Abstract
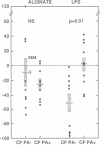
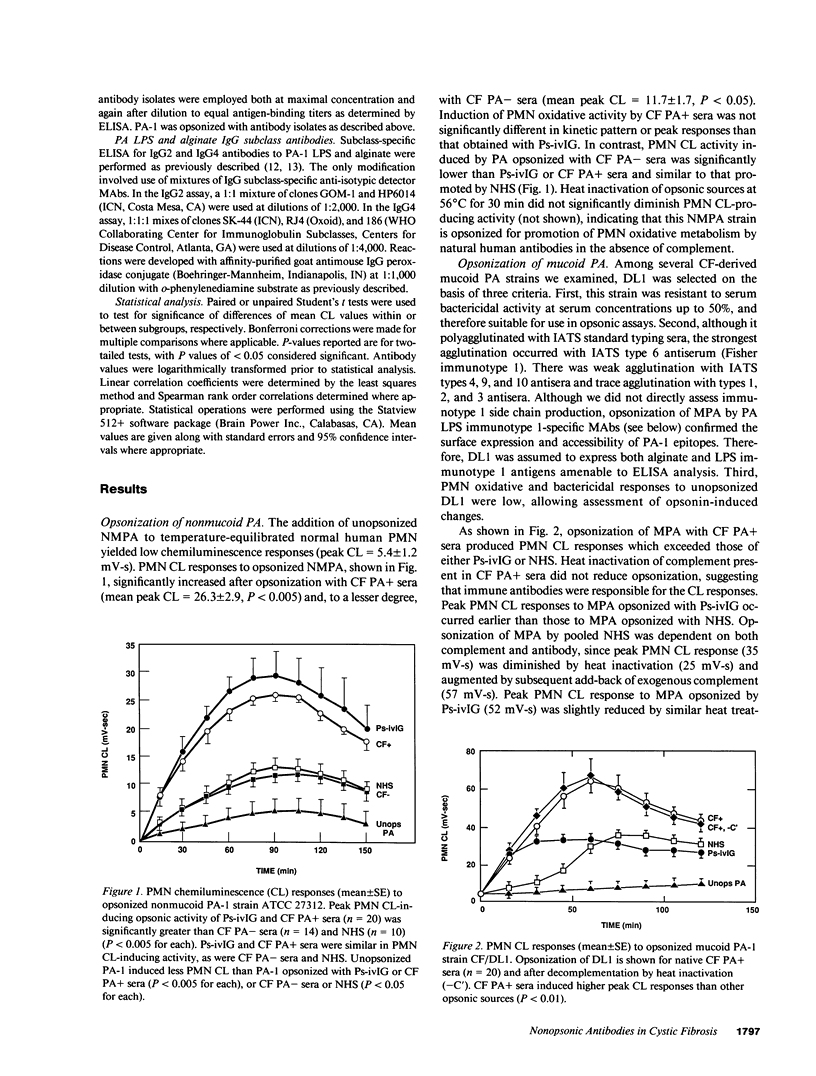
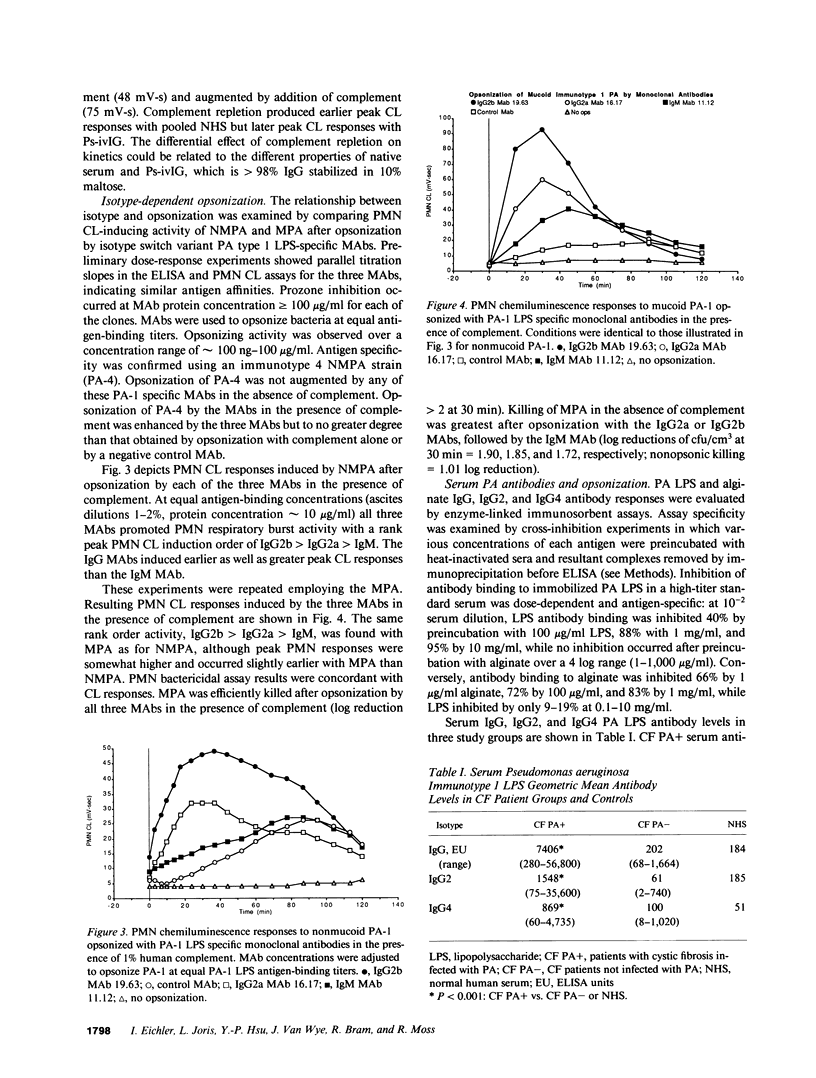
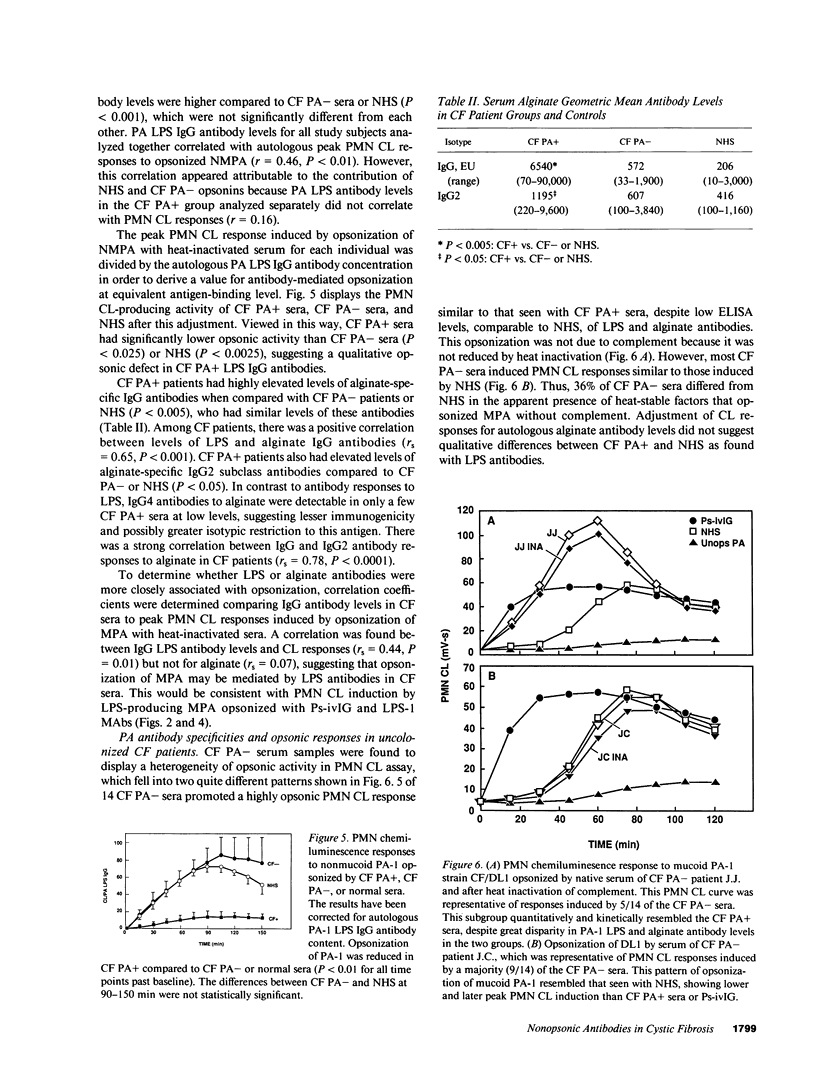
Antibody opsonins from cystic fibrosis (CF) patients were investigated using nonmucoid and mucoid lipopolysaccharide (LPS) immunotype 1 Pseudomonas aeruginosa as bacterial ligands and PMN phagocytes. CF sera were compared to normal sera, polyvalent PA LPS hyperimmune globulin, and isotype switch variant monoclonal antibodies (MAbs) specific for type 1 PA LPS. Sera from PA-infected CF patients (CF PA+) had elevated levels of PA LPS and alginate IgG antibodies and promoted significantly greater antibody-dependent PMN chemiluminescence responses than sera from uninfected CF patients (CF PA-) or normal human sera (NHS). After adjustment for autologous IgG PA LPS antibody content, however, CF PA+ sera had less antibody-dependent opsonic activity than sera from CF PA- patients (P less than 0.025) or NHS (P less than 0.0025), suggesting qualitative opsonic defects of IgG PA LPS antibodies in CF PA+ sera. Antigen-specific immunoprecipitation of PA LPS antibodies enhanced opsonization by 40% of CF PA+ sera while uniformly reducing that from CF PA- sera (P less than 0.01), indicating LPS-specific nonopsonic antibodies in some CF PA+ sera. Alginate antibodies were not critical opsonins in most uninfected CF patient sera. PA LPS IgG antibodies isolated by immunoaffinity chromatography from NHS, hyperimmune globulin, and CF PA- sources were opsonic and had greater activity at equal antigen-binding concentration than identical antibodies isolated from infected CF patients (P less than 0.01-0.05); the majority of isolates from CF PA+ sera did not promote PMN oxidative responses above nonopsonic baseline. A potential isotypic basis for these findings was supported by differences in PMN responses to PA opsonized with MAbs of identical specificity but differing isotypes. PA LPS-specific IgG antibodies inhibiting PMN oxidative responses in infected patient sera demonstrate antigen-specific immunomodulation of host responses by chronic bacterial parasitism in CF, which may play a role in the pathophysiology of lung disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Lieberman M. M. Kinetic analysis of microbe opsonification based on stimulated polymorphonuclear leukocyte oxygenation activity. Infect Immun. 