Abstract
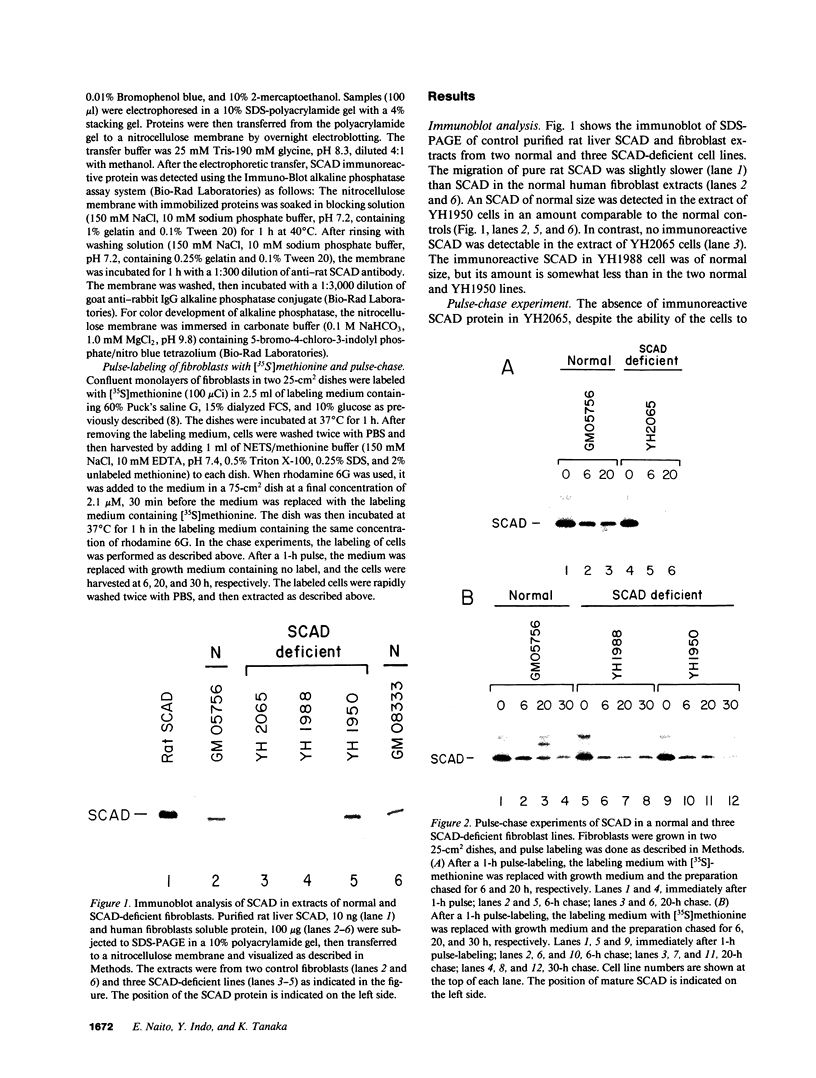
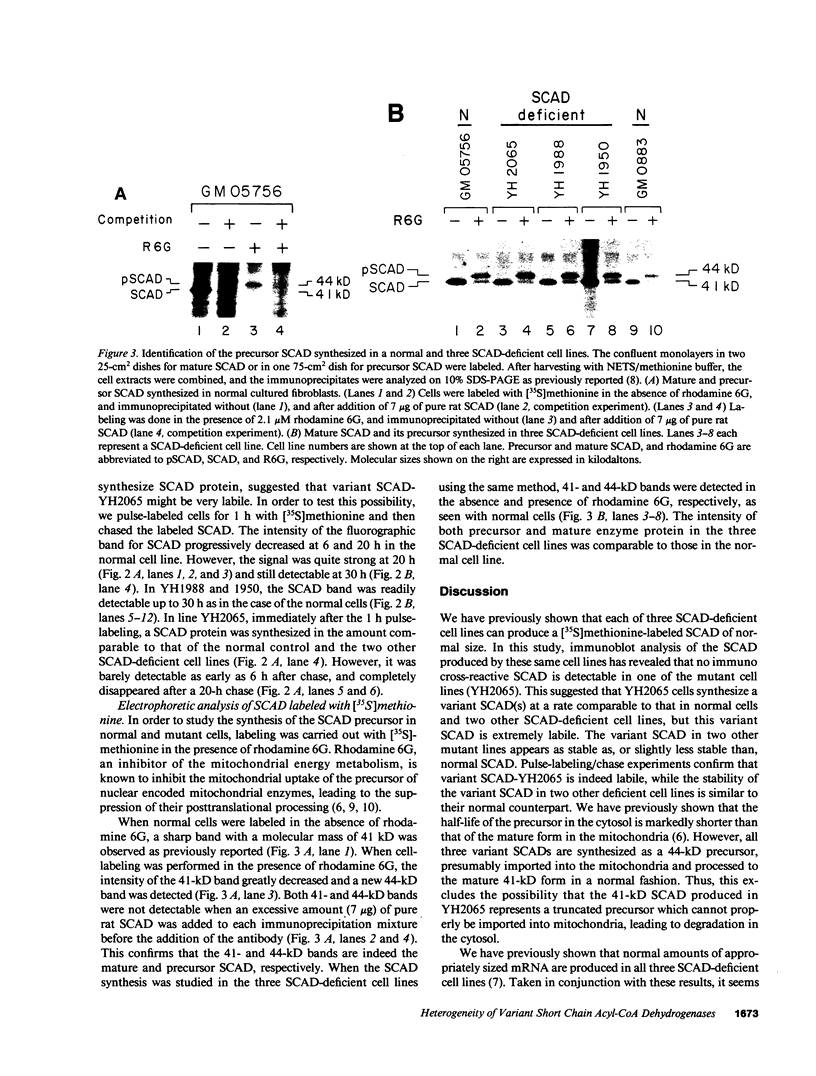
Using a [35S]methionine labeling/immunoprecipitation technique, we have previously shown that cultured skin fibroblast from three patients with short chain acyl-CoA dehydrogenase (SCAD) deficiency each synthesize a normal-sized (41 kD) variant SCAD in an amount comparable to that of normal cells. In the current study, these same cell lines were reexamined with immunoblot analysis. In one cell line (YH2065) no SCAD protein was detectable. In the other two deficient cell lines, the amount of variant SCAD was similar to, or only slightly less than, normal. These results suggested that SCAD-YH2065 is labile. In the pulse-labeling experiments, labeled SCAD was readily detectable for at least 30 h in a normal control and two other SCAD-deficient cell lines. In contrast, the labeled SCAD band in YH2065 cells was barely detectable at 6 h and undetectable at 20 h. [35S]Methionine-labeling in the presence of rhodamine 6G demonstrated that SCAD-YH2065 was synthesized as a 44-kD precursor and imported normally into mitochondria, as were the normal SCAD and two other variant SCADs, excluding the possibility that SCAD-YH2065 is a truncated precursor that cannot be imported into mitochondria. These results indicate that the mutations responsible for SCAD deficiency are heterogeneous, and emphasize the importance of using both radiolabeling and immunoblotting when evaluating such genetic defects at the protein level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amendt B. A., Greene C., Sweetman L., Cloherty J., Shih V., Moon A., Teel L., Rhead W. J. Short-chain acyl-coenzyme A dehydrogenase deficiency. Clinical and biochemical studies in two patients. J Clin Invest. 1987 May;79(5):1303–1309. doi: 10.1172/JCI112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates P. M., Hale D. E., Finocchiaro G., Tanaka K., Winter S. C. Genetic deficiency of short-chain acyl-coenzyme A dehydrogenase in cultured fibroblasts from a patient with muscle carnitine deficiency and severe skeletal muscle weakness. J Clin Invest. 1988 Jan;81(1):171–175. doi: 10.1172/JCI113290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Kraus J. P., Rosenberg L. E. Immunochemical studies of fibroblasts from patients with methylmalonyl-CoA mutase apoenzyme deficiency: detection of a mutation interfering with mitochondrial import. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1421–1424. doi: 10.1073/pnas.84.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Dabrowski C., Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. Identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J Biol Chem. 1983 Jan 25;258(2):1066–1076. [PubMed] [Google Scholar]
- Ikeda Y., Keese S. M., Fenton W. A., Tanaka K. Biosynthesis of four rat liver mitochondrial acyl-CoA dehydrogenases: in vitro synthesis, import into mitochondria, and processing of their precursors in a cell-free system and in cultured cells. Arch Biochem Biophys. 1987 Feb 1;252(2):662–674. doi: 10.1016/0003-9861(87)90072-5. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Keese S. M., Tanaka K. Molecular heterogeneity of variant isovaleryl-CoA dehydrogenase from cultured isovaleric acidemia fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7081–7085. doi: 10.1073/pnas.82.20.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Okamura-Ikeda K., Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. Isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. J Biol Chem. 1985 Jan 25;260(2):1311–1325. [PubMed] [Google Scholar]
- Matsubara Y., Indo Y., Naito E., Ozasa H., Glassberg R., Vockley J., Ikeda Y., Kraus J., Tanaka K. Molecular cloning and nucleotide sequence of cDNAs encoding the precursors of rat long chain acyl-coenzyme A, short chain acyl-coenzyme A, and isovaleryl-coenzyme A dehydrogenases. Sequence homology of four enzymes of the acyl-CoA dehydrogenase family. J Biol Chem. 1989 Sep 25;264(27):16321–16331. [PubMed] [Google Scholar]
- Morita T., Mori M., Ikeda F., Tatibana M. Transport of carbamyl phosphate synthetase I and ornithine transcarbamylase into mitochondria. Inhibition by rhodamine 123 and accumulation of enzyme precursors in isolated hepatocytes. J Biol Chem. 1982 Sep 25;257(18):10547–10550. [PubMed] [Google Scholar]
- Naito E., Ozasa H., Ikeda Y., Tanaka K. Molecular cloning and nucleotide sequence of complementary DNAs encoding human short chain acyl-coenzyme A dehydrogenase and the study of the molecular basis of human short chain acyl-coenzyme A dehydrogenase deficiency. J Clin Invest. 1989 May;83(5):1605–1613. doi: 10.1172/JCI114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]