Abstract
BACKGROUND
Seizures are common in children, but the causes and recurrence risk for children with a nonfebrile first seizure remain poorly understood.
OBJECTIVE
In a prospective longitudinal study of children who presented with a first-time seizure, we investigated the viral etiology of associated infectious illnesses and sought to determine the risk of recurrent seizures stratified by fever and type of illness.
PATIENTS AND METHODS
Children (aged 6 months to 6 years) were enrolled at the time of evaluation for their first seizure and followed monthly for up to 5 years. Seizure and illness data were collected through parent interviews and medical-record reviews. Stool, serum, and cerebrospinal fluid collected within 48 hours of the first seizure were evaluated for viral gastrointestinal pathogens.
RESULTS
Of the 117 children enrolled, 78 (67%) had febrile seizures, 34 (29%) had nonfebrile-illness seizures, and 5 (4%) had unprovoked seizures. Children with nonfebrile-illness seizures were more likely than those with febrile seizures to have acute gastroenteritis (47% and 28%, respectively; P = .05). No significant differences in seizure recurrence were found between children with or without a fever at first seizure. Children with acute gastroenteritis at first seizure, regardless of fever, had a lower risk of seizure recurrence compared with children with other acute illnesses (hazard ratio: 0.28; 95% confidence interval: 0.09–0.80).
CONCLUSIONS
Our results confirm the role of gastrointestinal illness as a distinguishing feature in childhood seizures. Children with this distinct presentation have a low rate of seizure recurrence and few neurologic complications.
Keywords: seizures, febrile, rotavirus, norovirus, child, gastroenteritis
Febrile seizures are the most common seizures of childhood. These seizures affect 2% to 4% of children in the United States and are generally considered to have a good prognosis.1,2 Reports have suggested the presence of another, distinct category of provoked seizures, nonfebrile-illness seizures (or “afebrile benign convulsions”), which are not associated with a fever but occur in the presence of other symptoms of infection.3,4
For parents whose child has had a first-time seizure, and for the physicians who treat them, the most salient concern once the child is stabilized regards the risk of recurrence. That risk has been well defined for febrile seizures but remains poorly defined for nonfebrile-illness seizures.5 Retrospective analyses and case reports have shown that a subset of these seizures may be associated with diarrheal illness and that children who suffer a first seizure associated with a nonfebrile illness may have a lower risk of seizure recurrence compared with children with an unprovoked first seizure.3,4,6–17 However, prospective, systematically collected data are scarce in regard to the long-term prognosis of children with nonfebrile-illness seizures, and few studies have included comprehensive viral testing on children with first-time seizures.
To systematically describe associated viral etiologies and to determine the risk of recurrent seizures in children with nonfebrile-illness seizures, we performed a prospective longitudinal study of children who presented with a first-time seizure.
PATIENTS AND METHODS
Subject Enrollment
Patients who met the inclusion criteria for this study included children aged 6 months through 6 years who presented to the emergency department of Seattle Children’s Hospital (Seattle, WA) with a first-time seizure and had a parent or legal guardian available to provide informed consent in English. Children were excluded if they had seizures caused by trauma, meningitis, encephalitis, toxic ingestion, hypoxia (or “breath-holding spells”), brain tumors, other known medical conditions that predispose to seizures (such as microcephaly, macrocephaly, and brain malformation), or infantile spasms. Study staff members were available in the emergency department and inpatient units from 8:00 AM to midnight daily from February 18, 2005, until May 31, 2008. A seizure was defined as a witnessed event that the treating physician considered likely to be an epileptic seizure and that was characterized by a paroxysmal change in motor activity with or without an associated change in mental status. At the time of study enrollment children were categorized into 3 study groups according to seizure characteristics: febrile seizure (seizure was accompanied by a body temperature of ≥38.0°C within 24 hours before or 2 hours after the index seizure), nonfebrile-illness seizure (symptoms of illness such as rhinorrhea, cough, diarrhea, vomiting, or rash occurred within the week before the seizure, but no fever was present within 24 hours before or 2 hours after the first seizure), and unprovoked seizure (seizure was not accompanied by symptoms of illness at the time of or during the week before the seizure).
Index-Seizure Data
After informed consent was obtained from the child’s guardian, research personnel conducted an interview with the guardian to collect clinical information about the study patient’s index seizure, medical history, and demographic characteristics. On days 2 and 7 after the patient was discharged from the hospital, families were contacted again to collect information on resolution of illness symptoms.
Medical-record abstraction was used to collect data on laboratory values, clinical course, and electroencephalogram (EEG) and brain-imaging findings. The guardian also completed a Child Development Inventory, a validated measure of children’s cognitive and physical development.18
Illness Symptom Data Collection
Trained study staff interviewed guardians about specific illness symptoms within 7 days of the index seizure. Symptom interviews were repeated on days 2 and 7. To assess the relationship between concurrent acute illness and seizure characteristics and prognosis, children’s accompanying illness symptoms were categorized as follows: acute gastrointestinal illness (diarrhea, with or without vomiting, that developed within the 7 days before or after the seizure) and acute nongastrointestinal illness (illness symptoms, including runny nose, cough, or rash, that developed within the 7 days before or after the seizure without acute gastrointestinal symptoms).