1984 Aug;45(2):475–482. doi: 10.1128/iai.45.2.475-482.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Fick R. B., Jr, Fino L. Antibody to multiple mucoid strains of Pseudomonas aeruginosa in patients with cystic fibrosis, measured by an enzyme-linked immunosorbent assay. Pediatr Res. 1986 Nov;20(11):1085–1090. doi: 10.1203/00006450-198611000-00005. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Shedd D. G. The role of complement in the opsonization of mucoid and non-mucoid strains of Pseudomonas aeruginosa. Pediatr Res. 1983 Dec;17(12):952–958. doi: 10.1203/00006450-198312000-00006. [DOI] [PubMed] [Google Scholar]
- Bender J. G., Florman A. L., Van Epps D. E. Correlation of serum opsonic activity in cystic fibrosis with colonization and disease state: measurement of opsonins to Pseudomonas aeruginosa by neutrophil superoxide anion generation. Pediatr Res. 1987 Oct;22(4):383–388. doi: 10.1203/00006450-198710000-00002. [DOI] [PubMed] [Google Scholar]
- Biggar W. D., Holmes B., Good R. A. Opsonic defect in patients with cystic fibrosis of the pancreas. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1716–1719. doi: 10.1073/pnas.68.8.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerbaum B., Kagumba A., Matthews L. W. Selective inhibition of phagocytic activity of rabbit alveolar macrophages by cystic fibrosis serum. Am Rev Respir Dis. 1973 Oct;108(4):777–783. doi: 10.1164/arrd.1973.108.4.777. [DOI] [PubMed] [Google Scholar]
- Brett M. M., Ghoneim A. T., Littlewood J. M. Serum IgG antibodies in patients with cystic fibrosis with early Pseudomonas aeruginosa infection. Arch Dis Child. 1987 Apr;62(4):357–361. doi: 10.1136/adc.62.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Kureishi A., Rabin H. R. Detection of antibodies to Pseudomonas aeruginosa alginate extracellular polysaccharide in animals and cystic fibrosis patients by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Aug;18(2):276–282. doi: 10.1128/jcm.18.2.276-282.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R. Immunoglobulin G: functional sites. Mol Immunol. 