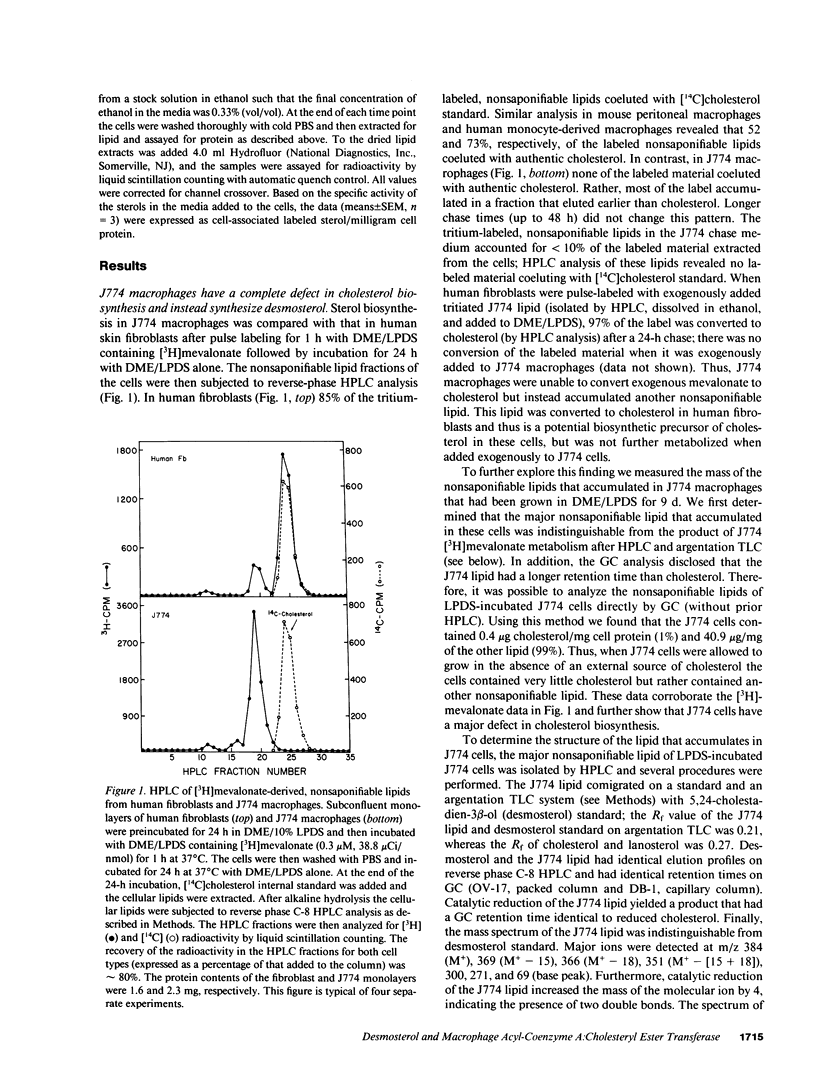
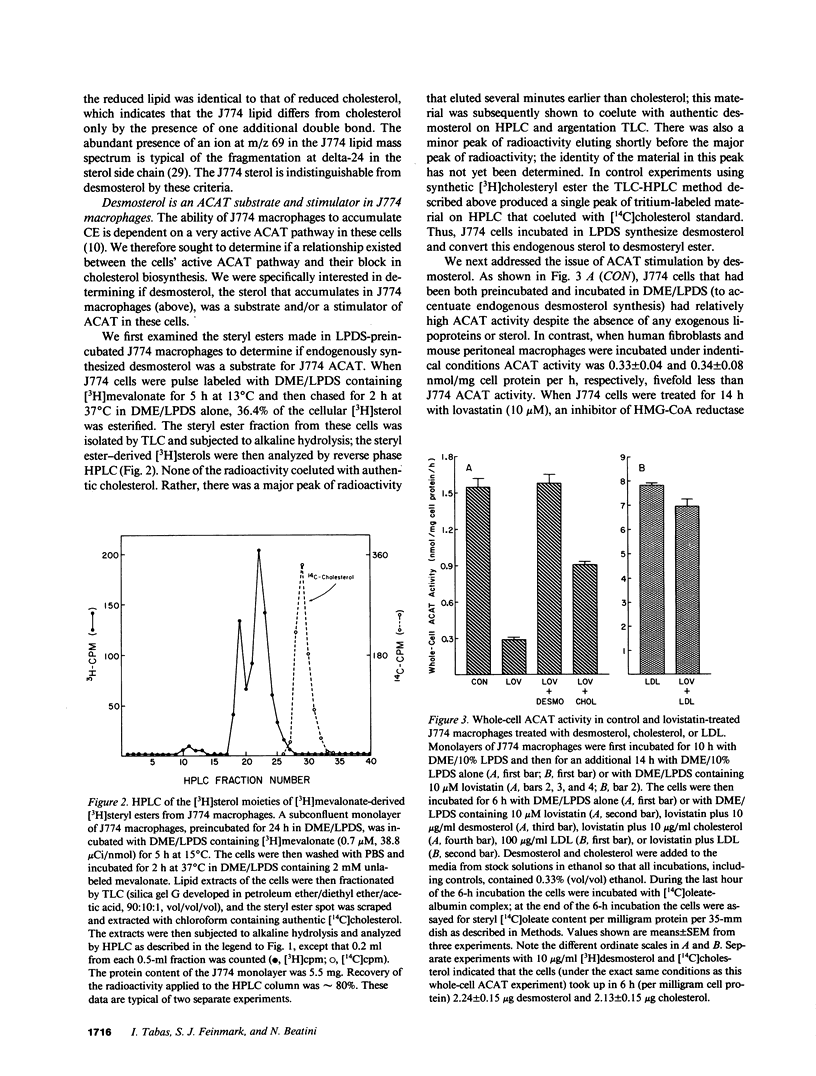
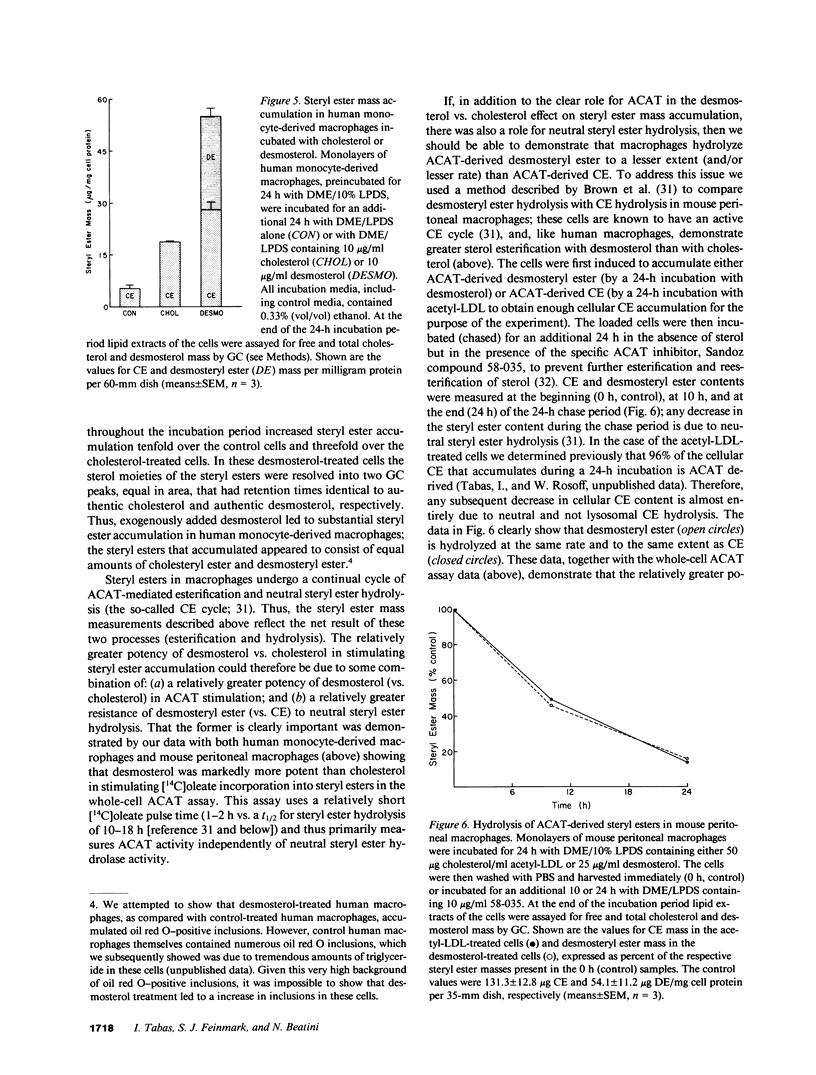
Abstract
The acyl-CoA: cholesterol acyl transferase (ACAT) reaction in macrophages is a critical step in atherosclerotic foam cell formation, but little is known about the reaction's sterol substrate specificity. In this report we examine the macrophage ACAT reactivity of the shellfish sterol, desmosterol, and other sterols found in man because of shellfish ingestion or in association with the foam cell diseases sitosterolemia and cerebrotendinous xanthomatosis (CTX). We first show that the J774 macrophage, a foam cell model with a hyperactive ACAT pathway, synthesizes desmosterol instead of cholesterol and that both endogenous and exogenous desmosterol are substrates and stimulators of the ACAT reaction in these cells. When exogenous desmosterol was added to human monocyte-derived macrophages, ACAT was stimulated 29- and 4-fold compared with control and cholesterol-treated cells, respectively. Steryl ester mass accumulation in desmosterol-treated human macrophages was 10-fold greater than in control cells and 3-fold greater than in cholesterol-treated cells. Another shellfish sterol, 24-methylene cholesterol, also stimulated ACAT in human macrophages, but most of the xanthomatosis-related sterols did not stimulate ACAT. These data suggest that: (a) the shellfish sterols desmosterol and 24-methylene cholesterol may be atherogenic; and (b) the excessive foam cell formation seen in sitosterolemia and CTX cannot be explained by ACAT hyperreactivity of their associated sterols.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J., STEINBERG D. Deposition of desmosterol in the lesions of experimental atherosclerosis. Lancet. 1962 Mar 17;1(7229):572–572. doi: 10.1016/s0140-6736(62)91549-0. [DOI] [PubMed] [Google Scholar]
