. Data are mean (± SEM) of the preference score in rats trained and tested at ZT20. Shown are comparisons between rats given repeated reinstatement with no photoperiod switches (No Switch) and rats from Figure 2C given repeated reinstatement when photoperiods were switched (Switch) to longer days (A) and shorter days (B) and then back to 12L:12D. Rats shifted to longer days in (A) showed no differences with rats under the No Switch condition, while rats shifted to shorter days in (B) showed suppressed reinstatement compared with rats under the No Switch condition. For No Switch, N = 14. *P < 0.05, compared with initial reinstatement under 12L:12D.; +P < 0.05, compared with the No Switch group on the same day of testing.
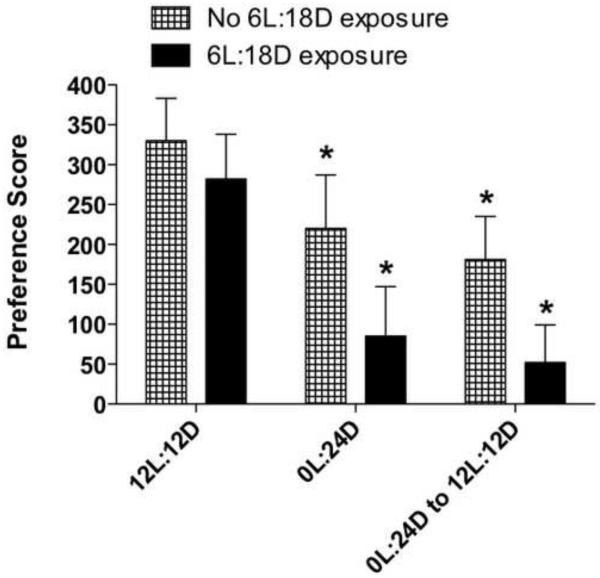
To determine if the suppression in reinstatement we observed at ZT20 in rats exposed to a shorter photoperiod was dependent on a stepwise change in photoperiod (i.e.,12L:12D to 6L:18D to 0L:24D) or was independent of the first step in the sequence (6L:18D), an additional group of rats was trained for CPP and extinguished as before. In this experiment, rats were taken directly from the 12L:12D photoperiod to constant darkness, and then switched back to 12L:12D. Figure 4 shows a comparison of these rats with those held under the same photoperiods but first given a transition photoperiod of 6L:18D (from Figure 2C). The results show that there was a decrease in cocaine-primed reinstatement in rats that were switched directly from the 12L:12D to constant darkness and then back to the 12L:12D schedule (one-way ANOVA, p < 0.008), indicating that there was a significant suppression of behavior without first transitioning rats through the 6L:18D photoperiod. These data indicate that the suppression of reinstatement may be at least partially dependent on the animal’s prior photoperiodic history.
Figure 4.
Cocaine-induced reinstatement of CPP is also suppressed by omitting the 6L:18D photoperiod shift. Data are mean (± SEM) of the preference score in rats trained and tested at ZT20. Comparison between rats given one less photoperiod shift at 6L:18D (No 6L:18D exposure) with rats from Figure 2C given the 6L:18D photoperiod shift prior to the shift at 24D. Both groups demonstrated suppressed reinstatement compared with reinstatement under their original 12L:12D schedule. For the No 6L:18D exposure group, N = 9. *P < 0.05, compared with initial reinstatement under 12L:12D.
Since normal behaviors and cocaine-induced behaviors (including reinstatement) are clearly dependent on dopamine, we also assessed whether photoperiod itself can alter DAT and TH. Figure 5A-H shows the results from western blot analysis of DAT and TH protein levels in the mPFC, nucleus accumbens, dorsal striatum, and VTA collected at ZT20 from drug-naïve rats. Significant changes in DAT and TH levels were found only in the mPFC and dorsal striatum, with the greatest magnitude of changes in the mPFC. No differences in DAT or TH protein levels were found in the nucleus accumbens or VTA.