1985 Mar;22(3):161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- Cassino R. J., Sordelli D. O., Macri C. N., Kohan M., Dillon M. H., Pivetta O. H. Pulmonary nonspecific defense mechanisms in cystic fibrosis. I. Phagocytic capacity of alveolar macrophages and neutrophils. Pediatr Res. 1980 Nov;14(11):1212–1215. doi: 10.1203/00006450-198011000-00012. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Roby R. E. Protective activity of an intravenous immune globulin (human) enriched in antibody against lipopolysaccharide antigens of Pseudomonas aeruginosa. Am J Med. 1984 Mar 30;76(3A):168–174. doi: 10.1016/0002-9343(84)90337-1. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Reynolds H. Y. Use of Pseudomonas aeruginosa lipopolysaccharide immunoadsorbents to prepare high potency, mono-specific antibodies. J Immunol Methods. 1980;38(1-2):103–116. doi: 10.1016/0022-1759(80)90335-x. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Olchowski J., Squier S. U., Merrill W. W., Reynolds H. Y. Immunoglobulin-G subclasses in cystic fibrosis. IgG2 response to Pseudomonas aeruginosa lipopolysaccharide. Am Rev Respir Dis. 1986 Mar;133(3):418–422. doi: 10.1164/arrd.1986.133.3.418. [DOI] [PubMed] [Google Scholar]
- Fomsgaard A., Conrad R. S., Galanos C., Shand G. H., Høiby N. Comparative immunochemistry of lipopolysaccharides from typable and polyagglutinable Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis. J Clin Microbiol. 1988 May;26(5):821–826. doi: 10.1128/jcm.26.5.821-826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard A., Høiby N., Shand G. H., Conrad R. S., Galanos C. Longitudinal study of antibody response to lipopolysaccharides during chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. Infect Immun. 1988 Sep;56(9):2270–2278. doi: 10.1128/iai.56.9.2270-2278.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mouat E. C., Speert D. P. Quantitation and identification of antibodies to outer-membrane proteins of Pseudomonas aeruginosa in sera of patients with cystic fibrosis. J Infect Dis. 1984 Feb;149(2):220–226. doi: 10.1093/infdis/149.2.220. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. J., Loren A. B., Scott P. J., Niwa Y., Yokoyama M. M. Demonstration of neutrophil dysfunction in the serum of patients with cystic fibrosis. J Clin Lab Immunol. 1981 Sep;6(2):137–139. [PubMed] [Google Scholar]