Sample Collection
Serum, stool, and residual cerebrospinal fluid (CSF) samples were collected on site within 48 hours of the index seizure. If stool samples were not available during hospital admission, they were collected by parents and sent by courier to the study site. All samples were stored at −80°C.
Laboratory Testing for Viral Gastrointestinal Pathogens
Stool samples and sera were diluted 1:10 in phosphate-buffered saline and then tested for rotavirus antigen by Premier Rotaclone enzyme immunoassay (Meridian Biosciences, Cincinnati, OH). RNA was extracted from stool samples by using the MagMAX viral RNA-isolation kit (Ambion, Austin, TX) on the Thermo Scientific KingFisher Flex instrument (Thermo Scientific, Waltham, MA). RNA from sera and CSF was extracted by using the RNaid kit (Q-Biogene, Montreal, Quebec, Canada). Samples were tested by real-time reverse transcription–polymerase chain reaction (RT-PCR) for the rotavirus NSP3 gene on the ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA) modified from the methods of Freeman et al19 and/or by a conventional RT-PCR that targeted the rotavirus VP6 gene20 to confirm positive samples. Positive stool samples were then genotyped for the VP4 and VP7 genes by using consensus primers described previously.21–23
For norovirus, viral RNA was extracted from 50 μL of clarified 10% fecal suspension, serum, or CSF, as described for rotavirus, and tested for norovirus RNA by TaqMan real-time RT-PCR as described.24 For samples with adequate remaining volume, testing for enterovirus, parechovirus, adenovirus, and bocavirus was performed at the University of Washington molecular diagnostics laboratory. Stool specimens were thawed at 4°C, and samples were obtained by immersion of a Dacron swab into the specimen. The swab was broken off into 1 mL of sterile Hanks’ balanced salt solution. The mixture was vortexed well and centrifuged for 10 minutes. Volumes of 20 and 200 μL of supernatant were removed, and the total nucleic acids in each aliquot were extracted by a minikit (Qiagen, Valencia, CA) using the spin protocol for body fluids. An extraction and amplification control RNA was added to each specimen during extraction to monitor for adequate RNA extraction and the presence of PCR inhibitors.25 All nucleic acid specimens were tested by in-house real-time RT-PCR assays for the detection of enteroviruses, parechoviruses, and the control RNA25 and by previously described real-time PCR assays for the detection of adenoviruses25 and bocavirus.26
Long-term Follow-up
After the index seizure, data on recurrent seizures were collected once per month by interview of guardians by telephone or e-mail.
Statistical Analysis
Subject characteristics, seizure characteristics, and viral test results were described according to study group (febrile, nonfebrile illness, unprovoked) and compared by using χ2 and t tests, and Fisher’s exact and Mann-Whitney tests were used for nonparametric comparisons. Time to recurrent seizures was described by using Kaplan-Meier failure estimates and plots. Risk of seizure recurrence was compared between study groups by using Cox regression with a time-dependent covariate for use of antiepileptic medication. Recurrence estimates were calculated as time to a child’s first recurrence of any type of seizure and as time to a child’s first nonfebrile recurrence; however, non-febrile recurrences were uncommon in this study population. In response to recent reports of a unique seizure category observed with acute gastroenteritis, we also performed a secondary analysis to evaluate our study participants on the basis of concurrent acute illness symptoms at the time of first seizure. Analyses of subject characteristics, seizure characteristics, and risk of recurrence were repeated according to type of illness at enrollment, according to use of the definitions for acute gastrointestinal illness and acute nongastrointestinal illness described above.
RESULTS
Demographic and Clinical Characteristics
A total of 308 children were screened at the time of their first seizure. Of these children, 148 were deemed ineligible after their initial screening for reasons that included: non–English-speaking guardian (n = 48), no guardian available for consent or sample collection (n = 28), chronic medical condition that increased the risk of seizure (n = 18), acute medical condition that increased the risk of seizure (n = 11), and preexisting developmental delay (n = 8). Guardians declined enrollment for 43 children. A total of 117 children were enrolled and evaluated. Of the 117 children enrolled, 78 (67%) had febrile first seizures, 34 (29%) had nonfebrile-illness first seizures, and 5 (4%) had unprovoked first seizures (Table 1). Among the 32 children for whom the Child Development Inventory was completed, no differences in development were found for any domain.
TABLE 1.
