Abstract
CD40 is required for T-dependent humoral immunity, but can also contribute to the pathogenesis of autoimmunity and B cell malignancy. The TNF receptor associated factor (TRAF)2 and TRAF6 adaptor proteins are positive regulators of CD40 signaling required to activate downstream kinase cascades and transcription factors. In contrast, TRAF3 can serve as a negative regulator of CD40 signaling, and CD40 signals are amplified in TRAF3−/− B cells. We previously reported a gain-of-function polymorphism of the human CD40 receptor, hCD40-P227A, which signals in an amplified manner to B lymphocytes. Here, we show that hCD40-P227A binds more TRAF3 and TRAF5, as well as certain associated proteins, than Wt-CD40. Studies in TRAF-deficient B cell lines revealed that hCD40-P227A uses TRAF3 as a positive rather than negative regulator. Although located outside of any known TRAF binding sites, the P227A polymorphism can alter TRAF binding and dramatically changes the role played by TRAF3 in CD40 signaling.
Introduction
CD40, a member of the Tumor Necrosis Factor Receptor (TNFR) superfamily of molecules, is constitutively expressed on a variety of cell types, including macrophages, dendritic cells and B cells (1), and can also be inducibly expressed on additional cell types following activation (2). CD154, the ligand for CD40, is expressed transiently on the surface of activated T cells (2), and its binding to CD40 on B cells is required for T-dependent humoral immunity (1, 2). However, CD40:CD154 interactions also contribute to the pathogenesis of many autoimmune diseases, including Graves’ disease (GD), multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) (3, 4). A Kozak sequence single nucleotide polymorphism (SNP) in the 5’-UTR of CD40 is associated with GD in multiple ethnic groups, and enhances expression of CD40 protein on the cell surface (5, 6). Recently, SNPs in the 5’-UTR and 2nd intron of human CD40 have been associated with incidence of MS and RA (7, 8). These SNPs are in complete linkage disequilibrium with each other and with the GD-associated SNP, indicating that each arose independently and each may be associated with a distinct functional modification. How these genetic changes affect CD40 protein expression or function is unknown (7, 8).
The cytoplasmic domain of CD40 lacks intrinsic enzymatic activity, instead transducing intracellular signals by binding to TRAFs 1, 2, 3, 5, and 6 (1). Studies in TRAF-deficient B cell lines revealed that TRAF2 and TRAF6 are positive regulators of CD40 signaling, while TRAF3 is a negative regulator (9–11). Latent membrane protein 1 (LMP1) is an Epstein-Barr Virus (EBV)-encoded CD40 mimic which activates many of the same signaling pathways as CD40, yet does so in an amplified and sustained manner compared to CD40 (10, 12). LMP1 also binds TRAF2 and TRAF3, recruiting 2–3 fold more TRAF3 to its cytoplasmic domain than CD40, yet uses TRAF3 as a positive rather than negative regulator, and binds TRAF3 in a manner that is overlapping but distinct from that of CD40 (10, 13). Thus, different receptors can utilize the same TRAFs to activate cellular signaling events, but the distinct nature of their interactions and different TRAF roles can regulate the magnitude and duration of activation.
We and colleagues previously identified a gain-of-function allele of human(h) CD40, hCD40-P227A, which is highly overrepresented in individuals of Mexican and South American descent (14). SLE patients with this genetic heritage are known to be predisposed to more severe forms of SLE (15), although it is unclear whether the hCD40-P227A allele plays a causal role, as SLE is multifactorial in origin, a common characteristic of human autoimmune disease. The proline-to-alanine amino acid substitution at position 227 of CD40 is three amino acids upstream of the TRAF6 binding site (Figure 1A) in the cytoplasmic domain of CD40 (14). Signaling via hCD40-P227A relative to endogenous or transfected Wt-CD40 receptors in mouse(m) or human B cell lines results in increased phosphorylation of JNK and its substrate c-Jun increased production of the pro-inflammatory cytokines IL-6 and TNF-α, increased Ig production, and enhanced ability to cooperate with signals from the B cell antigen receptor (BCR) leading to enhanced synergistic production of IL-6 and Ig (14). The activity of hCD40-P227A is remarkably similar to that of LMP1, which also signals in an amplified and sustained manner compared to Wt-CD40. Thus, we hypothesized that hCD40-P227A could be using TRAFs differently from Wt-CD40, perhaps in a manner analogous to LMP1.
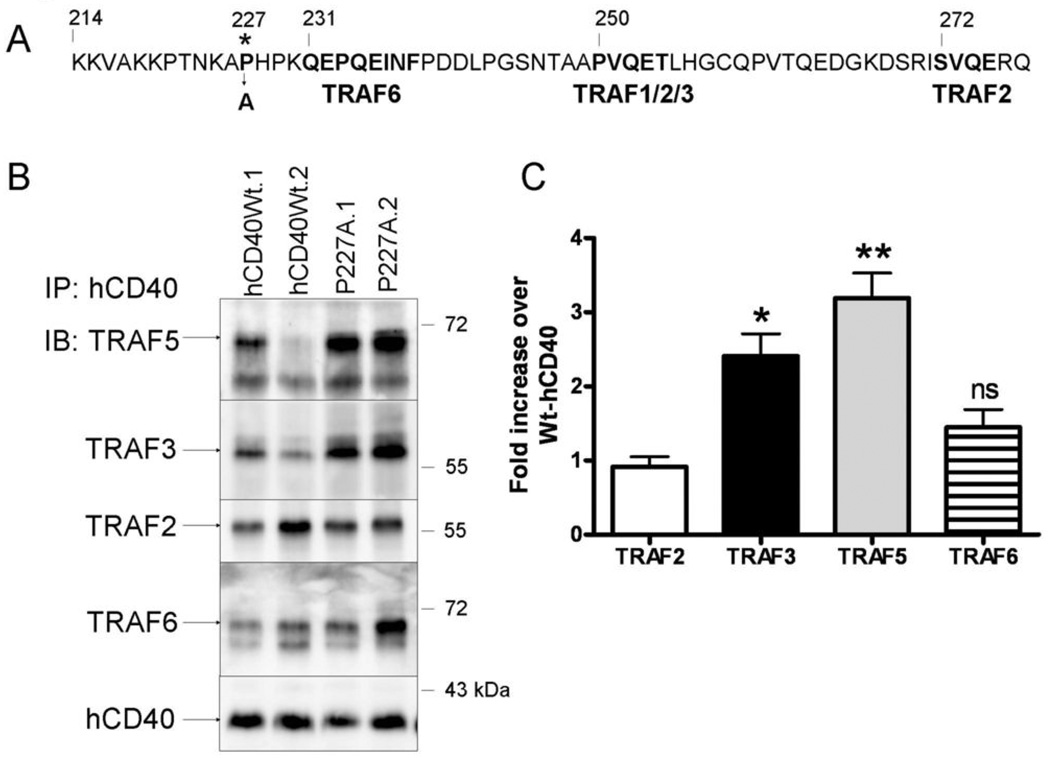
Figure 1. TRAF binding by hCD40-P227A vs. hCD40Wt.
A. Protein sequence of the hCD40 cytoplasmic domain, highlighting the P227A mutation and its proximity to known TRAF binding sites (bold type).
B. CH12.LX cells stably expressing matched amounts of hCD40Wt (hCD40Wt.1, hCD40Wt.2) or hCD40-P227A (P227A.1, P227A.2) were stimulated with agonistic anti-hCD40 Ab coated magnetic beads as described in Methods for 15 minutes, lysed, and the receptor complex isolated by magnetic field immunoprecipitation. Proteins were resolved by SDS-PAGE and immunoblotted for TRAF5. The membrane was stripped and re-probed for the following proteins in sequence: TRAF6, TRAF2, TRAF3, and hCD40 as a loading control. Results are representative of 3 independent experiments.