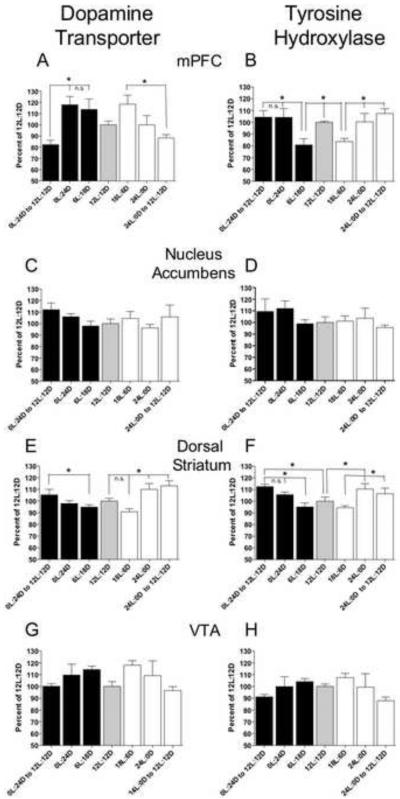
Figure 5.
Protein levels of DAT and TH are altered in the mPFC and the dorsal striatum by photoperiod shifts in drug-naïve rats. Data are mean (± SEM) of DAT and TH protein levels expressed as a percent of 12L:12D levels in the mPFC (A, B) the nucleus accumbens, (C, D) the dorsal striatum, (E, F) and the VTA (G, H). Differences were found in the mPFC and dorsal striatum for both DAT and TH, while no changes were found in the nucleus accumbens or the VTA. Significant differences between groups are indicated by an asterisk. N = 6/group.
In the mPFC (Figure 5A), a one-way ANOVA revealed a significant effect of photoperiod on DAT levels (F6,41 = 4.64, p < 0.0014). The levels of DAT were reduced when rats were switched back to the 12L:12D photoperiod from a shorter photoperiod in comparison to when they were maintained on either a short photoperiod (6L:18D) or constant darkness. Similarly, DAT levels were reduced when animals were returned to the 12L:12D photoperiod in comparison to when they switched from a longer photoperiod (18L:6D).
The effect of photoperiod changes on TH in the mPFC (Figure 5B) were somewhat opposite to the effect observed with DAT in this brain region (F6,41 = 4.13, p < 0.0031). Compared with the starting 12L:12D photoperiod, either a shorter photoperiod (6L:18D) or a longer photoperiod (18L:6D) suppressed TH protein levels, and these levels were restored in animals that were exposed to constant darkness or constant light. After the switch back to the 12L:12D photoperiod, no changes in TH were noted, with levels similar to those from animals held under the original 12L:12D photoperiod.
In the dorsal striatum, a one-way ANOVA revealed a significant influence of photoperiod on DAT levels (F6,41 = 5.13, p < 0.0007; Figure 5E) and TH levels (F6,41 = 4.31, p < 0.0024; Figure 5F); these changes were generally smaller in magnitude than those found in the mPFC. A shorter photoperiod produced a significant increase in DAT levels between rats held under a 6L:18D photoperiod and rats that were switched back from constant darkness to 12L:12D. Somewhat similarly, a longer photoperiod produced a significant increase in DAT levels in the dorsal striatum of rats held under 12L:12D and also of rats held under 18L:6D compared with rats held under constant light and with rats that were switched from constant light back to 12L:12D. Overall, the pattern of DAT protein changes in the dorsal striatum was virtually opposite to that found in the mPFC.
The direction and magnitude of changes in TH protein in the dorsal striatum were similar to those of DAT in the dorsal striatum. A shorter photoperiod produced an increase in TH levels when compared to rats that were either exposed to shorter photoperiod and returned to a 12L:12D photoperiod, rats that were initially held under 12L:12D, and to rats that were held under a shorter photoperiod (6L:18D) (Figure 5F). A longer photoperiod produced an increase in TH levels in rats held under constant light (24L) compared with rats initially held under 12L:12D. In addition, rats returned to 12L:12D after being held under a longer photoperiod exhibited an increase in TH levels compared with rats held under a longer photoperiod.
No significant changes were found in the nucleus accumbens (Figure 5C,D) or the VTA (Figure 5G,H).
DISCUSSION
The main findings from this study are the following: 1) Photoperiod impacts cocaine-primed reinstatement of CPP in a time-of-day dependent fashion. A complete suppression of reinstatement is observed when rats are exposed to shorter photoperiods (at ZT4 and ZT20). 2) Exposure to a short photoperiod, but not a long photoperiod, followed by a return to the original 12L:12D photoperiod under which rats were trained results in a failure to reinstate with cocaine. 3) The levels of DAT and TH protein in drug-naïve rats are altered by changes in photoperiod directly; this occurred most profoundly in the mPFC and to a lesser extent in the dorsal striatum, with no changes in the nucleus accumbens or VTA. 4) An endogenous circadian rhythm of cocaine reinstatement, like that of cocaine self-administration, is observed in animals housed in complete darkness.