Demographics of Study Population According to Enrollment Group
| Overall (N = 117) | Febrile (N = 78) | Afebrile (N = 34) | Unprovoked (N = 5) | |
|---|---|---|---|---|
| Age, mean (SD), mo | 24 (15) | 22 (13) | 24 (12) | 54 (19) |
| Male, n (%) | 63 (54) | 41 (53) | 18 (53) | 4 (80) |
| Daycare attendance, n (%) | 40 (34) | 24 (31) | 14 (41) | 2 (40) |
| Unknown | 2 (3) | |||
| Race, n (%) | ||||
| White | 82 (70) | 57 (73) | 21 (62) | 4 (80) |
| Asian | 15 (13) | 9 (12) | 6 (18) | 0 (0) |
| Black or African American | 3 (3) | 2 (3) | 1 (3) | 0 (0) |
| Other | 13 (11) | 8 (10) | 4 (12) | 1 (20) |
| Unknown/not reported | 4 (3) | 2 (3) | 2 (6) | 0 (0) |
| Mother’s education, n (%) | ||||
| Some high school | 2 (2) | 1 (1) | 1 (3) | 0 (0) |
| High school | 14 (12) | 8 (10) | 5 (15) | 1 (20) |
| Some college or community college | 15 (13) | 9 (12) | 3 (9) | 3 (60) |
| College | 36 (31) | 25 (32) | 11 (32) | 0 (0) |
| Graduate or professional school | 35 (30) | 26 (33) | 8 (24) | 1 (20) |
| Unknown/not reported | 15 (13) | 9 (12) | 6 (18) | 0 (0) |
| Father’s education, n (%) | ||||
| Some high school | 2 (2) | 1 (1) | 1 (3) | 0 (0) |
| High school | 11 (9) | 6 (8) | 4 (12) | 1 (20) |
| Some college or community college | 13 (11) | 11 (14) | 2 (6) | 0 (0) |
| College | 31 (27) | 20 (26) | 10 (29) | 1 (20) |
| Graduate or professional school | 41 (35) | 29 (37) | 10 (29) | 2 (40) |
| Unknown/not reported | 19 (16) | 11 (14) | 7 (21) | 1 (20) |
| Annual household income, n (%) | ||||
| <10 000 | 6 (5) | 4 (5) | 2 (6) | 0 (0) |
| 10–25 000 | 7 (6) | 3 (4) | 3 (9) | 1 (20) |
| 25–50 000 | 13 (11) | 6 (8) | 5 (15) | 2 (40) |
| 50–75 000 | 17 (15) | 13 (17) | 4 (12) | 0 (0) |
| >75 000 | 56 (48) | 40 (51) | 15 (44) | 1 (20) |
| Unknown/not reported | 18 (15) | 12 (15) | 5 (15) | 1 (20) |
| Received immunization in month prior to seizure, n (%) | 27 (23) | 19 (24) | 7 (21) | 1 (20) |
| Unknown/not reported | 22 (19) | 14 (18) | 8 (24) | 0 (0) |
| History of medical issues, n (%) | 32 (27) | 22 (28) | 8 (24) | 2 (40) |
| Unknown/not reported | 2 (2) | 0 (0) | 1 (3) | 1 (20) |
| History of seizures in immediate family, n (%) | 22 (19) | 19 (24) | 3 (9) | 0 (0) |
| Unknown/not reported | 74 (63) | 47 (60) | 22 (65) | 5 (100) |
| Mean follow-up time, mo | 28 | 27 | 30 | 41 |
Index-Seizure Characteristics
Children in the nonfebrile-illness group were more likely to experience additional seizures during the 24 hours after their index seizure (59% compared with 28% in the febrile group; P = .002) but were not more likely to experience additional seizures during the following week (6% in both groups) (Table 2). Initial febrile seizures and nonfebrile-illness seizures were similar in duration (median: 2 minutes in both groups) and proportion with focal presentation (9% and 6%, respectively; P = .72). Children with first nonfebrile-illness seizures were less likely than children with first febrile seizures or first unprovoked seizures to have abnormal blood glucose results (14% and 71%, respectively; P = .002) according to normal laboratory ranges (Table 2); however, no child had a glucose level below 41 mg/dL.
TABLE 2.
