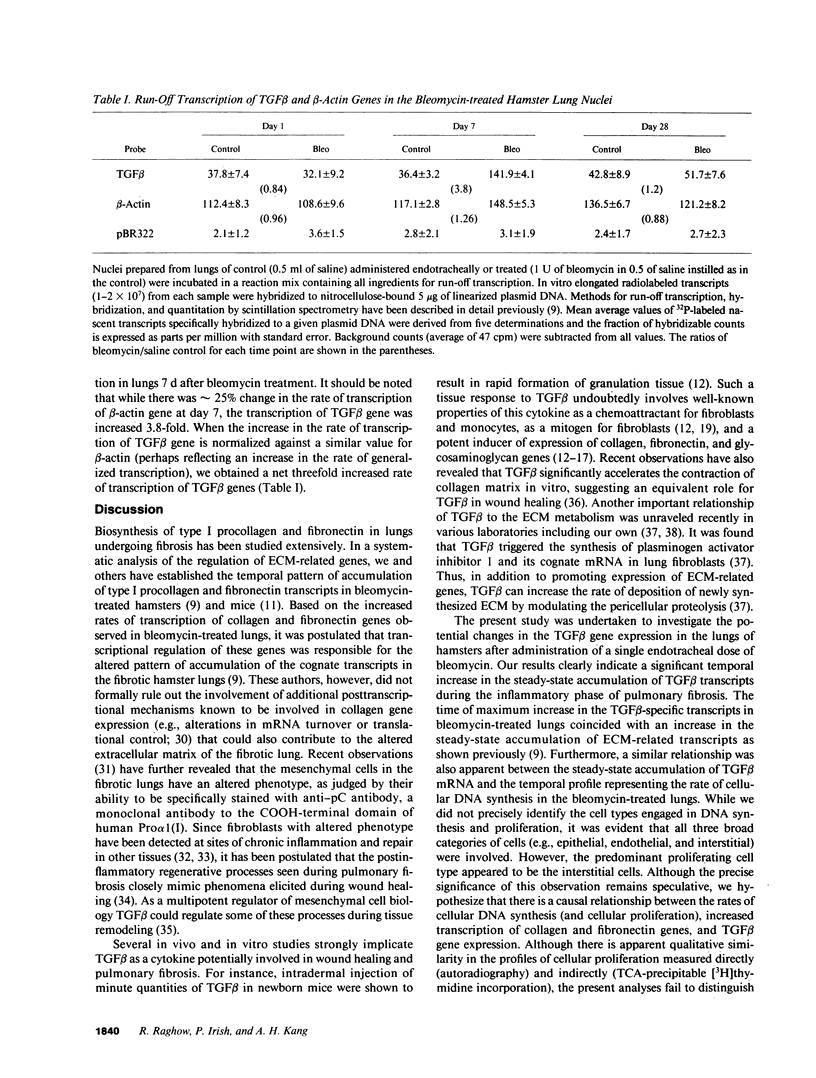
Abstract
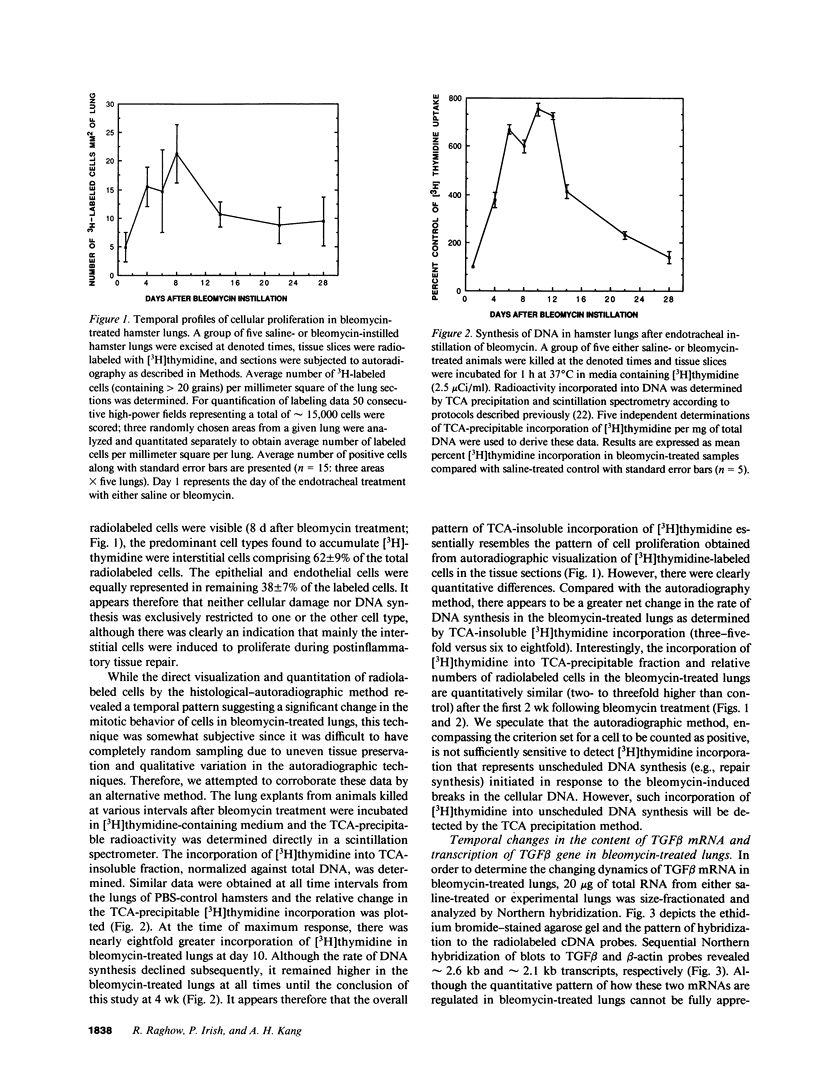
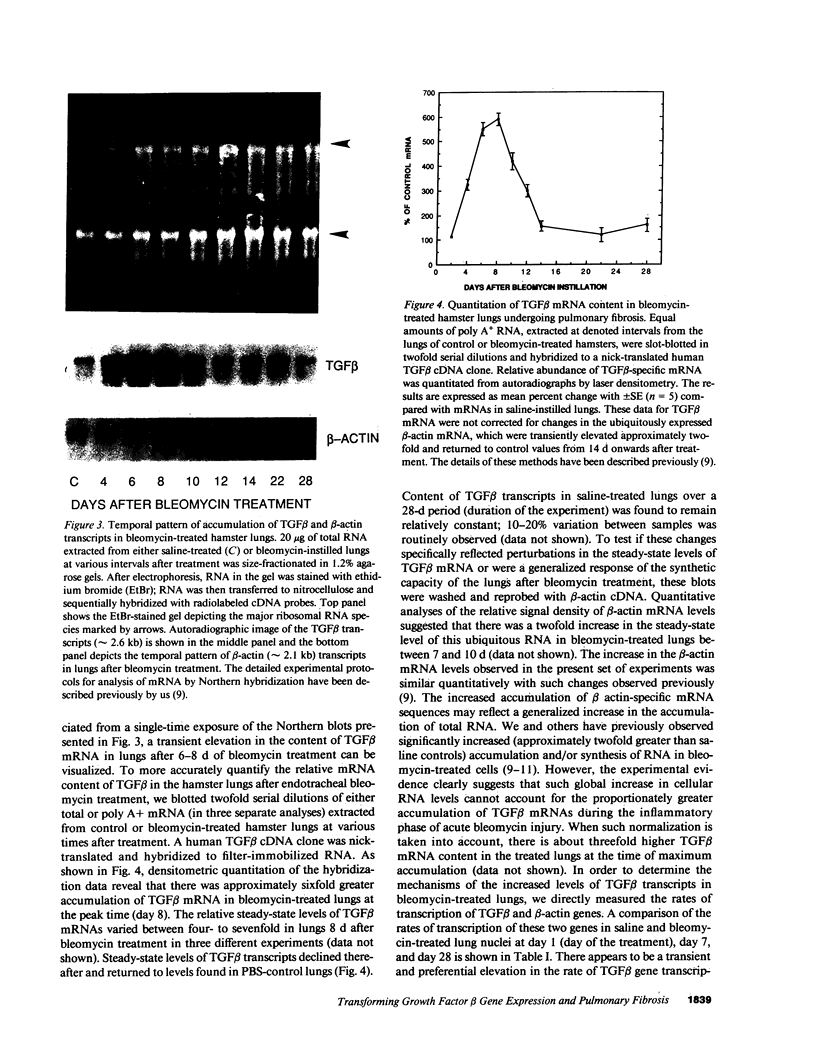
The number of mesenchymal cells, as well as their ability to synthesize extracellular matrix (ECM) components, greatly increase in the interstitium of fibrotic lungs. We have previously shown that the transcription of type I procollagen and fibronectin genes in the lungs is preferentially elevated during the early stages of bleomycin-induced pulmonary fibrosis (Raghow, R., S. Lurie, J. M. Seyer, and A. H. Kang. 1985, J. Clin. Invest. 76:1734-1739. Since a cytokine-like transforming growth factor beta (TGF beta) that is capable of enhancing mesenchymal cell proliferation and ECM synthesis could be potentially involved in this process, we investigated the temporal relationship between the regulation of TGF beta gene transcription and cellular proliferation in the bleomycin-treated hamster lungs. We observed a transient 5-7-fold increase in the accumulation of TGF beta transcripts, a concomitant 3-4-fold elevation in the cellular proliferation, and 8-10-fold stimulation of DNA synthesis in these lungs; all three parameters peaked around day 10 after bleomycin administration. Based on these results, we conclude that regulation of TGF beta gene expression may contribute significantly to the early events that lead to bleomycin-induced pulmonary fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. G., Kuhn C., 3rd, McDonald J. A., Mecham R. P. Lung connective tissue. Int Rev Connect Tissue Res. 1983;10:249–331. doi: 10.1016/b978-0-12-363710-9.50011-3. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Overton J. E., Marino B. A., Uitto J., Starcher B. C. Collagen biosynthesis in bleomycin-induced pulmonary fibrosis in hamsters. J Lab Clin Med. 1980 Dec;96(6):943–953. [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Fine A., Goldstein R. H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987 Mar 15;262(8):3897–3902. [PubMed] [Google Scholar]