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Receptor-dependent hydrolysis of cholesteryl esters contained in plasma low density lipoprotein. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2925–2929. doi: 10.1073/pnas.72.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- COPIUS-PEEREBOOM J. W., BEEKES H. W. THE ANALYSIS OF MIXTURES OF ANIMAL AND VEGETABLE FATS. V. SEPARATION OF STEROL ACETATES BY THIN-LAYER CHROMATOGRAPHY IN REVERSED-PHASE SYSTEMS AND ON SILICA GEL G-SILVER NITRATE LAYERS. J Chromatogr. 1965 Jan;17:99–113. doi: 10.1016/s0021-9673(00)99839-x. [DOI] [PubMed] [Google Scholar]
- Connor W. E., Lin D. S. Absorption and transport of shellfish sterols in human subjects. Gastroenterology. 1981 Aug;81(2):276–284. [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Galli G., Maroni S. Mass spectrometric investigations of some unsaturated sterols biosynthetically related to cholesterol. Steroids. 1967 Sep;10(3):189–197. doi: 10.1016/0039-128x(67)90046-3. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Goldstein J. L. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J Clin Invest. 1980 Nov;66(5):1094–1100. doi: 10.1172/JCI109938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtenstein A. H., Brecher P. Esterification of cholesterol and 25-hydroxycholesterol by rat liver microsomes. Biochim Biophys Acta. 1983 May 16;751(3):340–348. doi: 10.1016/0005-2760(83)90292-8. [DOI] [PubMed] [Google Scholar]