Influence of photoperiod on CPP reinstatement
Our results demonstrate that a shift in photoperiod can dramatically alter cocaine-primed reinstatement in rats. This effect was dependent on the time of day at which rats were trained and tested for reinstatement. Previous studies have demonstrated time-of-day differences in cocaine- or amphetamine-induced behaviors, including locomotor sensitization (Gaytan et al., 1999, Abarca et al., 2002, Uz et al., 2002, Akhisaroglu et al., 2004, Sleipness et al., 2005), CPP (Abarca et al., 2002, Kurtuncu et al., 2004, Webb et al., 2009b) and self-administration (Deneau et al., 1969, Negus et al., 1995, Roberts and Andrews, 1997, Baird and Gauvin, 2000, Roberts et al., 2002, Bass et al., 2010). Other work has demonstrated that performance on a learning task is better when animals are trained and tested at the same time of day compared to when training and testing times differ (Cain et al., 2004a, Cain et al., 2004b). We can add to this list, photoperiod history in the control of reinstatement behavior based on our findings that reinstatement was suppressed in rats exposed to shorter daylengths, whereas it was not in rats switched to longer daylengths
We now confirm using the CPP paradigm that this process is endogenously generated by virtue of being expressed under conditions of constant darkness. The lack of clear rhythmicity under conditions of constant light is somewhat unexpected given that cocaine self-administration exhibits a free-running rhythm under these conditions, albeit with a longer period and greatly suppressed intake (Bass et al., 2010). Thus, it is possible that even the slight elevation we observed at 20h after lights-on represents the endogenous peak in cocaine reinstatement under our current conditions. This is not contradictory, as the peak would be expected to occur later due to the slowing of the endogenous oscillator by light. Nevertheless, more training and testing times will be required to confirm this hypothesis.
Interestingly, we did not find any photoperiod-induced changes in rats trained and tested at ZT12. This lack of effect may be related to what we have termed the “reward potential” in rats (Sleipness et al., 2007a), which we hypothesized is manifest as the synchronized expression of motivated behavior (feeding, reproduction) to those times of day when an animal is most likely to survive when engaged in them. For rats housed on a 12L:12D light cycle, the peak of reward was observed at the beginning of dark onset (ZT12). Even in constant darkness, the peak in reinstatement occurred at 12hr after lights-off. Apparently, changing the photoperiod was not able to influence cocaine-primed reinstatement even when ZT12 occurred during the light phase (e.g., under 18L:6D) because it is apparently phase-locked. This phenomenon could also reflect the proposed role of the circadian system in learning and memory (Daan, 2000).
Reinstatement of CPP is dependent on spatial memory, and therefore the suppression of cocaine-primed reinstatement observed at ZT4 and ZT20 may be due to a memory deficit induced by shifting photoperiods rather than to a suppression of the reinforcing efficacy of cocaine. Previous studies have examined the impact of phase shifts on memory performance (Childs and Redfern, 1981, Tapp and Holloway, 1981, Fekete et al., 1985, Fekete et al., 1986, Reijmers et al., 2001, Loh et al., 2010). In these studies, the photoperiod remained the same, but the light cycle was shifted, so that rats were exposed to a jet-lag scenario. It is doubtful that our results were due strictly to phase shifting since animals were given adequate time to reentrain their activity cycles and thus adjust to a shift (Yamazaki et al., 2000, Reddy et al., 2002). Nevertheless, the incorporation of photoperiod history may be able to impact future performance and thus is worth considering in this context.
Tapp and Holloway (1981) found that a 6 hr or 12 hr phase shift in rats immediately after passive avoidance training impaired performance when rats were tested a few days later, during the period of resynchronization. However, this effect persisted even after activity rhythms of rats were re-entrained to the new light cycle. Since animals that were phase shifted 5 days after training and then tested a few days later during circadian disorganization did not demonstrate amnesia, these results pointed to an effect of phase shifts on memory consolidation processes. The same study showed that retention of the passive avoidance memory was dependent on the pattern of re-entrainment of circadian activity rhythms; rats that demonstrated smooth shifts to the new cycle showed less impairment than rats that demonstrated abrupt shifts to the new cycle. In the present study, rats were already trained for CPP prior to altering their photoperiods, and thus the suppressive effect of photoperiodic shifts on reinstatement would not likely be related to an effect on the consolidation of memory. Instead, the suppressive effects of these shifts on CPP behavior may be related to the process of reconsolidation.