- Jacobson M. A., Radolf J. D., Young L. S. Human IgG antibodies to Pseudomonas aeruginosa core lipopolysaccharide determinants are detected in chronic but not acute pseudomonas infection. Scand J Infect Dis. 1987;19(6):649–660. doi: 10.3109/00365548709117200. [DOI] [PubMed] [Google Scholar]
- LeBlanc C. M., Bortolussi R., Issekutz A. C., Gillespie T. Opsonization of mucoid and non-mucoid Pseudomonas aeruginosa by serum from patients with cystic fibrosis assessed by a chemiluminescence assay. Clin Invest Med. 1982;5(2-3):125–128. [PubMed] [Google Scholar]
- Meshulam T., Obedeanu N., Merzbach D., Sobel J. D. Phagocytosis of mucoid and nonmucoid strains of Pseudomonas aeruginosa. Clin Immunol Immunopathol. 1984 Aug;32(2):151–165. doi: 10.1016/0090-1229(84)90117-x. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Lewiston N. J., Curd J. G., Milgrom H., Hart S., Dyer B., Larrick J. W. Association of systemic immune complexes, complement activation, and antibodies to Pseudomonas aeruginosa lipopolysaccharide and exotoxin A with mortality in cystic fibrosis. Am Rev Respir Dis. 1986 Apr;133(4):648–652. doi: 10.1164/arrd.1986.133.4.648. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Sullivan M. M., Lewiston N. J. Altered antibody isotype in cystic fibrosis: possible role in opsonic deficiency. Pediatr Res. 1986 May;20(5):453–459. doi: 10.1203/00006450-198605000-00015. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Van Eede P. H., Van Leeuwen A. M., Lewiston N. J., De Lange G. Altered antibody isotype in cystic fibrosis: impaired natural antibody response to polysaccharide antigens. Pediatr Res. 1987 Dec;22(6):708–713. doi: 10.1203/00006450-198712000-00020. [DOI] [PubMed] [Google Scholar]
- Moss R. B. Hypergammaglobulinemia in cystic fibrosis. Role of Pseudomonas endobronchial infection. Chest. 1987 Apr;91(4):522–526. doi: 10.1378/chest.91.4.522. [DOI] [PubMed] [Google Scholar]
- Naegel G. P., Young K. R., Jr, Reynolds H. Y. Receptors for human IgG subclasses on human alveolar macrophages. Am Rev Respir Dis. 1984 Mar;129(3):413–418. doi: 10.1164/arrd.1984.129.3.413. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Schmeling D., Lindemann M., Verhoef J., Quie P. G. Complement-mediated phagocytosis of Pseudomonas aeruginosa. J Lab Clin Med. 1978 Dec;92(6):883–894. [PubMed] [Google Scholar]