C. Amounts of associating TRAFs were normalized to the amount of receptor precipitated. Data are presented as a fold increase of TRAF bound to the average of both hCD40-P227A clones relative to both clones of hCD40-Wt and are the mean ± SE of three separate experiments. * = p<0.05; ** = p<0.005; ns =not significant by Student’s t-test.
To address this hypothesis, we stably expressed hCD40-P227A in TRAF2−/−, TRAF3−/−, or TRAF6−/− mouse B cell lines known to be good models for CD40-mediated signals (10, 11, 16), and examined TRAF association and TRAF dependence of CD40 functions. TRAF3 displayed enhanced association with hCD40-P227A compared to Wt-CD40. Of particular interest, this enhanced association corresponded to a change in the function of TRAF3 from a Wt-CD40 inhibitor to a stimulatory factor for hCD40-P227A. These findings reveal the important principle that structural changes outside of canonical TRAF binding sites can alter TRAF binding and lead to striking alterations in TRAF function.
Materials and Methods
Cells
The CD40-responsive mature mouse B cell lines A20.2J (17) and CH12.LX (18), their subclones lacking TRAF2 (T2−/−) (16), TRAF3 (T3−/−) (10), or TRAF6 (T6−/−) (11), and subclones stably expressing hCD40-Wt (16), hCD40-LMP1 (10), hCD40Δ55 (19) or hCD40-P227A (14) have been described in the references cited. Cells were grown in RPMI 1640 supplemented with 10% FCS (Atlanta Biologicals, Norcross, GA), 10 µM 2-ME (GIBCO, Grand Island, NY), penicillin and streptomycin (B-cell medium, BCM). 400 µg/ml G418 (Research Products International, Mt. Prospect, IL) was added to maintain transfected cDNA. Subclones expressing similar levels of transfected CD40 as determined by flow cytometry were selected for experiments, with 2 or more individual clones tested for each B cell function (Supplementary Fig 1). Hi5 insect cells infected with WT or hCD154-expressing baculoviruses have been described (14, 19, 20), and provide a source of trimeric, membrane-bound CD154 without overgrowing cell cultures, as insect cells normally grow at room temperature and die at 37°C, forming membrane fragments.
Stable transfection of B cell lines
A20.2J and A20.T6−/− cells were transfected with the plasmid construct hCD40-P227A.neo* and selected with 600 µg/ml G418 (11, 14). CH12.T3−/− and CH12.T2−/− cells were transfected with hCD40-P227A.neo* and selected with 400 µg/ml G418 (10, 14, 16). Surface expression of transfected CD40 was determined by flow cytometry (14). Subclones expressing similar levels of transfected CD40 were selected for experiments, with 2 or more individual clones tested for each B cell function (Supplementary Fig 1).
Abs and chemicals
Stimulatory Abs included 1C10 (rat anti-mCD40 IgG2a), G28-5 (mouse anti-hCD40 IgG1), and the isotype controls mAB72 (rat anti-human α-L-fucosidase IgG2a) and MOPC21 (myeloma protein; mouse IgG1). The 1C10 hybridoma was a kind gift of Dr. Frances Lund (University of Rochester, Rochester, NY). The G28-5, mAB72, and MOPC21 hybridomas were purchased from ATCC (Manassas, VA). Hybridoma supernatants were purified by saturated ammonium sulfate precipitation and the mAbs quantified by isotype-specific sandwich ELISA.
The following Abs were used for immunoblotting: rabbit anti-TRAF5 (H-257), anti-TRAF3 (H-122), rabbit anti-JNK1/2 (FL), and rabbit anti-Act1 (H-300) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-TRAF2 and chicken anti-TRAF6 (Medical and Biological Laboratories, Watertown, MA); rabbit anti-CD40 (Stressgen, Ann Arbor, MI), and rabbit anti-cIAP1 (Proteintech, Chicago, IL); mouse anti-actin (Millipore, Billerica, MA). S12 mouse IgG2a anti-LMP1 Ab was the gift of Dr. F. Wang, Harvard University, Boston, MA. Polyclonal sheep anti-GST-hCD40 was prepared by Elmira Biologicals (Iowa City, IA) as described previously (21). HRP-conjugated polyclonal goat anti–mouse, goat anti–rabbit, and donkey anti–chicken and donkey anti-sheep Abs were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Sheep erythrocytes (SRBC) used in IgM secretion assays were purchased from Elmira Biologicals (Iowa City, IA). Guinea pig complement was purchased from Invitrogen Life Technologies (Carlsbad, CA).
IgM and cytokine assays
The IgM produced by the CH12.LX B cell line recognizes phosphatidylcholine, a major constituent of SRBC membranes (22), allowing Ig-secreting cells to be quantified by direct plaque-forming cell (Pfc) assay on a lawn of sheep erythrocytes (SRBCs) as described (14). The quantitative mouse IL-6 ELISA has been described previously (14).
JNK and c-Jun phosphorylation
The JNK and c-Jun phosphorylation assays were performed as described previously (14). Cells were stimulated at a ratio of 1 Hi5 (Wt or CD154-expressing): 5 B cells. Whole cell lysates were prepared by addition of 100 µl 2× SDS-PAGE sample buffer, sonication, and heating to 95°C for 10 min.
CD40 immunoprecipitation and detection in cell lysates
CD40 and associated TRAFs were immunoprecipitated with G28-5 coated Protein G paramagnetic beads (Invitrogen) as described previously, with the following modifications (11). Cells (25×106) were resuspended in 1 mL BCM and stimulated with 20 µl of Ab-coated beads for 15 min. at 37°C. Cells were pelleted by centrifugation, the medium discarded, and lysed in ice-cold buffer containing 0.5% Triton X-100 as described previously (11). Beads were pelleted using a magnet and washed several times in ice-cold lysis buffer. To aid in quantification of bands present on immunoblots, CD40 was deglycosylated by incubating beads with PNGase F (New England Biolabs, Ipswich, MA) according to manufacturer’s instructions. Proteins were eluted from magnetic beads by addition of 20 µl 2× SDS-PAGE sample buffer, for a total sample volume of 40 µl, and heat denatured at 95°C for 10 min.
Immunoblotting
10 µl of whole-cell lysate (for JNK phosphorylation assays) or 15 µl of immunoprecipitate (for CD40 immunoprecipitation experiments) was resolved by 10% SDS-PAGE. Samples were transferred to Immobilon-P membranes (Millipore) as described previously (14). Membranes were blocked with 10% nonfat dry milk for 1h at 25°C, incubated with primary Ab overnight at 4°C, washed 3×, and incubated with species-specific HRP-conjugated secondary Ab (Jackson Immunoresearch) for 4h at 25°C. Immunoblots were developed with a chemiluminescent substrate (Pierce Biotechnology, Rockford, IL), read on a low-light digital camera (LAS-4000; Fujifilm Medical Systems USA, Stamford, CT), and quantified using Image Guage software (Fujifilm). Phosphorylated JNK was normalized to the amount of total JNK on the same immunoblot. Phosphorylated c-Jun was normalized to the amount of actin on the same immunoblot. Immunoprecipitated proteins were normalized to the amount of receptor immunoprecipitated in each sample on the same immunoblot (14).
Results
Binding of TRAFs and cIAP1 by Wt-hCD40 and hCD40P227A
The hCD40-P227A mutation is located outside of all known TRAF binding sites, yet the hCD40-P227A receptor exhibits amplified signaling relative to Wt-hCD40 (14, 23, 24); Figure 1A). Although we originally could not detect consistent differences in TRAF2, TRAF3, or TRAF6 binding by hCD40-P227A by conventional, relatively non-quantitative co-immunoprecipitation, we re-evaluated TRAF recruitment by this receptor using a more sensitive immunoprecipitation method, utilizing magnetic beads (11). All cell lines used in these studies expressed similar levels of hCD40 receptor on the cell surface as determined by flow cytometry (Supplementary Fig 1). Using this method, we consistently observed that hCD40-P227A bound two-fold more TRAF3 and TRAF5 than Wt-hCD40 following stimulation (Figure 1B,C). Normalized to the amount of CD40 precipitated (Figure 1C), this difference was highly reproducible. Although the P227A mutation is closest to the membrane-proximal TRAF6 binding site, TRAF2 and TRAF6 recruitment to hCD40-P227A was similar to Wt-CD40 (Figure 1C)(14). TRAFs did not associate with magnetic beads coated with an isotype control Ab (mouse IgG1 isotype), nor with a CD40 receptor which lacks the C-terminal 55 amino acids corresponding to the entire cytoplasmic domain (Supplementary Fig 2 and Fig 3).