Memory reconsolidation begins when original memories are reactivated into a labile state. In the labile state, memories are susceptible to disruption by a variety of pharmacological agents (Misanin et al., 1968, Lewis, 1979, Mactutus et al., 1979, Judge and Quartermain, 1982, Nader et al., 2000, Sara, 2000, Dudai, 2004, Alberini, 2005, Tronson et al., 2006, Taylor et al., 2009). In drug abuse studies, disruption of reconsolidation is examined by reactivating memory of the drug-associated context, cue, and/or the drug itself (interoceptive cues) in the presence of a pharmacological agent. Later, in the absence of this agent, animals are tested for suppressed drug-seeking behavior on a test for reinstatement induced by the same stimulus used to reactivate the memory. Studies have shown that numerous agents suppress cocaine-seeking behavior in the CPP model (Miller and Marshall, 2005, Bernardi et al., 2006, Milekic et al., 2006, Valjent et al., 2006, Robinson and Franklin, 2007, Brown et al., 2008, Fricks-Gleason and Marshall, 2008, Itzhak, 2008, Wang et al., 2008, Zhai et al., 2008, Yu et al., 2009). Because phase shifts can alter the rhythms of proteins in the brain (Field et al., 2000, Yamazaki et al., 2000, Angeles-Castellanos et al., 2007), phase shifts may be similar to pharmacological agents in their ability to disrupt the reconsolidation process, which in the present experiment would be manifest as a suppression of CPP reinstatement upon subsequent testing. Thus, simply reactivating the memory by cocaine-primed reinstatement under certain photoperiods different from the one in which training originally occurred might prevent reconsolidation of the salient cocaine-associated memory and dampen subsequent cocaine-primed reinstatement. Therefore, similar to the “time-stamp” described in fear conditioning (Cain et al., 2004a, Cain et al., 2008), a “photoperiod-stamp” may also exist.
Other studies suggest that photoperiod shifts may impair the retrieval of memory. For example, Fekete et al. (1985; 1986) found that passive avoidance performance was impaired only transiently during the time before rats could re-entrain to the new light cycle. In addition, rats trained under their standard light cycle and phase shifted just prior to testing for memory retention showed impaired performance; both of these results suggest that the retrieval of memory was impaired. A retrieval impairment could explain the present findings, but unlike the findings of Fekete et al. (1985; 1986), rats in our study did not demonstrate recovery of retrieval at ZT4 and ZT20 even after allowing rats the necessary time to re-entrain to the new light cycle, indicating that photoperiod switches and phase shifts (within the same photoperiod) utilize potentially different mechanisms.
Influence of photoperiod on DAT and TH protein levels
The photoperiodic control of rewarding behaviors has received little attention despite the fact that photoperiod is well known to impact many physiological events including reproduction, migration, hibernation, and coat characteristics (pelage). Reproduction is considered highly dependent upon dopamine for its actions although the populations of DA neurons involved differ from those of the reward system (i.e., tuberoinfundibular and A15 vs. mesolimbic/mesocortical) (Goodman et al., 2010). Our laboratory and others have reported time-of-day differences in DAT and TH protein levels (Sleipness et al., 2007b, Webb et al., 2009a, Webb et al., 2009b) and in DAT function (Sleipness et al., 2008) in the mPFC and nucleus accumbens. Several other groups have also demonstrated diurnal or circadian changes in dopamine and its metabolites (O’Neill and Fillenz, 1985, Paulson and Robinson, 1994, Schade et al., 1995, Paulson and Robinson, 1996, Feenstra et al., 2000, Castaneda et al., 2004, Webb et al., 2009a) and in dopamine D2 receptor function (Tonissaar et al., 2006). However, as far as we know, the influence of altered photoperiod on DAT and TH within the mesocortical/mesolimbic DA system has not been examined. The impact of photoperiodic shifts at ZT20 (the only time tested here) on TH and DAT protein concentrations was greatest within the mPFC as compared with the dorsal striatum, which generally showed smaller changes. The nucleus accumbens and VTA showed no changes across different photoperiods. These results thus reveal a regional specificity in photoperiodic influences and point to a selective engagement of brain region rather than a non-specific effect. In the mPFC, DAT levels were highest for both short (6L:18D) and long (18L:6D) photoperiods when TH levels were lowest, indicating that DAT function is highest at a time when dopamine synthesis is low, which would be expected to produce an overall decrease in basal dopamine levels in the mPFC. While it is unknown how these putative alterations in basal dopamine in the absence of cocaine would translate into effects on place-preference behavior during cocaine-primed reinstatement, the mPFC has been shown to play a key role in cocaine-primed reinstatement for both CPP (Capriles et al., 2003, Sanchez et al., 