Characteristics of Primary Seizure Event According to Primary-Seizure Enrollment Group and Gastrointestinal Illness Group
| Overall (N = 117) | Enrollment-Seizure Group |
Illness Group |
||||
|---|---|---|---|---|---|---|
| Unprovoked (n = 5) | Febrile (n = 78) | Nonfebrile Illness (n = 34) | Non-GI Illness (n = 74) | GI Illness (n = 38) | ||
| Median duration of first seizure, median (IQR), min | 2 (0.6–4) | 2.5 (2–3) | 2 (0.5–5) | 2 (1–3) | 2 (0.75–5) | 2 (0.5–3) |
| Seizure duration ≥ 15 min, n (%) | 9 (8) | 0 (0) | 7 (9) | 2 (6) | 6 (8) | 3 (8) |
| Multiple seizures in first 24 h, n (%) | 43 (37) | 1 (20) | 22 (28) | 20 (59) | 20 (27) | 22 (58) |
| P | .002 | .001 | ||||
| Additional seizures in first week, n (%) | 7 (6) | 0 (0) | 5 (6) | 2 (6) | 5 (7) | 1 (5) |
| Focal primary seizure, n (%) | 10 (9) | 1 (20) | 7 (9) | 2 (6) | 9 (12) | 0 (0) |
| Unknown | 1 (1) | 1 (1) | .024 | |||
| Abnormal findingsa | ||||||
| Glucose, n/N (%) | 16/33 (48) | 2/2 (100) | 12/17 (71) | 2/14 (14) | 9/16 (56) | 5/15 (33) |
| P | .002 | |||||
| Sodium, n/N (%) | 4/38 (11) | 0/2 (0) | 2/17 (12) | 2/19 (11) | 0/16 (0) | 4/20 (20) |
| Calcium, n/N (%) | 1/25 (4) | 0/2 (0) | 1/9 (11) | 0/14 (0) | 0/11 (0) | 1/12 (8) |
| Magnesium, n/N (%) | 3/20 (15) | 0/2 (0) | 1/5 (20) | 2/13 (15) | 1/8 (13) | 2/10 (20) |
| Phosphorous, n/N (%) | 3/22 (14) | 0/2 (0) | 0/6 (0) | 3/14 (21) | 1/10 (10) | 2/10 (20) |
| Computed tomography, n/N (%) | 4/20 (20) | 0/1 (0) | 3/11 (27) | 1/8 (13) | 4/14 (29) | 0/5 (0) |
| MRI, n/N (%) | 6/8 (75) | 1/2 (50) | 4/5 (80) | 1/1 (100) | 5/5 (100) | 0/1 (0) |
| EEG, n/N (%) | 14/32 (44) | 3/4 (75) | 5/11 (45) | 6/17 (35) | 8/19 (42) | 3/9 (33) |
| Specific EEG abnormalities | ||||||
| Slowing, n (n focal) | 8 (4) | 1 (1) | 4 (2) | 3 (1) | 5 (3) | 2 (0) |
| Epileptiform discharge, n (n focal) | 7 (6) | 2 (2) | 2 (2) | 3 (2) | 4 (4) | 1 (0) |
GI indicates gastrointestinal; IQR, interquartile range; n/N, number of children with abnormalities per number of children with test performed.
The ranges of abnormal laboratory values were as follows: low glucose, 41–61 mg/dL; high glucose, 107–271 mg/dL; low sodium, 131–134 mEq/L; low calcium, 16 mg/dL; low magnesium, 1.5–1.7 mg/dL; low phosphorus, 3.6–3.8 mg/dL; high phosphorus, 7.4 mg/dL.
Illness Symptoms Concurrent With Index Seizure
Acute gastrointestinal illness (onset within 7 days before or after the index seizure) was present in a larger proportion of the nonfebrile-illness-seizure group compared with the febrile-illness-seizure group (47% and 28%, respectively; P = .05) (Table 3). Overall, 36 of 38 children with acute gastrointestinal illness developed diarrhea within the 5 days that led up to the index seizure, and 2 children developed diarrhea at days 2 and 3 after the index seizure. Both of these children presented with fever and rhinorrhea at the time of seizure, and 1 had a 2-day history of vomiting.
TABLE 3.
Acute Illnesses and Viral Pathogens That Accompanied First Seizure, According to Study Group
| Febrile (N = 78) | Nonfebrile Illness (N = 34) | P | |
|---|---|---|---|
| Acute illness type, n (%) | |||
| Acute diarrheal illness | 22 (28) | 16 (47) | .05 |
| Acute upper respiratory illness | 25 (32) | 3 (9) | .009 |
| Stool specimen results | |||
| Rotavirus, n/N (%) | 6/65 (9) | 7/25 (28) | .02 |
| Rotavirus genotype | |||
| G1P[8] | 3 | 5 | |
| G3P[8] | 2 | 0 | |
| G9P[8] | 1 | 0 | |
| Unknowna | 0 | 2 | |
| Norovirus, n/N (%) | 8/64 (13) | 7/23 (30) | .05 |
| Norovirus type | |||
| GI | 1 (2) | 0 | .55 |
| GII | 7 (11) | 7 (30) | .03 |
| Enterovirus, n/N (%) | 11/43 (26) | 0/12 | .05 |
| Parechovirus, n/N (%) | 1/43 (2) | 0/12 | .59 |
| Adenovirus, n/N (%) | 13/43 (30) | 1/12 (8) | .12 |
| Bocavirus, n/N (%) | 3/43 (7) | 1/12 (8) | .87 |
| Multiple infections, n | |||
| Rotavirus/parechovirus | 1 | 0 | |
| Rotavirus/bocavirus | 0 | 1 | |
| Enterovirus/adenovirus | 2 | 0 | |
| Norovirus/adenovirus | 0 | 1 | |
| Norovirus/enterovirus/adenovirus | 1 | 0 | |
| Norovirus/enterovirus/adenovirus/bocavirus | 1 | 0 | |
| Serum specimen results, n/N (%) | |||
| Rotavirus | 1/6 (17) | 0/12 (0) | .33 |
| Norovirus | 0/6 (0) | 0/12 (0) | — |
| CSF specimen results, n/N (%) | |||
| Rotavirus | 0/3 (0) | 1/2 (50) | .40 |
| Norovirus | 0/3 (0) | 0/2 (0) | — |
Five children in the unprovoked seizure enrollment group did not have accompanying illness symptoms, by definition. n/N indicates number of children with a positive result per number of children with test performed.