- Macauley S. K., Billheimer J. T., Ritter K. S. Sterol substrate specificity of acyl coenzyme A:cholesterol acyltransferase from the corn earworm, Heliothis zea. J Lipid Res. 1986 Jan;27(1):64–71. [PubMed] [Google Scholar]
- Ross A. C., Go K. J., Heider J. G., Rothblat G. H. Selective inhibition of acyl coenzyme A:cholesterol acyltransferase by compound 58-035. J Biol Chem. 1984 Jan 25;259(2):815–819. [PubMed] [Google Scholar]
- Rothblat G. H., Buchko M. K. Effect of exogenous steroids on sterol synthesis in L-cell mouse fibroblasts. J Lipid Res. 1971 Nov;12(6):647–652. [PubMed] [Google Scholar]
- Rothblat G. H., Burns C. H., Conner R. L., Landrey J. R. Desmosterol as the major sterol in L-cell mouse fibroblasts grown in sterol-free culture medium. Science. 1970 Aug 28;169(3948):880–882. doi: 10.1126/science.169.3948.880. [DOI] [PubMed] [Google Scholar]
- Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971 Dec;75(6):843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- Salen G., Kwiterovich P. O., Jr, Shefer S., Tint G. S., Horak I., Shore V., Dayal B., Horak E. Increased plasma cholestanol and 5 alpha-saturated plant sterol derivatives in subjects with sitosterolemia and xanthomatosis. J Lipid Res. 1985 Feb;26(2):203–209. [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Suckling K. E., Stange E. F. Role of acyl-CoA: cholesterol acyltransferase in cellular cholesterol metabolism. J Lipid Res. 1985 Jun;26(6):647–671. [PubMed] [Google Scholar]