We previously demonstrated that signaling via Wt-CD40 or hCD40-P227A results in similar ability to stimulate degradation of TRAF2 and TRAF3 (14). Here, Wt-CD40 and hCD40-P227A also recruited similar amounts of cIAP1, an E3 ubiquitin ligase, which is consistent with the ability of these receptors to induce TRAF2 and TRAF3 degradation with equivalent kinetics and magnitude (14, 25) (Figure 2A,B). We also compared hCD40-P227A to a hybrid molecule with the cytoplasmic signaling domain of LMP1 (hCD40-LMP1). We have previously demonstrated that this molecule associates with TRAFs and induces signaling similarly to that of Wt LMP1 (25). In contrast to Wt-CD40, hCD40-LMP1 signaling does not induce TRAF2 or TRAF3 degradation (25) and this receptor binds very small amounts of cIAP1 (Figure 2A,B), suggesting that the amount of cIAP1 recruited to the cytoplasmic tail of a receptor regulates the ability to degrade TRAFs. Figure 2A and 2B also demonstrate that TRAF2 and TRAF6 are recruited equally well to the cytoplasmic domains of hCD40-Wt and hCD40-P227A after stimulation, and less efficiently to the cytoplasmic domain of hCD40-LMP1.
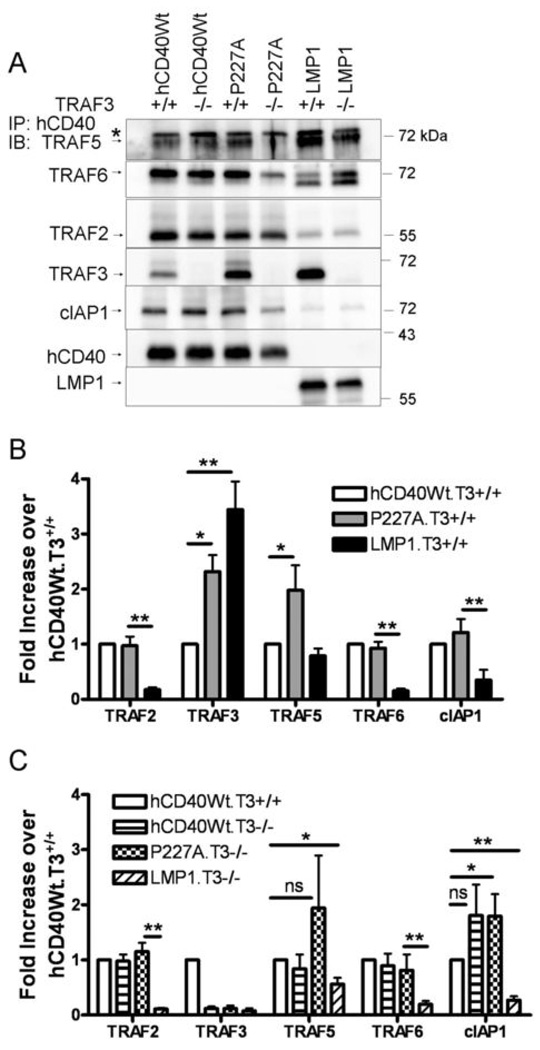
Figure 2. TRAF binding by hCD40-Wt, hCD40-P227A, and hCD40-LMP1 in TRAF3+/+ vs. TRAF3−/− B cells.
A. T3+/+ or T3−/− CH12.LX cells stably expressing matched amounts of hCD40Wt, hCD40-P227A (P227A), or hCD40-LMP1 (LMP1) were stimulated as in Figure B with magnetic beads for 15 min. and the receptor complex isolated as in Fig. 1. Proteins were resolved by SDS-PAGE and immunoblotted for TRAF5. The membrane was stripped and re-probed for the following proteins in sequence: TRAF6, TRAF2, TRAF3, and hCD40 or LMP1 as a loading control. A separate membrane was probed for cIAP1; loading controls were similar to those shown for the TRAF5 immunoblot. Results are representative of 5 independent experiments. At least two subclones of each cell line were tested with similar results, however, the apparent decrease of TRAF5, TRAF6, and cIAP1 binding to this particular subclone of P227A.T3−/− was not reproducible in replicate experiments. * = nonspecific band.
B. Amounts of associating TRAFs and cIAP1 were normalized to the amount of receptor precipitated from TRAF3+/+ B cells. Data are presented as a fold increase of molecule bound to hCD40-P227A and hCD40-LMP1 relative to hCD40-Wt (which was set equal to 1 for each molecule) and are the mean ± SE of 3–5 separate experiments. * = p<0.05; ** = p<0.005; ns =not significant by Student’s t-test.
C. Amounts of associating TRAFs and cIAP1 were normalized to the amount of receptor precipitated from TRAF3−/− B cells. Data are presented as a fold increase of molecule bound to hCD40Wt.T3−/−, hCD40P227A.T3−/−, and hCD40-LMP1.T3−/− relative to hCD40Wt.T3+/+ (which was set equal to 1 for each molecule) and are the mean ± SE of 3–5 separate experiments. * = p<0.05; ** = p<0.005; ns =not significant by Student’s t-test.
Interestingly, hCD40-P227A recruited amounts of TRAF3 which were intermediate between TRAF3 association with Wt-hCD40 and hCD40-LMP1 (Figure 2A,B). TRAF3 is a negative regulator of Wt-CD40 signaling (9), and Wt-CD40 signaling is amplified in TRAF3−/− B cells (10). Therefore, the elevated TRAF3-hCD40-P227A binding seemed inconsistent with this receptor’s known gain-of-function activity (14). This raised the possibility that hCD40-P227A uses TRAF3 as a positive regulator, in a manner similar to the C-terminal cytoplasmic domain of LMP1 (10). To test this idea, we stably expressed hCD40-P227A in subclones of the mature mouse B cell lines CH12.LX or A20.2J which have been rendered completely and specifically deficient in either TRAF3 (10), TRAF2 (16), or TRAF6 (11) via somatic cell gene targeting. Our previous studies of hCD40-P227A show that it signals similarly in either mouse or human B cells (14). TRAF1/2 and TRAF3/5 molecules can form heterotrimers which affect CD40 function in transformed epithelial cell lines when the receptor and TRAF molecules are overexpressed (26, 27). We thus wished to test whether the lack of TRAF3 affected recruitment of TRAFs or other molecules to the cytoplasmic domains of stimulated hCD40-Wt, hCD40-P227A, or hCD40-LMP1 receptors. Immunoprecipitation of hCD40 and hCD40-P227A from TRAF3−/− B cells revealed that recruitment of TRAF2, TRAF6, and cIAP1 to each receptor was TRAF3-independent (Figure 2A,B). However, TRAF5 recruitment to hCD40-P227A and hCD40-LMP1 was partially reduced in TRAF3−/− B cells (Figure 2B and 2C), while the recruitment of TRAF5 to hCD40-Wt was TRAF3-independent. Although TRAF6 and cIAP1 recruitment to hCD40-P227A appears to be partially TRAF3 dependent in the representative experiment shown, this difference was not consistent in replicate experiments (Figure 2C). These data suggest that the hCD40-P227A polymorphism significantly alters the recruitment of TRAF3 and TRAF5 to the receptor, resulting in a complex with notable features of the signaling complex associated with LMP1, rather than Wt-CD40.