Three rotavirus tests were performed clinically without sample available for detailed genotype testing. Results for 2 of these samples were positive; both samples were from children in the nonfebrile-illness group.
Children with acute gastrointestinal illness experienced multiple seizures within the first 24 hours significantly more often than children with febrile seizures (58% and 27%, respectively; P = .001) (Table 2). This association persisted after we controlled for fever in a logistic regression model (adjusted odds ratio: 3.3; 95% confidence interval [CI]: 1.4–7.7). None of the 38 primary seizures in the presence of acute gastrointestinal illness had focal presentation, compared with 9 (12%) of the 74 seizures in the nongastrointestinal illness group (P = .02) (Table 2).
Viral Pathogens
Stool samples were available for rotavirus and norovirus testing from 64 children with a febrile seizure, 23 children with a nonfebrile-illness seizure, and 4 children with a first unprovoked seizure (Table 3). Rotavirus tests were performed for 3 additional children at the clinical laboratory of Seattle Children’s Hospital, and 2 were positive for rotavirus. Children with a first nonfebrile-illness seizure were more likely than those with a first febrile seizure to have a stool sample test positive for rotavirus (P = .02) and for norovirus (P = .05). No viruses were detected in 4 available stool samples from children with unprovoked seizures.
A total of 19 children (including 18 with acute illness) had serum samples and 5 children had CSF samples available for testing for rotavirus and norovirus (Table 3). Five children with rotavirus-positive stool samples had available serum samples, 1 of which tested positive for rotavirus RNA. This sample was from a child with a first febrile seizure and acute gastrointestinal illness. Only 1 CSF sample was available from the group of children with rotavirus-positive stool. This sample was positive for rotavirus RNA and was from a child with a first nonfebrile-illness seizure and acute gastrointestinal illness.
Fifty-nine stool samples had sufficient material for additional virus testing (Table 3). Enterovirus and parechovirus were detected only in the febrile group, whereas adenovirus and bocavirus were detected in both the febrile and nonfebrile-illness groups. At least 1 virus was detected in 46 children, and multiple viruses were detected in 7 children (5 in the febrile group and 2 in the afebrile group). No coinfections involving both rotavirus and norovirus were observed (Table 3).
EEG Evaluations
EEGs were performed at the discretion of the consulting neurologist or primary care physician in 32 of 117 children. The EEG was performed an average of 26 days (interquartile range: 3–40) after the index seizure. The proportion of children with EEGs recorded within 3 days of the index seizure was not significantly different between children with normal and children with abnormal EEG findings (22% and 36%, respectively; P = .45, Fisher’s exact test). Abnormalities were identified on 14 of 32 EEGs (Table 2). Slowing or epileptiform discharges were identified in 3 children with first febrile seizure, 3 children with first nonfebrile-illness seizure, and 3 children with first unprovoked seizure. Of the 9 children with acute gastrointestinal illness who underwent EEG, 3 had EEG results that revealed abnormalities, but none showed focal slowing or epileptiform discharges (Table 2).
Subsequent Seizures
Children were followed for a total of 101 324 days (range: 1–1684 days; mean: 866 days). Mean follow-up time was similar among all study groups (Table 1).
The Kaplan-Meier failure estimates for any second seizure after the first week was 60% (95% CI: 25%–95%) for children with a first unprovoked seizure, 24% (95% CI: 12%–44%) for children with a first nonfebrile-illness seizure and 31% (95% CI: 21%–43%) for children with a first febrile-illness seizure. With the use of a Cox proportional hazards model to compare the risk of a second seizure, the prognosis for nonfebrile-illness seizures was not significantly different from that of febrile-illness seizures after we controlled for use of antiepileptic medication (Table 4). However, children who experienced acute gastrointestinal illness, regardless of fever, at the time of the index seizure had a significantly reduced risk of recurrent seizures, even after we controlled for the presence of fever at the time of the index seizure (Table 4). When we examined data for children with illness-associated seizures and controlled for antiepileptic drug use, we found that rotavirus and norovirus infection each conferred a reduced risk of seizure recurrence that was not statistically significant (hazard ratio: 0.30 [95% CI: 0.04–2.23] [rotavirus] and 0.49 [95% CI: 0.11–2.10] [norovirus]). Overall, the Kaplan-Meier failure estimates for a second seizure of any type were 11% (95% CI: 4%–28%) for children with gastrointestinal illness and 40% (95% CI: 29%–53%) for children with nongastrointestinal illness (Fig 1).