- Tabas I., Boykow G. C., Tall A. R. Foam cell-forming J774 macrophages have markedly elevated acyl coenzyme A:cholesterol acyl transferase activity compared with mouse peritoneal macrophages in the presence of low density lipoprotein (LDL) despite similar LDL receptor activity. J Clin Invest. 1987 Feb;79(2):418–426. doi: 10.1172/JCI112828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Rosoff W. J., Boykow G. C. Acyl coenzyme A:cholesterol acyl transferase in macrophages utilizes a cellular pool of cholesterol oxidase-accessible cholesterol as substrate. J Biol Chem. 1988 Jan 25;263(3):1266–1272. [PubMed] [Google Scholar]
- Tabas I., Weiland D. A., Tall A. R. Inhibition of acyl coenzyme A:cholesterol acyl transferase in J774 macrophages enhances down-regulation of the low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl-coenzyme A reductase and prevents low density lipoprotein-induced cholesterol accumulation. J Biol Chem. 1986 Mar 5;261(7):3147–3155. [PubMed] [Google Scholar]
- Tabas I., Weiland D. A., Tall A. R. Unmodified low density lipoprotein causes cholesteryl ester accumulation in J774 macrophages. Proc Natl Acad Sci U S A. 1985 Jan;82(2):416–420. doi: 10.1073/pnas.82.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskos J. M., Bowen W. D., Shafiee A., Fischer R. T., Gaylor J. L. Cytochrome P-450-dependent oxidation of lanosterol in cholesterol biosynthesis. Microsomal electron transport and C-32 demethylation. J Biol Chem. 1984 Nov 10;259(21):13402–13412. [PubMed] [Google Scholar]
- Wong H. Y., Vroman H. E., Mendez H. C. Atherogenic aspects of desmosterol metabolism caused by prolonged triparanol administration. Life Sci. 1966 Apr;5(7):629–637. doi: 10.1016/0024-3205(66)90294-3. [DOI] [PubMed] [Google Scholar]