- Goldstein R. H., Lucey E. C., Franzblau C., Snider G. L. Failure of mechanical properties to parallel changes in lung connective tissue composition in bleomycin-induced pulmonary fibrosis in hamsters. Am Rev Respir Dis. 1979 Jul;120(1):67–73. doi: 10.1164/arrd.1979.120.1.67. [DOI] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Kelley J., Chrin L., Shull S., Rowe D. W., Cutroneo K. R. Bleomycin selectively elevates mRNA levels for procollagen and fibronectin following acute lung injury. Biochem Biophys Res Commun. 1985 Sep 16;131(2):836–843. doi: 10.1016/0006-291x(85)91315-4. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Raghow R., Sawdey M., Loskutoff D. J., Postlethwaite A. E., Kang A. H., Moses H. L. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta. Divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988 Mar 5;263(7):3111–3115. [PubMed] [Google Scholar]
- Krieg T., Perlish J. S., Fleischmajer R., Braun-Falco O. Collagen synthesis in scleroderma: selection of fibroblast populations during subcultures. Arch Dermatol Res. 1985;277(5):373–376. doi: 10.1007/BF00509236. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980 Jul;11(4):353–366. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- McCullough B., Collins J. F., Johanson W. G., Jr, Grover F. L. Bleomycin-induced diffuse interstitial pulmonary fibrosis in baboons. J Clin Invest. 1978 Jan;61(1):79–88. doi: 10.1172/JCI108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Matheke M. L., Crouch E., Koo M., Kuhn C., 3rd A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest. 1986 Nov;78(5):1237–1244. doi: 10.1172/JCI112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray V., Martin R. F. The sequence specificity of