2003, Zavala et al., 2003) and self-administration (McFarland and Kalivas, 2001, Park et al., 2002, McFarland et al., 2003). In addition, recent work in the DAT knockout mouse supports the notion that dopamine is important for memory recall under partial cue conditions (Li et al., 2010). Given that animals tested during dark periods may perceive cues differently from when they were presented in light conditions but at the same ZT, it is suggested that the suppression of DAT by short photoperiods may mediate this disruption. The levels of basal DAT protein in the mPFC under the 12L:12D cycle may not recover completely once rats have been shifted to the shorter photoperiod, indicating that either the shorter photoperiod under 6L:18D and/or the switch to constant darkness alters DAT levels. This hypothesized process may thus partly underlie the suppression of reinstatement under this photoperiod and after the return to 12L:12D. Similarly, the decreased levels of basal TH in the mPFC after the shorter photoperiod (6L:18D) facilitate the suppression of reinstatement, potentially either by a memory-related mechanism or a devaluation of cocaine’s reinforcing effects during reinstatement under 6L:18D. However, the changes in DAT and TH appeared to be nearly symmetrical between rats switched to a longer daylength and those switched to a shorter daylength, suggesting that changes in these molecules are not entirely sufficient to explain the suppressed reinstatement found only after shorter daylengths.
The main changes in the dorsal striatum were an increase in both DAT and TH levels after rats were taken through all photoperiod switches such that the levels of these molecules were higher when they were returned to 12L:12D compared with when they were housed under their original 12L:12D schedule (with exception of the shorter daylength for DAT). Once again, however, these changes were generally symmetrical for animals switched to shorter or longer daylengths, suggesting that any changes in DAT or TH likely only partially explain the suppressed reinstatement after shorter, but not longer, daylengths.
Of note is that DAT and TH were assessed only under baseline conditions; that is, these animals had no cocaine history, and cocaine was not present at the time of analysis. Thus, it is possible that there is an interaction between photoperiod and the presence of cocaine during reinstatement such that the function of these molecules is altered. This would be expected to involve neurons in the VTA and NAc. Intriguingly, we have previously reported that rats initially held under 12L:12D and then housed in complete darkness displayed an unusual pattern of dopamine clearance in the mPFC (Sleipness et al., 2008). Cocaine, which inhibited mPFC dopamine clearance at all times of day in rats held under 12L:12D, showed no inhibition of clearance when rats were switched to complete darkness. Furthermore, cocaine significantly enhanced dopamine uptake at 16hr after lights-off (Sleipness et al., 2008). Thus, altering light:dark ratios can profoundly alter the clearance of dopamine in the presence of cocaine.
The changes in TH and DAT protein levels may be linked to corresponding changes in gene expression within the brain regions examined. Photoperiod changes are reflected in shifts of the daily rhythm of several clock genes and proteins expressed in the SCN (Sumova et al., 1995, Messager et al., 2000, Lincoln et al., 2002, Tournier et al., 2009). A role for Clock in the mesolimbic DA system is now well established (McClung et al., 2005, Roybal et al., 2007) and implicates the clock genes directly in the regulation of the brain’s mesolimbic DA system. An interaction between clock genes and dopamine would therefore be expected to account for the complement of cocaine-related behaviors in animals exposed to a variety of different photoperiodic or seasonal conditions.
In summary, we found that cocaine-induced reinstatement is influenced in both circadian (i.e., time-of-day) and photoperiodic (i.e., time-of-year) fashion. Photoperiod history appears to be more important than absolute photoperiod for determining preference since all rats began and ended their photoperiod trials in exactly the same way. Photoperiod also caused changes in both DAT and TH levels in the mPFC and dorsal striatum, suggesting these proteins may mediate some of the photoperiodic effects on rewarding behaviors such as cocaine-primed reinstatement, although this remains to be tested. Alterations in DAT and/or TH would be expected to change local concentrations of DA in the mPFC and dorsal striatum; therefore, if a “threshold” is required for cocaine’s rewarding effects (Norman et al., 1999) the combination of photoperiod history and photoperiodic suppression of DAT/TH could prevent reward from being expressed. Together, our findings lend support to the hypothesis that photoperiod may contribute to the seasonal expression of certain illicit behaviors in humans living at different latitudes and suggest that altering the light:dark ratio could provide a novel method for modulating the reward potential of drugs of abuse.