TABLE 4.
Cox Regression Models for Risk of Future Seizure Recurrence of Any Type
| Model | Data Included | Covariates | Risk of a First Recurrence of Any Type, Hazard Ratio (95% CI) |
|---|---|---|---|
| 1 | Illness-associated first seizures (n = 112) | First seizure category | |
| Febrile | 1.00 | ||
| Nonfebrile illness | 0.73 (0.31–1.73) | ||
| 2 | Illness-associated first seizures (n = 112) | First Seizure with accompanying illness | |
| Acute non-GI Illness | 1.00 | ||
| Acute GI illness | 0.28 (0.09–0.80) | ||
| 3 | Illness-associated first seizures (n = 112) | Illness accompanying first seizure | |
| Acute non-GI illness | 1.00 | ||
| Acute GI illness | 0.28 (0.10–0.82) | ||
| First seizure category: | |||
| Febrile | 1.00 | ||
| Nonfebrile illness | 0.94 (0.39–2.24) | ||
| 4 | Illness-associated first seizures with testing performed (n = 89) | Rotavirus-negative/norovirus-negative | 1.00 |
| Rotavirus-positive or norovirus-positive | 0.40 (0.12–1.35) |
Cox regression estimates the risk of a recurrent seizure accounting for subject characteristics and time since first seizure. The interpretation of the hazard ratio is analogous to a relative risk. All models were controlled for use of antiepileptic medication as a time-varying covariate. No subsequent nonfebrile seizures were observed in children with first febrile seizures or in children with acute gastrointestinal illness. GI indicates gastrointestinal.
FIGURE 1.
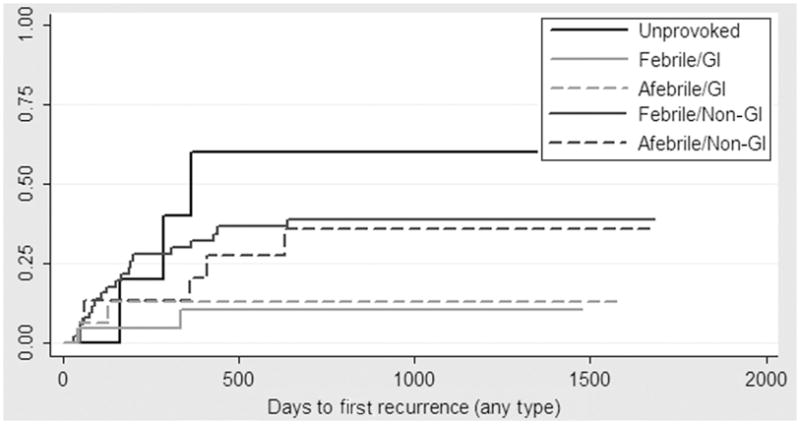
Kaplan-Meier failure for seizure recurrence, according to study group and acute illness. GI indicates gastrointestinal.
Of the recurrent seizures that occurred, only 6 were nonfebrile, 2 in children with a first unprovoked seizure and 4 in children with a first nonfebrile-illness seizure without acute gastrointestinal illness (Fig 2). The Kaplan-Meier failure estimates for a future nonfebrile seizure were 40% (95% CI: 12%–87%) for children with a first unprovoked seizure and 14% (95% CI: 5%–32%) for children with a first nonfebrile-illness seizure; no recurrent nonfebrile seizures were observed in children with a first febrile-illness seizure (Fig 2). For children with nongastrointestinal illness, the Kaplan-Meier failure estimate for a nonfebrile recurrence was 9% (95% CI: 4%–19%). No nonfebrile recurrent seizures occurred in children with acute gastrointestinal illness at the time of their index seizure.
FIGURE 2.
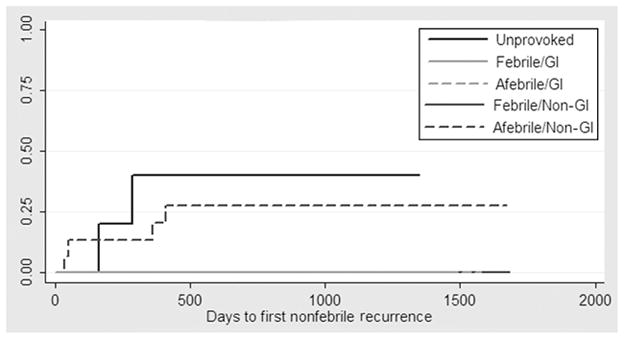
Kaplan-Meier failure for first nonfebrile seizure recurrence, according to study group and acute illness. No subsequent nonfebrile seizures were observed in children with first febrile seizures or in children with acute gastrointestinal illness. GI indicates gastrointestinal.