bleomycin-induced DNA damage in intact cells. J Biol Chem. 1985 Sep 5;260(19):10389–10391. [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., Thrall R. S., Williams C. Bleomycin-induced pulmonary fibrosis. Effects of steroid on lung collagen metabolism. Am Rev Respir Dis. 1981 Oct;124(4):428–434. doi: 10.1164/arrd.1981.124.4.428. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A. E., MacDonald R. J., Harding J. D., Pictet R. L., Rutter W. J. Accumulation of the predominant pancreatic mRNAs during embryonic development. J Biol Chem. 1979 Mar 25;254(6):2154–2159. [PubMed] [Google Scholar]
- Quinones F., Crouch E. Biosynthesis of interstitial and basement membrane collagens in pulmonary fibrosis. Am Rev Respir Dis. 1986 Dec;134(6):1163–1171. doi: 10.1164/arrd.1986.134.6.1163. [DOI] [PubMed] [Google Scholar]
- Raghow R., Gossage D., Seyer J. M., Kang A. H. Transcriptional regulation of type I collagen genes in cultured fibroblasts by a factor isolated from thioacetamide-induced fibrotic rat liver. J Biol Chem. 1984 Oct 25;259(20):12718–12723. [PubMed] [Google Scholar]
- Raghow R., Kang A. H., Pidikiti D. Phenotypic plasticity of extracellular matrix gene expression in cultured hamster lung fibroblasts. Regulation of type I procollagen and fibronectin synthesis. J Biol Chem. 1987 Jun 15;262(17):8409–8415. [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987 Apr;79(4):1285–1288. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Thompson J. P. Molecular mechanisms of collagen gene expression. Mol Cell Biochem. 1989 Mar 16;86(1):5–18. doi: 10.1007/BF00231686. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976 Jun;57(6):1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan S. Wound healing. Int Rev Connect Tissue Res. 1981;9:1–26. doi: 10.1016/b978-0-12-363709-3.50007-3. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]