TRAF requirements for JNK activation by hCD40-P227A
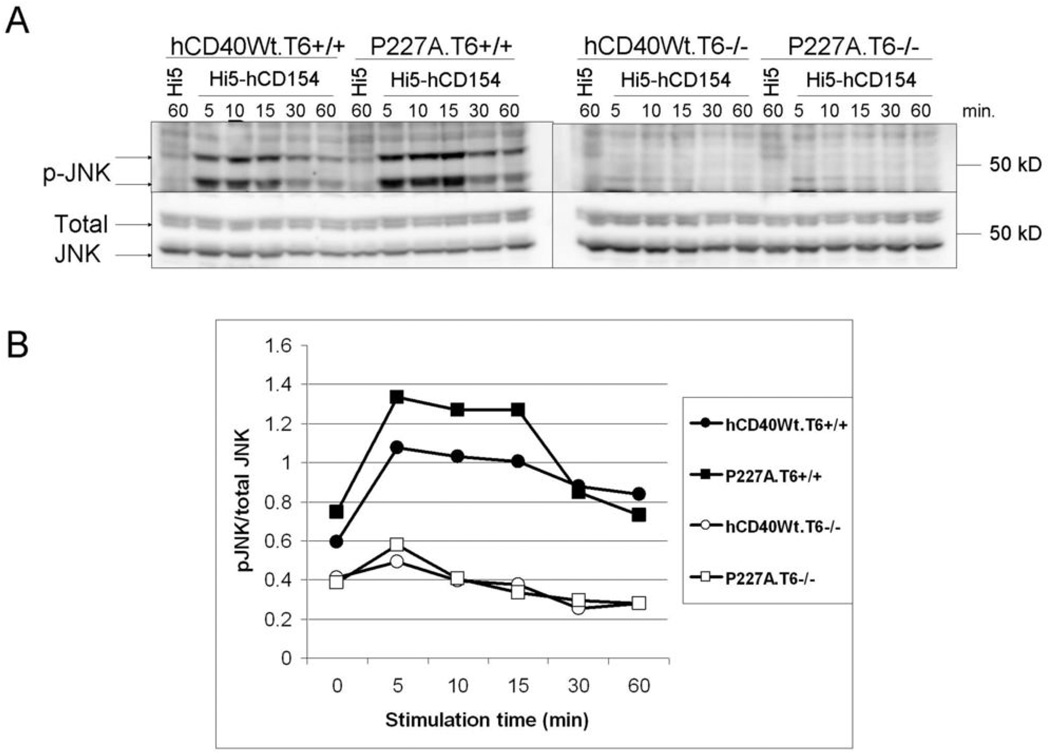
Previous work revealed that hCD40-P227A signaling selectively amplifies the JNK pathway relative to Wt-CD40, although NF-κB, ERK, p38, and Akt pathways are unaltered (14), and that TRAF6 is required for the activation of JNK by Wt-CD40 (11). To test whether TRAF6 was required for hCD40-P227A-induced JNK activation, we stimulated TRAF6+/+ or TRAF6−/− A20 cells stably expressing similar levels of hCD40Wt or hCD40-P227A with the CD40 ligand, CD154, and measured JNK phosphorylation (11, 14). JNK activation was nearly abrogated in TRAF6−/− cells following either hCD40-Wt or hCD40-P227A signaling (Figure 3). This indicates that like Wt-CD40, hCD40-P227A requires TRAF6 for JNK activation, as does Wt-CD40 (11). Similarly, hCD40-P227A-mediated JNK activation in TRAF2−/− CH12.LX B cells was reduced (data not shown), as previously reported for Wt-CD40 (9, 16).
Figure 3. Role of TRAF6 in JNK phosphorylation following hCD40-P227A signaling.
A. A20.2J cells stably expressing hCD40Wt. (hCD40Wt.T6+/+ or hCD40Wt.T6−/−) or hCD40-P227A (P227A.T6+/+ or P227A.T6−/−) were stimulated for the indicated times with insect cells infected with WT baculovirus (Hi5), or insect cells infected with a baculovirus encoding hCD154 (Hi5-hCD154). Total cell lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated JNK, followed by total JNK as a loading control. Data are representative of three separate experiments. At least two clones of each cell line were tested with similar results.
B. Images were quantified and normalized to the amount of total JNK. The zero time point depicted in the graph is the “60 minute Hi5” lane shown in 2A.
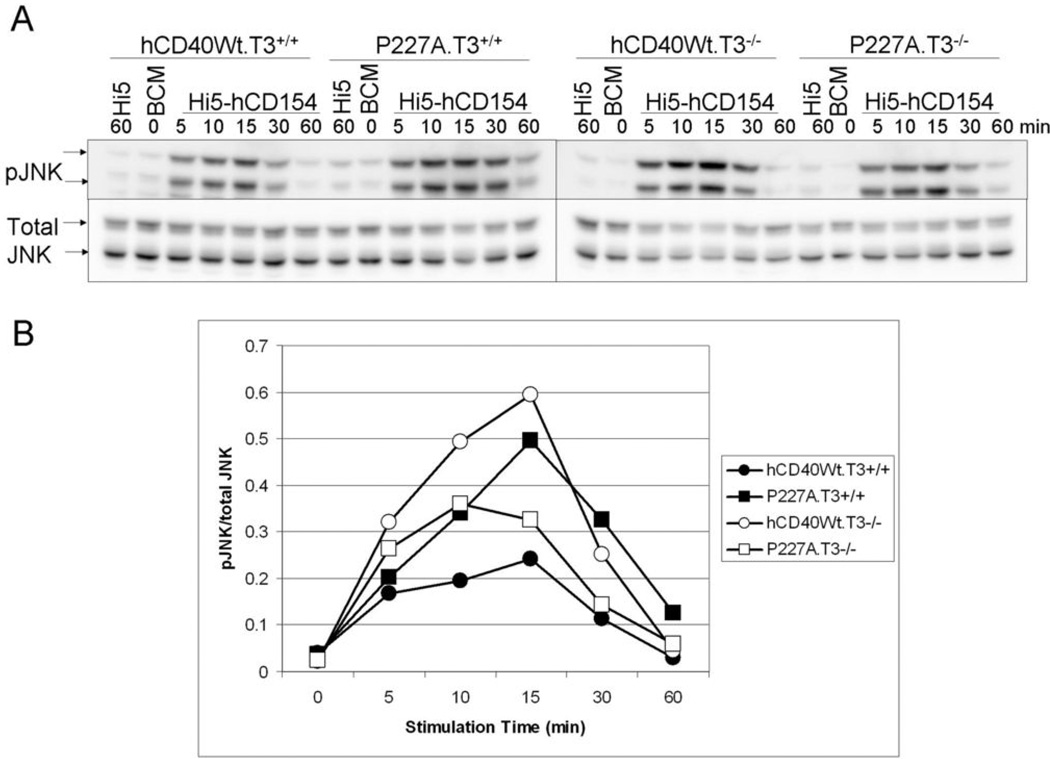
We next evaluated the role of TRAF3 in JNK activation by hCD40-P227A vs. Wt-hCD40, using TRAF3+/+ or TRAF3−/− B cell lines stably expressing similar levels of hCD40-Wt or hCD40-P227A. Consistent with previously published results, hCD40-P227A signaling in TRAF3-sufficient B cells resulted in an approximate doubling in JNK phosphorylation when compared to Wt-CD40 signaling (Figure 4;(14)). Signaling via Wt-CD40 in TRAF3−/− cells was amplified, as was signaling via endogenous mCD40 (data not shown), consistent with previous identification of TRAF3 as a negative regulator of Wt-CD40 signaling (9, 10). In sharp contrast, hCD40-P227A signaling in TRAF3−/− cells was reduced in comparison to the activity of Wt-CD40 in the same cells and compared to the activity of P227A in TRAF3-sufficient cells (Figure 4).
Figure 4. Role of TRAF3 in JNK phosphorylation following hCD40-P227A signaling.