DISCUSSION
In this prospective study of first-time seizures in young children, we found that children whose first seizures were associated with a nonfebrile illness had a prognosis similar to that of children whose first seizures were associated with a febrile illness. We also found that the type of illness syndrome that accompanied the index seizure was an important prognostic indicator. Overall, seizure recurrence was highest among children with first unprovoked seizures, followed by children with febrile or nonfebrile-illness first seizures without acute gastrointestinal symptoms. Seizure recurrence was lowest among children who had acute gastrointestinal symptoms at the time of their first seizure (Fig 1).
Komori et al13 previously defined benign convulsions with gastroenteritis as afebrile tonic-clonic convulsions in healthy children, which occur between the first and fifth sick day of viral gastroenteritis and are typically associated with normal electrolyte and glucose levels and a low risk of recurrence. Although this definition stipulates that the index seizure be afebrile, our data support the conclusion that it is the acute gastrointestinal illness that predicates a lower risk of future recurrences, regardless of whether fever was present at the time of seizure.
Lee and Ong,4 who performed a study in Singapore, reported a low risk of subsequent nonfebrile seizures in children with gastrointestinal illness compared with children with respiratory infections or nonspecific fever. We were able to confirm these findings in our study, conducted in a different setting almost 10 years later, by using standardized in-person symptom interviews at the time of the first seizure and detailed viral testing on children with and without gastrointestinal illness. By using monthly parent interviews to obtain data on recurrent seizures, we were able to obtain detailed data on long-term prognosis and also to minimize recall bias. Thus, we obtained data on recurrent seizures for which the patients may not have presented for medical care, data that may have been missed in earlier studies. In our study, as in the study by Lee and Ong, antiepileptic medication was prescribed as clinically indicated and may have influenced the crude results if medication rates differed between study groups. Our findings were consistent even after we controlled for periods of antiepileptic use in a Cox proportional hazards model.
The proportion of unprovoked first seizures ascertained in our study was low compared with proportions reported for earlier studies.3,4 The low incidence of unprovoked seizures in our study patients may have resulted from our structured and detailed in-person interviews regarding patient symptoms. Seizures that occurred in children with an illness, especially those without an associated fever, may have been mis-classified as unprovoked seizures in previous studies in which medical records were used for case identification. Through the use of interviews, we also were able to confirm that each child had no previous seizures before the seizure that was identified as the index seizure in our investigation. It is notable that, in the cases we investigated, diarrhea started as early as 5 days before the seizure, an observation that is comparable to findings of other reported studies.13 Our findings highlight the importance of a detailed review of gastrointestinal symptoms that occurred in the week before an index seizure to differentiate between gastrointestinal-illness–associated seizures and unprovoked seizures.
The results of this study and of other similar investigations support the recognition of a distinct type of childhood seizure associated with gastrointestinal illness; however, the mechanism behind this association remains un-clear. We cannot implicate fever as the cause, because 58% of children with gastrointestinal-illness–associated seizuresin our study did not have a fever in the 24 hours before or 2 hours after the first seizure. Electrolyte concentrations were normal in most children with gastrointestinal illness (Table 2), which suggests that metabolic derangement was an unlikely cause. Our findings were not limited to a single pathogen; we found a large but statistically insignificant reduction in the risk of seizure recurrence in children with rotavirus or norovirus infection.
CONCLUSIONS
The results of this study confirm the role of gastrointestinal illness as a distinguishing factor in childhood seizures. This distinct seizure type is associated with a significantly lower rate of seizure recurrence and few neurologic complications. Although the mechanism behind these seizures remains unclear, our results confirm the good prognosis and low risk of seizure recurrence for children who present with a first-time seizure associated with an acute gastrointestinal illness.
Acknowledgments
This research was funded in part by an anonymous donor to Children’s Hospital and by grant UL1RR025014 from the National Center for Research Resources, a component of the National Institutes of Health.
We thank Anne Berg, PhD, for valuable input in the design of this study.