RESEARCH HIGHLIGHTS.
This study examined if daylength (photoperiod) affects cocaine CPP in rats.
The findings reveal that short, but not long, photoperiods suppress cocaine CPP.
Cocaine CPP is sensitive to prior photoperiod history.
Proteins involved in regulating dopamine in the brain are affected by daylength.
ACKNOWLEDGMENTS
The authors wish to thank Ms. Jenny Baylon and Jenny Browning for assistance with the manuscript. This work was supported by NIH grant DA023202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol Biochem Behav. 2004;79:37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Wever R. Human circadian rhythms: a multioscillatory system. Fed Proc. 1976;35:236–232. [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65:289–299. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Bass CE, Jansen HT, Roberts DCS. Free-running rhythms of cocaine self-adminstration in rats held under constant lighting conditions. Chronobiology International: The Journal of Biological & Medical Rhythm Research. 2010;27:535–548. doi: 10.3109/07420521003664221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bredow S, Guha-Thakurta N, Taishi P, Obal F, Jr., Krueger JM. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4:84–90. doi: 10.1159/000097325. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Are humans seasonally photoperiodic? J Biol Rhythms. 2004;19:180–192. doi: 10.1177/0748730404264658. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, Chou T, Ralph MR. Circadian modulation of performance on an aversion-based place learning task in hamsters. Behav Brain Res. 2004a;150:201–205. doi: 10.1016/j.bbr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cain SW, Ko CH, Chalmers JA, Ralph MR. Time of day modulation of conditioned place preference in rats depends on the strain of rat used. Neurobiol Learn Mem. 2004b;81:217–220. doi: 10.1016/j.nlm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Cain SW, McDonald RJ, Ralph MR. Time stamp in conditioned place avoidance can be set to different circadian phases. Neurobiol Learn Mem. 2008;89:591–594. doi: 10.1016/j.nlm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Childs G, Redfern PH. A circadian rhythm in passive avoidance behavior: the effect of phase shift and the benzodiazepines. Neuropharmacology. 1981;20:1365–1366. [PubMed] [Google Scholar]
- Daan S. Learning and circadian behavior. J Biol Rhythms. 2000;15:296–299. doi: 10.1177/074873000129001396. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–121. discussion 121-115, 281-114. [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience. 2000;100:741–748. doi: 10.1016/s0306-4522(00)00319-5. [DOI] [PubMed] [Google Scholar]
- Fekete M, Van Ree JM, De Wied D. The ACTH-(4-9) analog ORG 2766 and desglycinamide9-(Arg8)-vasopressin reverse the retrograde amnesia induced by disrupting circadian rhythms in rats. Peptides. 1986;7:563–568. doi: 10.1016/0196-9781(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Fekete M, van Ree JM, Niesink RJ, de Wied D. Disrupting circadian rhythms in rats induces retrograde amnesia. Physiol Behav. 1985;34:883–887. doi: 10.1016/0031-9384(85)90008-3. [DOI] [PubMed] [Google Scholar]
- Field MD, Maywood ES, O’Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Curr Biol. 2008;18:R784–R794. doi: 10.1016/j.cub.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan O, Lewis C, Swann A, Dafny N. Diurnal differences in amphetamine sensitization. Eur J Pharmacol. 1999;374:1–9. doi: 10.1016/s0014-2999(99)00243-5. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. J Neuroendocrinol. 2010;22:674–681. doi: 10.1111/j.1365-2826.2010.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. J Neuroendocrinol. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann N Y Acad Sci. 2008;1139:350–357. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- Judge ME, Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol Behav. 1982;28:585–590. doi: 10.1016/0031-9384(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Kang SW, Thayananuphat A, Bakken T, El Halawani ME. Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience. 2007;150:223–233. doi: 10.1016/j.neuroscience.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–205. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Langworthy RH, McKelvie AR. Seasonal use of marijuana and cocaine by arrestees in Anchorage, Alaska. University of Alaska Anchorage; Anchorage, Alaska: 2005. [Google Scholar]