A. CH12.LX cells stably expressing similar amounts of hCD40-Wt (hCD40Wt.T3+/+, hCD40WT.T3−/−) or hCD40-P227A (P227A.T3+/+, P227A.T3−/−) were stimulated for the indicated times with medium (BCM; 0 time point), insect cells infected with WT baculovirus (Hi5), or insect cells infected with a baculovirus encoding hCD154 (Hi5-hCD154). Total cell lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated JNK, followed by total JNK as a loading control. Data are representative of 5 independent experiments. At least two clones of each cell line were tested with similar results.
B. Images were quantified and normalized to the amount of total JNK. Results shown are representative of 5 independent experiments, with at least two clones of each cell line tested with similar results.
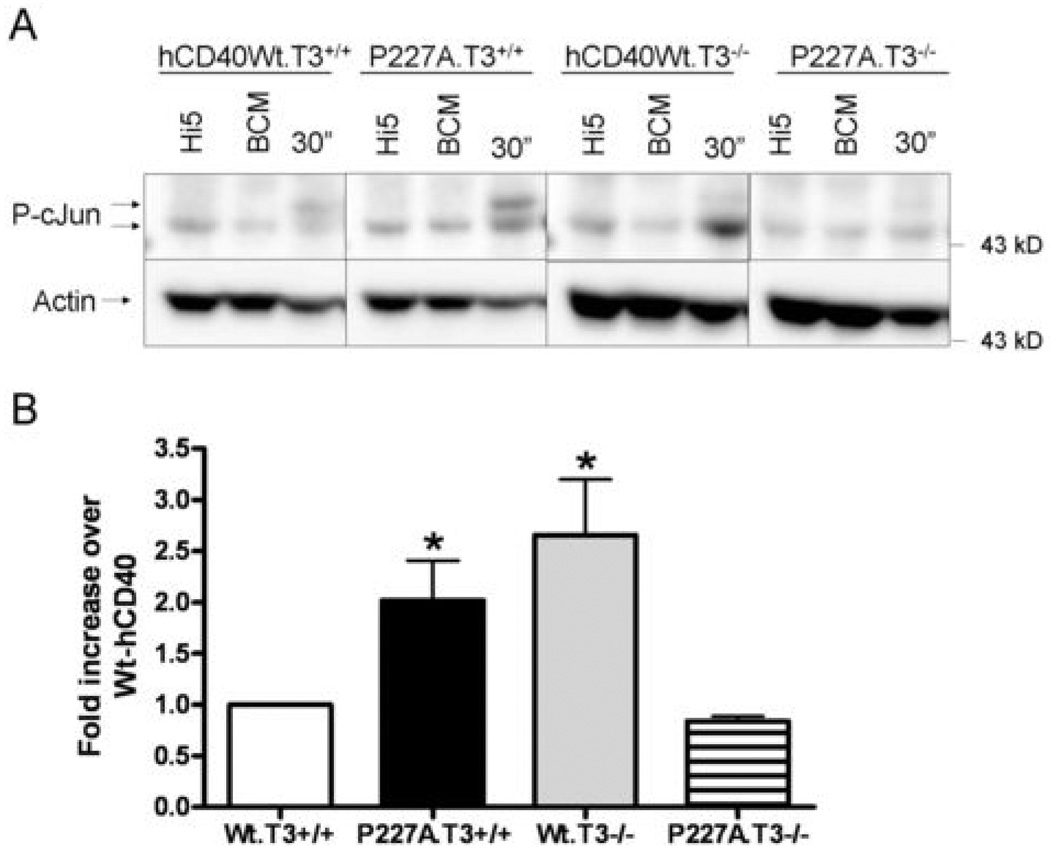
JNK activation induced by CD40 is rapid and transient, so relative differences can be difficult to evaluate quantitatively. As a more stable measure of JNK activation, we also examined phosphorylation of its major substrate c-Jun at Ser63 after CD40 signaling. Consistent with previously published results (14), hCD40-P227A signaling reproducibly resulted in ~2-fold more c-Jun phosphorylation after 30 minutes of signaling than that induced by hCD40Wt (Figure 5). In TRAF3−/− cells, Wt-CD40 signaling resulted in ~2-fold more c-Jun phosphorylation than in TRAF3-sufficient cells (Figure 5), supporting the role of TRAF3 as a negative regulator of Wt-CD40 signaling. In sharp contrast, however, hCD40-P227A signaling in TRAF3-deficient cells resulted in substantially reduced c-Jun phosphorylation when compared to hCD40-P227A signaling in TRAF3+/+ cells. Notably, in the absence of TRAF3, hCD40-P227A signaling was reduced to Wt-CD40 levels. This indicates that, like LMP1, hCD40-P227A not only bound increased amounts of TRAF3 (Figures 1 and 2), but also used TRAF3 as a positive signaling regulator necessary to its enhanced function.
Figure 5. Role of TRAF3 in c-Jun phosphorylation following hCD40-P227A signaling.
A. CH12.LX cells stably expressing similar amounts of hCD40-Wt (hCD40Wt.T3+/+, hCD40WT.T3−/−) or hCD40-P227A (P227A.T3+/+, P227A.T3−/−) were stimulated for 30 min. with medium (BCM; 0 time point), insect cells infected with WT baculovirus (Hi5), or insect cells infected with a baculovirus encoding hCD154 (Hi5-hCD154). Total cell lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated c-Jun, followed by actin as a loading control. Data are representative of 3 independent experiments. At least two clones of each cell line were tested with similar results.
B. Images were quantified and normalized to actin. Data are presented as a fold increase of c-Jun phosphorylated, relative to hCD40Wt .T3+/+ (which was set equal to 1) and are the mean ± SE of 3 separate experiments. * = p<0.05 by Student’s t-test.