- Piedra P., Ogra P. L. Immunologic aspects of surface infections in the lung. J Pediatr. 1986 May;108(5 Pt 2):817–823. doi: 10.1016/s0022-3476(86)80751-x. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Desjardins D., Aguilar T., Barnard M., Speert D. P. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J Clin Microbiol. 1986 Aug;24(2):189–196. doi: 10.1128/jcm.24.2.189-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Saunders J. M., Ames P., Edwards M. S., Auerbach H., Goldfarb J., Speert D. P., Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987 Sep 24;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler T., Mansa B., Jensen T., Pedersen S. S., Høiby N., Koch C. Increased IgG2 and IgG3 concentration is associated with advanced Pseudomonas aeruginosa infection and poor pulmonary function in cystic fibrosis. Acta Paediatr Scand. 1988 Jul;77(4):576–582. doi: 10.1111/j.1651-2227.1988.tb10703.x. [DOI] [PubMed] [Google Scholar]
- Shryock T. R., Mollé J. S., Klinger J. D., Thomassen M. J. Association with phagocytic inhibition of anti-Pseudomonas aeruginosa immunoglobulin G antibody subclass levels in serum from patients with cystic fibrosis. J Clin Microbiol. 1986 Mar;23(3):513–516. doi: 10.1128/jcm.23.3.513-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Lawton D., Mutharia L. M. Antibody to Pseudomonas aeruginosa mucoid exopolysaccharide and to sodium alginate in cystic fibrosis serum. Pediatr Res. 1984 May;18(5):431–433. doi: 10.1203/00006450-198405000-00008. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Boxerbaum B., Demko C. A., Kuchenbrod P. J., Dearborn D. G., Wood R. E. Inhibitory effect of cystic fibrosis serum on pseudomonas phagocytosis by rabbit and human alveolar macrophages. Pediatr Res. 1979 Sep;13(9):1085–1088. doi: 10.1203/00006450-197909000-00030. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Wood R. E., Sherman J. M. Phagocytosis of Pseudomonas aeruginosa by polymorphonuclear leukocytes and monocytes: effect of cystic fibrosis serum. Infect Immun. 1982 Nov;38(2):802–805. doi: 10.1128/iai.38.2.802-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Hastings M. J., Easmon C. S., Cole P. J. Factor affecting the in vitro assessment of opsonization: a study of the kinetics of opsonization using the technique of phagocytic chemiluminescence. Immunology. 1980 Dec;41(4):903–911. [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]