- Leclerc B, Kang SW, Mauro LJ, Kosonsiriluk S, Chaiseha Y, El Halawani ME. Photoperiodic modulation of clock gene expression in the avian premammillary nucleus. J Neuroendocrinol. 2010;22:119–128. doi: 10.1111/j.1365-2826.2009.01942.x. [DOI] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Li F, Wang LP, Shen X, Tsien JZ. Balanced Dopamine Is Critical for Pattern Completion during Associative Memory Recall. PLoS One. 2010;5:e15401. doi: 10.1371/journal.pone.0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc Natl Acad Sci U S A. 2002;99:13890–13895. doi: 10.1073/pnas.212517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mactutus CF, Riccio DC, Ferek JM. Retrograde amnesia for old (reactivated) memory: some anomalous characteristics. Science. 1979;204:1319–1320. doi: 10.1126/science.572083. [DOI] [PubMed] [Google Scholar]
- Mari F, Politi L, Biggeri A, Accetta G, Trignano C, Di Padua M, Bertol E. Cocaine and heroin in waste water plants: a 1-year study in the city of Florence, Italy. Forensic Sci Int. 2009;189:88–92. doi: 10.1016/j.forsciint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- McClung CA. Role for the Clock gene in bipolar disorder. Cold Spring Harb Symp Quant Biol. 2007;72:637–644. doi: 10.1101/sqb.2007.72.031. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Hazlerigg DG, Mercer JG, Morgan PJ. Photoperiod differentially regulates the expression of Per1 and ICER in the pars tuberalis and the suprachiasmatic nucleus of the Siberian hamster. Eur J Neurosci. 2000;12:2865–2870. doi: 10.1046/j.1460-9568.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Morris RW. Circadian and circannual rhythms of emergency room drug-overdose admissions. Prog Clin Biol Res. 1987;227B:451–457. [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lukas SE, Mendelson JH. Diurnal patterns of cocaine and heroin self-administration in rhesus monkeys responding under a schedule of multiple daily sessions. Behav Pharmacol. 1995;6:763–775. [PubMed] [Google Scholar]
- Norman AB, Norman MK, Hall JF, Tsibulsky VL. Priming threshold: a novel quantitative measure of the reinstatement of cocaine self-administration. Brain Res. 1999;831:165–174. doi: 10.1016/s0006-8993(99)01423-7. [DOI] [PubMed] [Google Scholar]
- O’Neill RD, Fillenz M. Simultaneous monitoring of dopamine release in rat frontal cortex, nucleus accumbens and striatum: effect of drugs, circadian changes and correlations with motor activity. Neuroscience. 1985;16:49–55. doi: 10.1016/0306-4522(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschane D. Variability of substance abuse. Global variability of substance abuse: is latitude a unique etiological factor? Int J Circumpolar Health. 1998;57:228–238. [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994;108:624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Analysis of circadian patterns using automated on-line microdialysis. Neuropsychopharmacology. 1996;14:325–337. doi: 10.1016/0893-133X(95)00141-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organisation of living systems. Cold Spring Harbor Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Zetin M, Stamenkovic V, Kripke D, Bunney WEj. Seasonal affective disorder: prevalence varies with latitude and climate. Clinical Neuropharmacology. 1986;9:181–183. [PubMed] [Google Scholar]
- Pozdeyev NV, Lavrikova EV. Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J Mol Neurosci. 2000;15:1–9. doi: 10.1385/JMN:15:1:1. [DOI] [PubMed] [Google Scholar]
- Quello SB, Brady KT, Sonne SC. Mood disorders and substance use disorder: a complex comorbidity. Sci Pract Perspect. 2005;3:13–21. doi: 10.1151/spp053113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Ko CH, Antoniadis EA, Seco P, Irani F, Presta C, McDonald RJ. The significance of circadian phase for performance on a reward-based learning task in hamsters. Behav Brain Res. 2002;136:179–184. doi: 10.1016/s0166-4328(02)00131-6. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochem Biophys Res Commun. 2010;391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond RC, Warren M, Morris RW, Leikin JB. Periodicity of presentations of drugs of abuse and overdose in an emergency department. J Toxicol Clin Toxicol. 1992;30:467–478. doi: 10.3109/15563659209021561. [DOI] [PubMed] [Google Scholar]