Role of TRAF3 in enhanced IL-6 production following hCD40-P227A signaling
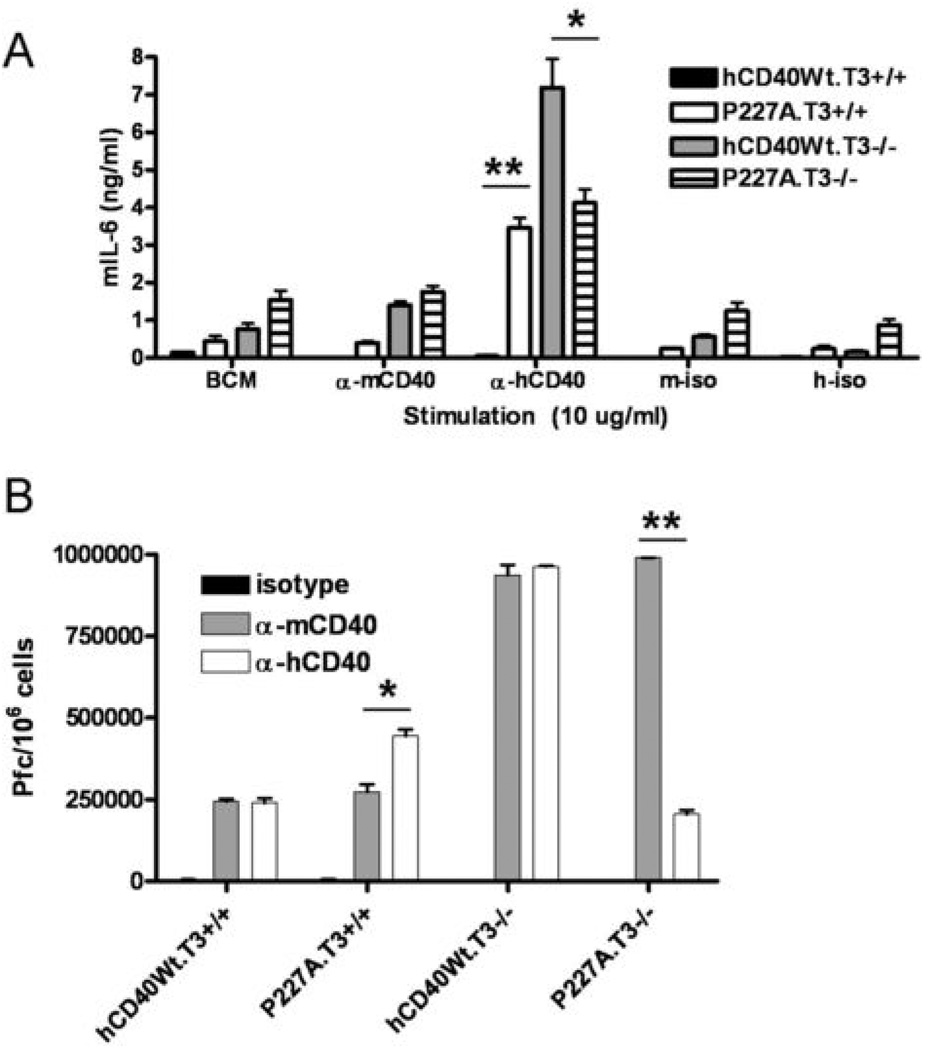
JNK phosphorylates c-Jun, which forms active AP-1 homodimers required for IL-6 production following CD40 signaling in B cells (28). Because hCD40-P227A-stimulated JNK and c-Jun phosphorylation were reduced to Wt-CD40 levels in TRAF3−/− cells, we next asked whether IL-6 production was also decreased. TRAF3+/+ or TRAF3−/− CH12.LX B cells expressing either Wt-hCD40 or hCD40-P227A were stimulated with agonistic anti-mCD40, anti-hCD40, or isotype control mAbs for 48 hours and IL-6 in culture supernatants was quantified by ELISA. While a membrane-bound source of CD154 is required to elicit IL-6 production following Wt-CD40 signaling in normal B cells and B cell lines (19), hCD40-LMP1 and hCD40-P227A can both induce IL-6 production in response to agonistic CD40 Abs (12, 14)(Figure 6A). Previous results showed that TRAF3 deficiency does not augment IL-6 production in B cell lines in response to CD154 (10). However, signaling via Wt-hCD40 in TRAF3−/− cells induced a large amount of IL-6 production in response to agonistic anti-CD40 antibody (Figure 6A), consistent with the previous demonstration that TRAF3 is a negative regulator of Wt-CD40 signaling (9, 10). In contrast, there was no augmented IL-6 production following hCD40-P227A signaling in TRAF3−/− B cells (p=ns, P227A.T3+/+ vs. P227A.T3−/−), consistent with the conclusion that TRAF3 was instead used as a positive signaling regulator. The observation that hCD40-P227A could still stimulate IL-6 production in TRAF3−/− cells is consistent with normal recruitment of TRAF6 to this receptor upon stimulation (29) (Figures 1 and 2).
Figure 6. Role of TRAF3 in secretion of IL-6 and IgM following hCD40-P227A or hCD40Wt signaling.
A. CH12.LX cells stably expressing matched amounts of both mCD40 and hCD40-Wt (hCD40Wt.T3+/+ or hCD40Wt.T3−/−), or hCD40-P227A (P227A.T3+/+ or P227A.T3−/−) were stimulated with medium only (BCM) or 10 µg/ml agonistic anti-mCD40, anti-hCD40, or isotype control mAbs (m-iso, h-iso) for 48 hours. Culture supernatants were analyzed by quantitative IL-6 ELISA. Data represent means ± s.d. of triplicate cultures, and are representative of three independent experiments. At least two separate subclones of each cell line were tested with similar results (not shown). *=p<0.05; **=p<0.001 by Student’s t-test.
B. CH12.LX cells stably expressing matched amounts of both mCD40 and hCD40-Wt hCD40-Wt (hCD40Wt.T3+/+ or hCD40Wt.T3−/−), or hCD40-P227A (P227A.T3+/+ or P227A.T3−/−) were stimulated with 2 µg/ml agonistic anti-mCD40, anti-hCD40, or isotype control mAbs for 72 hours. IgM-secreting cells in replicate cultures are enumerated (±SE) as described (14). Results are representative of 3 similar experiments. Two separate subclones of each cell line were tested with similar results (not shown). * = p<0.05; ** = p<0.001 by Student’s t-test.
Role of TRAF3 in enhanced hCD40-P227A-mediated Ig production
IL-6 secretion following CD40 signaling contributes to plasma cell differentiation and Ig production, as blocking IL-6 in CD40-stimulated B cell cultures reduces Ig production to basal levels (29). Because TRAF3 deficiency enhanced IL-6 production in response to Wt-CD40 signaling, but failed to enhance hCD40-P227A-mediated IL-6 production, we determined whether Ig production, the signature function of the B cell, was similarly affected by TRAF3 deficiency. When stimulated with CD40 agonists, the CH12.LX cell line produces and secretes IgM reactive to phosphorylcholine, an Ag present on the surface of SRBC. We stimulated TRAF3+/+ or TRAF3−/− B cells expressing similar levels of either Wt-hCD40 or hCD40-P227A and measured IgM secretion (14). Consistent with our previous results, hCD40-P227A signaling in TRAF3+/+ cells resulted in a doubling of IgM production, relative to Wt-hCD40 or to endogenous mCD40 signaling (p<0.05) (14) (Figure 6B). Wt-hCD40 or endogenous mCD40 signaling in TRAF3−/− cells resulted in a ~4-fold increase in IgM production (p<0.001), relative to signaling by these CD40 molecules in TRAF3+/+ cells, which is consistent with previously published data (10). In sharp contrast, however, hCD40-P227A signaling in TRAF3−/− cells resulted in much lower IgM production, similar to levels induced by WthCD40 in TRAF3-sufficient cells (p=ns, Wt-hCD40.T3+/+ vs. P227A.T3−/−) (Figure 6B).
Discussion
Results presented in this study indicate that hCD40-P227A used TRAF3 as a required positive regulator of signaling, and that TRAF3 was required for the gain-of-function activity of hCD40-P227A. TRAF3 was recruited more efficiently to hCD40-P227A than to hCD40-Wt following CD40 stimulation (Figures 1 and 2). This was initially surprising, considering the selective hyperactivation of the JNK pathway following hCD40-P227A signaling, and the previously demonstrated negative role of TRAF3 in Wt-CD40-induced JNK activation. Figures 2A and 2B demonstrate that TRAF2 and TRAF6 were recruited equally well to the cytoplasmic domains of hCD40-Wt and hCD40-P227A after stimulation, and less efficiently to the cytoplasmic domain of hCD40-LMP1. Notably, this is also the first demonstration that endogenous TRAF6 forms a complex with LMP1 in B cells. We reported that TRAF2 and TRAF3 degrade with equivalent magnitude and kinetics following either Wt-CD40 or CD40-P227A signaling in B cells (14), and Figures 2A and 2B demonstrate that similar recruitment of cIAP1 by CD40-P227A and Wt-hCD40 correlated with the ability to degrade TRAF2 and TRAF3. This increase in TRAF3 binding by hCD40-P227A is consistent with the use of TRAF3 as a positive, rather than a negative regulator of signaling, because JNK activation, IL-6 production, and Ig production were clearly reduced following P227A signaling in TRAF3−/− cells (Figures 3–6).
Several TRAFs are capable of activating the JNK pathway downstream of CD40 or LMP1-mediated signaling. TRAF2 and TRAF6 are required for optimal JNK activation following CD40 signaling, as demonstrated in previous work utilizing cells lacking either TRAF2 or TRAF6 (11). However, LMP1-mediated JNK activation requires TRAF3, but does not require TRAF2 (10). Results of this study suggest that the hCD40-P227A signaling pathway is overlapping but distinct from those mediated by either LMP1 or Wt-CD40, as TRAF2 (not shown), TRAF6 (Figure 3), and TRAF3 (Figures 4–6) are all required for optimal JNK activation by hCD40-P227A.