ABBREVIATIONS
- EEG
electroencephalogram
- CSF
cerebrospinal fluid
- CI
confidence interval
- RT-PCR
reverse transcription–polymerase chain reaction
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The article’s contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health or the National Center for Research Resources.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(suppl 2):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6):1281–1286. doi: 10.1542/peds.2008-0939. [DOI] [PubMed] [Google Scholar]
- 3.Zerr DM, Blume HK, Berg AT, et al. Nonfebrile illness seizures: a unique seizure category? Epilepsia. 2005;46(6):952–955. doi: 10.1111/j.1528-1167.2005.65204.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee WL, Ong HT. Afebrile seizures associated with minor infections: comparison with febrile seizures and unprovoked seizures. Pediatr Neurol. 2004;31(3):157–164. doi: 10.1016/j.pediatrneurol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Shinnar S, Hauser WA, Leventhal JM. Predictors of recurrent febrile seizures: a metaanalytic review. J Pediatr. 1990;116(3):329–337. doi: 10.1016/s0022-3476(05)82816-1. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Ami T, Sinai L, Granot E. Afebrile seizures and rotavirus gastroenteritis: an infrequently recognized association. Clin Pediatr (Phila) 2007;46(2):178–180. doi: 10.1177/0009922806290030. [DOI] [PubMed] [Google Scholar]
- 7.Chen SY, Tsai CN, Lai MW, et al. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin Infect Dis. 2009;48(7):849–855. doi: 10.1086/597256. [DOI] [PubMed] [Google Scholar]
- 8.DiFazio MP, Braun L, Freedman S, Hickey P. Rotavirus-induced seizures in childhood. J Child Neurol. 2007;22(12):1367–1370. doi: 10.1177/0883073807307083. [DOI] [PubMed] [Google Scholar]
- 9.Hongou K, Konishi T, Yagi S, Araki K, Miyawaki T. Rotavirus encephalitis mimicking afebrile benign convulsions in infants. Pediatr Neurol. 1998;18(4):354–357. doi: 10.1016/s0887-8994(97)00206-3. [DOI] [PubMed] [Google Scholar]
- 10.Iyadurai S, Troester M, Harmala J, Bodensteiner J. Benign afebrile seizures in acute gastroenteritis: is rotavirus the culprit? J Child Neurol. 2007;22(7):887–890. doi: 10.1177/0883073807304703. [DOI] [PubMed] [Google Scholar]
- 11.Johansen K, Hedlund KO, Zweygberg-Wirgart B, Bennet R. Complications attributable to rotavirus-induced diarrhoea in a Swedish paediatric population: report from an 11-year surveillance. Scand J Infect Dis. 2008;40(11–12):958–964. doi: 10.1080/00365540802415509. [DOI] [PubMed] [Google Scholar]
- 12.Kawano G, Oshige K, Syutou S, et al. Benign infantile convulsions associated with mild gastroenteritis: a retrospective study of 39 cases including virological tests and efficacy of anticonvulsants. Brain Dev. 2007;29(10):617–622. doi: 10.1016/j.braindev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Komori H, Wada M, Eto M, Oki H, Aida K, Fuji-moto T. Benign convulsions with mild gastroenteritis: a report of 10 recent cases detailing clinical varieties. Brain Dev. 1995;17(5):334–337. doi: 10.1016/0387-7604(95)00074-l. [DOI] [PubMed] [Google Scholar]
- 14.Narchi H. Benign afebrile cluster convulsions with gastroenteritis: an observational study. BMC Pediatr. 2004;4:2. doi: 10.1186/1471-2431-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiihara T, Watanabe M, Honma A, et al. Rota-virus associated acute encephalitis/encephalopathy and concurrent cerebellitis: report of two cases. Brain Dev. 2007;29(10):670–673. doi: 10.1016/j.braindev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Sugata K, Taniguchi K, Yui A, et al. Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics. 2008;122(2):392–397. doi: 10.1542/peds.2007-2290. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe T, Hara K, Kashiwagi M, Tamai H. Classification of benign infantile afebrile seizures. Epilepsy Res. 2006;70(suppl 1):S185–S189. doi: 10.1016/j.eplepsyres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Ireton H, Glascoe FP. Assessing children’s development using parents’ reports: the Child Development Inventory. Clin Pediatr (Phila) 1995;34(5):248. doi: 10.1177/000992289503400504. [DOI] [PubMed] [Google Scholar]
- 19.Freeman MM, Kerin T, Hull J, McCaustland K, Gentsch J. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J Med Virol. 2008;80(8):1489–1496. doi: 10.1002/jmv.21228. [DOI] [PubMed] [Google Scholar]
- 20.Iturriza Gomara M, Wong C, Blome S, Dessel-berger U, Gray J. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with sub-groups and evidence of independent segregation. J Virol. 2002;76(13):6596–6601. doi: 10.1128/JVI.76.13.6596-6601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouvea V, Glass RI, Woods P, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30(6):1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das BK, Gentsch JR, Cicirello HG, et al. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32(7):1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trujillo AA, McCaustland KA, Zheng DP, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol. 2006;44(4):1405–1412. doi: 10.1128/JCM.44.4.1405-1412.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44(7):2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. Human bocavirus in French children. Emerg Infect Dis. 2006;12(8):1251–1253. doi: 10.3201/eid1208.060213. [DOI] [PMC free article] [PubMed] [Google Scholar]