- Reddy AB, Field MD, Maywood ES, Hastings MH. Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J Neurosci. 2002;22:7326–7330. doi: 10.1523/JNEUROSCI.22-17-07326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Leus IE, Burbach JP, Spruijt BM, van Ree JM. Social memory in the rat: circadian variation and effect of circadian rhythm disruption. Physiol Behav. 2001;72:305–309. doi: 10.1016/s0031-9384(00)00434-0. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM. Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology (Berl) 1997;131:271–277. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Effects of anisomycin on consolidation and reconsolidation of a morphine-conditioned place preference. Behav Brain Res. 2007;178:146–153. doi: 10.1016/j.bbr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Rocchi MB, Miotto P, Preti A. Seasonal variation in suicides and in deaths by unintentional illicit acute drug intoxications. Addict Biol. 2004;9:255–263. doi: 10.1080/13556210412331292587. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr., McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine D1-like receptor activation in the rat medial prefrontal cortex alters stress-and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Kanofsky JD. Cocaine addiction: relationship to seasonal affective disorder. Int J Neurosci. 1992;64:195–201. doi: 10.3109/00207459209000545. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J, Golor G, Lemmer B. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats--influence on dopaminergic stimulation. Chronobiol Int. 1995;12:87–99. doi: 10.3109/07420529509064504. [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, de la Iglesia HO, Zlomanczuk P, Illnerova H. Encoding le quattro stagioni within the mammalian brain: photoperiodic orchestration through the suprachiasmatic nucleus. J Biol Rhythms. 2001;16:302–311. doi: 10.1177/074873001129002024. [DOI] [PubMed] [Google Scholar]
- Sher L. Relationships between seasonality and alcohol use: a genetic hypothesis. Med Hypotheses. 2002;59:85–88. doi: 10.1016/s0306-9877(02)00130-5. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Jansen HT, Schenk JO, Sorg BA. Time-of-day differences in dopamine clearance in the rat medial prefrontal cortex and nucleus accumbens. Synapse. 2008;62:877–885. doi: 10.1002/syn.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Res. 2005;1065:132–137. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiol Behav. 2007a;91:523–530. doi: 10.1016/j.physbeh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 2007b;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Sumova A, Travnickova Z, Peters R, Schwartz WJ, Illnerova H. The rat suprachiasmatic nucleus is a clock for all seasons. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7754–7758. doi: 10.1073/pnas.92.17.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taishi P, Bredow S, Guha-Thakurta N, Obal F, Jr., Krueger JM. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–1058. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JC, Chemineau P, Hernandez X, Migaud M, Malpaux B. Neuroendocrine interactions and seasonality. Domest Anim Endocrinol. 2002;23:87–100. doi: 10.1016/s0739-7240(02)00148-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonissaar M, Herm L, Rinken A, Harro J. Individual differences in sucrose intake and preference in the rat: circadian variation and association with dopamine D2 receptor function in striatum and nucleus accumbens. Neurosci Lett. 2006;403:119–124. doi: 10.1016/j.neulet.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Tournier BB, Birkenstock J, Pevet P, Vuillez P. Gene expression in the suprachiasmatic nuclei and the photoperiodic time integration. Neuroscience. 2009;160:240–247. doi: 10.1016/j.neuroscience.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Uitenbroek DG. Seasonal variation in alcohol use. J Stud Alcohol. 1996;57:47–52. doi: 10.15288/jsa.1996.57.47. [DOI] [PubMed] [Google Scholar]
- Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen S, Urbanoski K, Cairney J. Geographical variation in the prevalence of problematic substance use in Canada. Can J Psychiatry. 2007;52:426–433. doi: 10.1177/070674370705200704. [DOI] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Lehman MN, Coolen LM. Bidirectional interactions between the circadian and reward systems: is restricted food access a unique zeitgeber? Eur J Neurosci. 2009a;30:1739–1748. doi: 10.1111/j.1460-9568.2009.06966.x. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009b;24:465–476. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Westrin A, Lam RW. Seasonal affective disorder: a clinical update. Ann Clin Psychiatry. 2007;19:239–246. doi: 10.1080/10401230701653476. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yu LL, Wang XY, Zhao M, Liu Y, Li YQ, Li FQ, Wang X, Xue YX, Lu L. Effects of cannabinoid CB1 receptor antagonist rimonabant in consolidation and reconsolidation of methamphetamine reward memory in mice. Psychopharmacology (Berl) 2009;204:203–211. doi: 10.1007/s00213-008-1450-y. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- Zhai H, Wu P, Chen S, Li F, Liu Y, Lu L. Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behav Pharmacol. 2008;19:211–216. doi: 10.1097/FBP.0b013e3282fe88a0. [DOI] [PubMed] [Google Scholar]