It is notable that LMP1 recruits much more TRAF3 to its cytoplasmic domain upon signaling than Wt-CD40, and LMP1 is able to use TRAF3 as a positive signaling regulator. The P227A polymorphism of hCD40 not only increased the amount of TRAF3 recruited to the CD40 signaling complex, but also changed the role played by TRAF3 in downstream effects of CD40 engagement. Therefore, both LMP1 and CD40-P227A not only bind more TRAF3 but require this TRAF for optimal signal transduction, suggesting that the function of a TRAF may in part be determined by its concentration at the receptor complex, and/or the avidity with which it associates with a given receptor.
The hCD40-P227A polymorphism may alter TRAF3 binding and function in several ways, which are not mutually exclusive. It is possible that a new TRAF3 binding site is created by the alteration, and that TRAF3 is binding preferentially at this membrane-proximal site. The altered binding site may induce a conformational change in TRAF3 that alters its function, similar to the change in interaction shown in the crystal structure of TRAF3 binding to LMP1(13). Indeed, TRAF3 is not exclusively a negative regulator of receptor signaling, as LMP1, LT-βR, and certain innate immune receptors utilize TRAF3 as a positive regulator of signaling (10, 30–33).
Another possibility is that hCD40-P227A is recruiting additional B cell-specific molecules which are not recruited to Wt-hCD40 upon receptor signaling (or vice versa), and that these molecules indirectly influence TRAF3 binding in B cells. Interestingly, Leo et al. demonstrate that a fusion protein consisting of a GST-tagged CD40-P227A cytoplasmic domain binds TRAF2 and TRAF3 normally in transformed epithelial cells, suggesting that B cell- or hematopoietic cell-specific factors may influence TRAF3 binding (27). There is precedent for this, as a cytoplasmic domain mutant of CD40 shown to lack binding of both TRAFs 2 and 3 when overexpressed in transformed epithelial cells binds normal amounts of TRAF3 when expressed in B cells (1). Two such candidate proteins which may be differentially recruited to Wt-CD40 and hCD40-P227A are Act1 and T3JAM (34, 35). Act1 is found in a complex with CD40 and TRAF3 following CD40 signaling (36), yet it is unclear from these studies whether TRAF3 is required for Act1 binding to CD40 in B cells, as Act1 can also associate with TRAF6 (37). The role of Act1 in CD40 signaling is not yet firmly established. One strain of Act1−/− mice displays CD40-dependent autoimmunity and B cells derived from these mice display increased activation of NF-κB, ERK, p38, and JNK in response to CD40 agonists in vitro (36), yet another independently derived strain of Act1−/− mice on a similar genetic background does not share this phenotype (38). Interestingly, both hCD40-LMP1 and hCD40-P227A bound clearly increased amounts of Act1 following stimulation relative to Wt-hCD40 in co-immunoprecipitation experiments, when normalized to the amount of CD40 precipitated (Supplemental Figure 1). Furthermore, Act1 is recruited more efficiently to all three receptors in TRAF3−/− cells, suggesting that Act1 binding to hCD40-Wt, hCD40-P227A, and hCD40-LMP1 does not depend upon TRAF3 (Supplemental Figure 1). Further studies are needed to determine the role of Act1 in signaling by hCD40-P227A, LMP1, and CD40 in B cells.
T3JAM was originally identified by yeast two-hybrid studies using TRAF3 as bait (35). When overexpressed in transformed epithelial cells in conjunction with exogenously expressed TRAF3, T3JAM stimulates JNK but not NF-κB activity (35), similar to the selective augmentation of JNK phosphorylation by hCD40-P227A, yet the role of T3JAM in CD40 signaling is unknown. We have been unable to detect binding of T3JAM to CD40 in B cells upon stimulation, using commercially available antibodies (data not shown). We are currently employing a proteomics approach to identify additional factors that may be recruited differentially to hCD40-P227A vs. Wt-hCD40.
A third possibility is that the P227A alteration induces a conformational change in the receptor that introduces more stable contacts with TRAF3 (13). Previous studies show that although LMP1 and CD40 bind the same face of TRAF3, additional contacts are made between LMP1 and TRAF3 that stabilize TRAF-receptor binding (13). The site of the P227A SNP is located in an unstructured region of the CD40 cytoplasmic domain, as shown by secondary structure prediction programs and NMR spectra (39). Fragments of the CD40 cytoplasmic tail crystallized with TRAF2 or TRAF3 show that the tail only assumes secondary structure when complexed with TRAFs, suggesting that localized structure changes induced by the P227A amino acid alteration may not be detected in the absence of bound TRAF molecules. Furthermore, the P227A residue lies outside of the CD40 peptides co-crystallized with TRAF6, TRAF2, and TRAF3, making it impossible to determine from the structural data currently available what role, if any, this residue plays in TRAF interactions or binding affinity (39–41). We are currently producing purified CD40-P227A and TRAF molecules to address this question by biochemical and biophysical means.
In addition to increased recruitment of TRAF3 to hCD40-P227A, TRAF5 recruitment was also increased. Furthermore, TRAF5 binding by hCD40-P227A and hCD40-LMP1 following stimulation was reduced in TRAF3−/− B cells, suggesting that optimal recruitment of TRAF5 to these receptors requires TRAF3 (Figure 2). Therefore, the signaling defects observed following hCD40-P227A signaling in TRAF3−/− B cells could be due not only to the absence of TRAF3, but also to suboptimal recruitment of TRAF5. Recent studies in TRAF5−/− mice show that TRAF5 is required for LMP1 signaling in vivo (42). We are currently producing mCD40-hP227A transgenic mice, which can be bred onto the TRAF5−/− background to determine the contribution of this TRAF to P227A signaling in the context of the whole animal.
In summary, an experimental approach that exploits a sensitive immunoprecipitation technique as well as TRAF-deficient B cells allowed characterization of the molecular signaling requirements for a gain-of-function allele of human CD40 common in certain populations. Surprisingly, hCD40-P227A used TRAF3 as a positive rather than a negative regulator of signaling, and TRAF3 was required for its gain-of-function signaling outcomes in a manner analogous to that of the oncogenic EBV mimic, LMP1, which also shows enhanced B cell activation compared to Wt-CD40. Our studies indicate that mutations outside of the known TRAF binding sites can dramatically and selectively alter TRAF3 binding and function in CD40 signaling.
Supplementary Material
Acknowledgments
The authors wish to thank Drs. Jon Houtman and Bruce Hostager for helpful discussions and critical review of this manuscript.
Footnotes
Abbreviations used: RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; GD, Graves’ disease; SNP, single nucleotide polymorphism TRAF, TNF receptor associated factor; h, human; m, mouse; LMP1, latent membrane protein 1; SRBC, sheep red blood cells; BCM, B cell medium; JNK, c-Jun N-terminal kinase.
A. Peters was supported by a predoctoral fellowship from the American Heart Association (0815735G). G. Bishop is supported by grants from the National Institutes of Health (AI28847, AI49993, CA099997). This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Health, Services Research and Development, Merit Review Award 383 (to G.A.B.).
References
- 1.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Biol Med. 2007;597:131–151. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 2.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law C-L, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: The opportunities and challenges. In: Grewal IS, editor. Therapeutic Targets of the TNF Superfamily. Landes Bioscience; 2009. pp. 8–36. [DOI] [PubMed] [Google Scholar]
- 5.Tomer Y, Concepcion E, Greenberg DA. A C/T single-nucleotide polymorphism in the region of the CD40 gene is associated with Graves' disease. Thyroid. 2002;12:1129–1135. doi: 10.1089/105072502321085234. [DOI] [PubMed] [Google Scholar]
- 6.Kim TY, Park YJ, Hwang JK, Song JY, Park KS, Cho BY, Park DJ. A C/T polymorphism in the 5'-untranslated region of the CD40 gene is associated with Graves' disease in Koreans. Thyroid. 2003;13:919–925. doi: 10.1089/105072503322511319. [DOI] [PubMed] [Google Scholar]
- 7.Raychaudhuri S, Remmers EF, Lee AT, Hackett R, Guiducci C, Burtt NP, Gianniny L, Korman BD, Padyukov L, Kurreeman FA, Chang M, Catanese JJ, Ding B, Wong S, van der Helm-van Mil AH, Neale BM, Coblyn J, Cui J, Tak PP, Wolbink GJ, Crusius JB, van der Horst-Bruinsma IE, Criswell LA, Amos CI, Seldin MF, Kastner DL, Ardlie KG, Alfredsson L, Costenbader KH, Altshuler D, Huizinga TW, Shadick NA, Weinblatt ME, de Vries N, Worthington J, Seielstad M, Toes RE, Karlson EW, Begovich AB, Klareskog L, Gregersen PK, Daly MJ, Plenge RM. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet. 2008;40:1216–1223. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41:824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 9.Hostager BS, Bishop GA. Cutting Edge: Contrasting roles of TRAF2 and TRAF3 in CD40-mediated B lymphocyte activation. J. Immunol. 1999;162:6307–6311. [PubMed] [Google Scholar]
- 10.Xie P, Hostager BS, Bishop GA. Requirement for TRAF3 in signaling by LMP1, but not CD40, in B lymphocytes. J Exp Med. 2004;199:661–671. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland SR, Tremblay ML, Ellison JM, Stunz LL, Bishop GA, Hostager BS. A novel mechanism for TRAF6-dependent CD40 signaling. J. Immunol. 2007;179:4645–4653. doi: 10.4049/jimmunol.179.7.4645. [DOI] [PubMed] [Google Scholar]
- 12.Stunz LL, Busch LK, Munroe ME, Tygrett L, Sigmund C, Waldschmidt TW, Bishop GA. Expression of the LMP1 cytoplasmic tail in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity. 2004;21:255–266. doi: 10.1016/j.immuni.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Xie P, Welsh K, Li C, Ni C, Zhu X, Reed JC, Satterthwait AC, Bishop GA, Ely KR. LMP1 protein from EBV is a structural decoy in B lymphocytes for binding to TRAF3. J Biol Chem. 2005;280:33620–33626. doi: 10.1074/jbc.M502511200. [DOI] [PubMed] [Google Scholar]
- 14.Peters AL, Plenge R, Graham R, Altshuler D, Moser K, Gaffney PM, Bishop GA. A novel polymorphism of the human CD40 receptor with enhanced function. Blood. 2008;112:1863–1871. doi: 10.1182/blood-2008-02-138925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez M. A multiethnic, multicenter cohort of patients with SLE as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57:576–584. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 16.Hostager BS, Haxhinasto SA, Rowland SR, Bishop GA. TRAF2-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J. Biol. Chem. 2003;278:45382–45390. doi: 10.1074/jbc.M306708200. [DOI] [PubMed] [Google Scholar]
- 17.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 1979;122:549–555. [PubMed] [Google Scholar]
- 18.Bishop GA, Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc. Natl. Acad. Sci. (USA) 1986;83:7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baccam M, Bishop GA. Membrane-bound CD154, but not anti-CD40 mAbs, induces NF-κB independent B cell IL-6 production. Eur. J. Immunol. 1999;29:3855–3866. doi: 10.1002/(SICI)1521-4141(199912)29:12<3855::AID-IMMU3855>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Hostager BS, Bishop GA. Role of TRAF2 in the activation of IgM secretion by CD40 and CD120b. J. Immunol. 2002;168:3318–3322. doi: 10.4049/jimmunol.168.7.3318. [DOI] [PubMed] [Google Scholar]
- 21.Hostager BS, Catlett IM, Bishop GA. Recruitment of CD40, TRAF2 and TRAF3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- 22.Mercolino TJ, Arnold LW, Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J. Exp. Med. 1986;163:155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Cook WJ, Lin L, Noelle RJ. CD40 signaling through a newly identified TRAF2 binding site. J. Biol. Chem. 2003;278:45414–45418. doi: 10.1074/jbc.M309601200. [DOI] [PubMed] [Google Scholar]
- 24.Pullen SS, Miller HG, Everdeen DS, Dang TTA, Crute JJ, Kehry MR. CD40-TRAF interactions: Regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochem. 1998;37:11836–11845. doi: 10.1021/bi981067q. [DOI] [PubMed] [Google Scholar]
- 25.Graham JP, Moore CR, Bishop GA. Roles of the TRAF2/3 binding site in differential B cell signaling by CD40 and its viral oncogenic mimic, LMP1. J Immunol. 2009;183:2966–2973. doi: 10.4049/jimmunol.0900442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 27.Leo E, Welsh K, Matsuzawa S, Zapata JM, Kitada S, Mitchell RS, Ely KR, Reed JC. Differential requirements for TRAF family proteins in CD40-mediated induction of NF-κB and JNK activation. J. Biol. Chem. 1999;274:22414–22422. doi: 10.1074/jbc.274.32.22414. [DOI] [PubMed] [Google Scholar]
- 28.Baccam M, Woo S, Vinson C, Bishop GA. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: Involvement of NFkB, AP-1, and C/EBP. J. Immunol. 2003;170:3099–3108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 29.Jalukar SV, Hostager BS, Bishop GA. Characterization of the roles of TRAF6 in CD40-mediated B lymphocyte effector functions. J. Immunol. 2000;164:623–630. doi: 10.4049/jimmunol.164.2.623. [DOI] [PubMed] [Google Scholar]
- 30.Rooney IA, Butrovich KD, Glass AA, Borboroglu S, Benedict CA, Whitbeck JC, Cohen GH, Eisenberg RJ, Ware CF. The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem. 2000;275:14307–14315. doi: 10.1074/jbc.275.19.14307. [DOI] [PubMed] [Google Scholar]
- 31.Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu L-C, Wang GG, Kamps MP, Raz E, Wagner H, Häcker G, Mann M, Karin M. Specificity in TLR signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 32.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Critical role of TRAF3 in the TLR-dependent and independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 33.Van Arsdale TL, VanArsdale SL, Force WR, Walter BN, Mosialos G, Kieff E, Reed JC, Ware CF. LTβ receptor signaling complex: Role of TRAF3 recruitment in cell death and activation of NF-κB. Proc Natl Acad Sci (USA) 1997;94:2460–2465. doi: 10.1073/pnas.94.6.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine. 2008;41:105–113. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Dadgostar H, Doyle SE, Shahangian A, Garcia DE, Cheng G. T3JAM, a novel protein that specifically interacts with TRAF3 and promotes the activation of JNK(1) FEBS Lett. 2003;553:403–407. doi: 10.1016/s0014-5793(03)01072-x. [DOI] [PubMed] [Google Scholar]
- 36.Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott ML, Kotzin BL, Li X. Act1, a negative regulator in CD40 and BAFF-mediated B cell survival. Immunity. 2004;21:575–587. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Kanamori M, Kai C, Hayashizaki Y, Suzuki H. NF-κB activator Act1 associates with IL-1/Toll pathway adaptor molecule TRAF6. FEBS Let. 2002;532:241–246. doi: 10.1016/s0014-5793(02)03688-8. [DOI] [PubMed] [Google Scholar]
- 38.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, Siebenlist U. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni C, Welsh K, Leo E, Wu H, Reed JC, Ely KR. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. (USA) 2000;97:10395–10399. doi: 10.1073/pnas.97.19.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McWhirter SM, Pullen SS, Holton JM, Crute JJ, Kehry MR, Alber T. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc. Natl. Acad. Sci. (USA) 1999;96:8408–8413. doi: 10.1073/pnas.96.15.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M, Du K, Singh S, Pike JW, Darnay BG, Choi Y, Wu H. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–447. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 42.Kraus ZJ, Nakano H, Bishop GA. TRAF5 is a critical mediator of in vitro signals and in vivo functions of LMP1, the viral oncogenic mimic of CD40. Proc Natl Acad Sci (USA) 2009;106:17140–17145. doi: 10.1073/pnas.0903786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.