Abstract
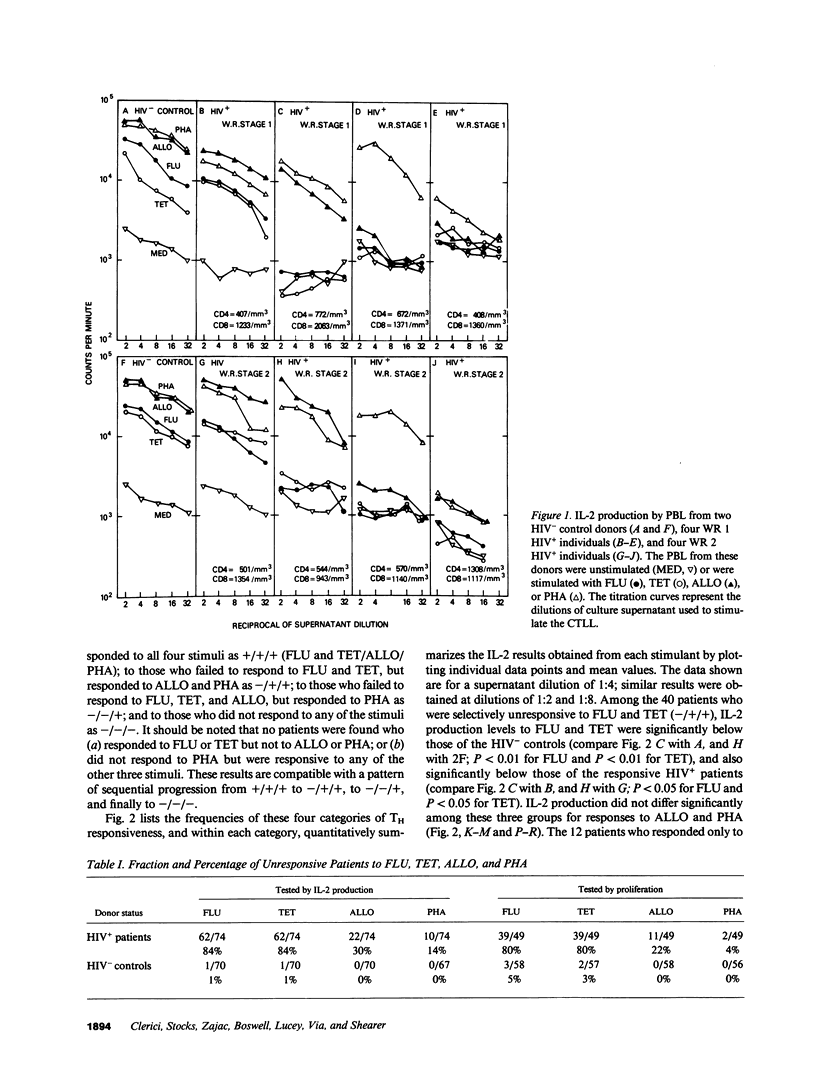
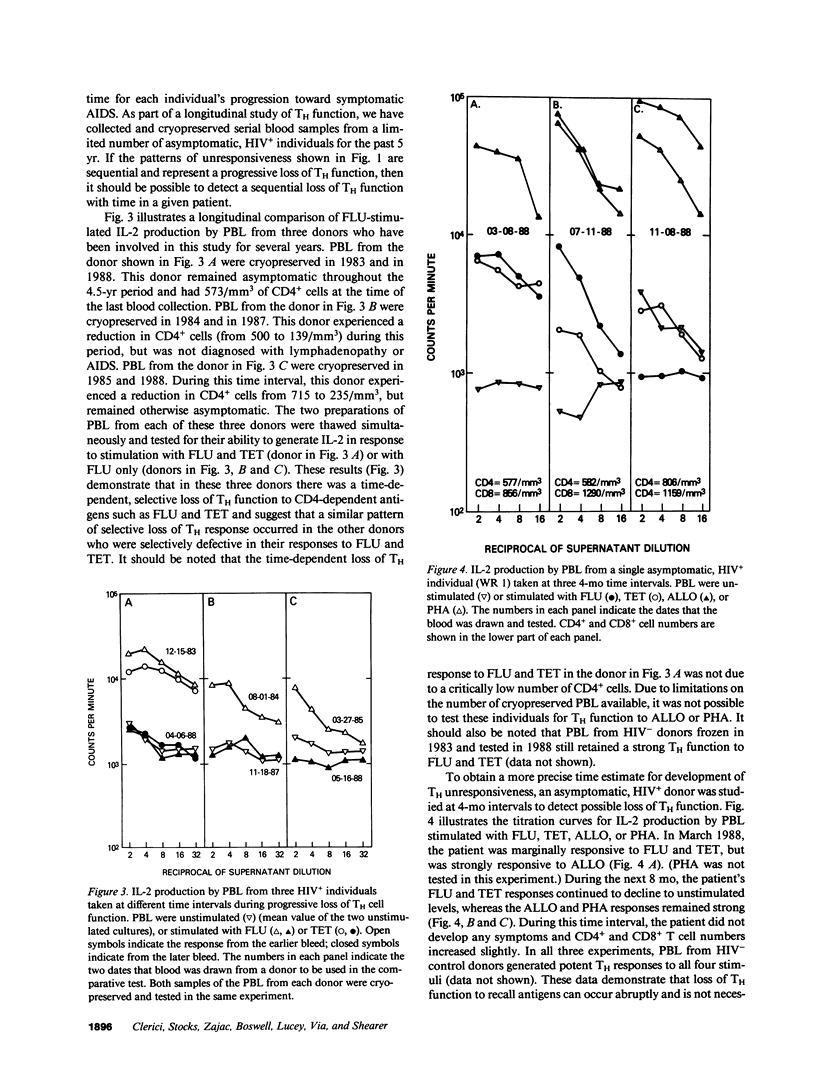
We have tested the T helper cell (TH) potential of asymptomatic, HIV seropositive (HIV+) patients, using an in vitro assay for IL-2 production. Peripheral blood leukocytes (PBL) from 74 HIV+ patients and 70 HIV- control donors were tested for TH function when stimulated with influenza A virus (FLU), tetanus toxoid (TET), HLA alloantigens (ALLO), or PHA. Of the HIV+ patients, four different response patterns were observed: (a) patients who responded to all four stimuli (16%); (b) patients who were selectively unresponsive to FLU and TET, but responded to ALLO and PHA (54%); (c) patients who were unresponsive to FLU, TET, or ALLO, but responsive to PHA (16%); and (d) patients who failed to respond to any of these stimuli (14%). Our results indicate a time-dependent progression from a stage responsive to all four stimuli to a stage unresponsive to any of the stimuli tested, progressing in the order outlined above. The earliest TH defect is the loss of responses to FLU and TET, indicating a selective defect in CD4+ MHC self-restricted TH function. The later loss of ALLO and PHA IL-2 responses suggests more severe TH dysfunction involving both CD4+ and CD8+ T cells. None of these patterns of TH unresponsiveness in asymptomatic HIV+ individuals were correlated with CD4+ cell numbers nor with Walter Reed staging criteria. This study indicates that the in vitro TH assay used can detect multiple stages of immune dysregulation early in the course of HIV infection and raises the possibility that staging of HIV+ patients should include in vitro TH functional analyses of the type described here.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen D. L., Lane H. C., Fauci A. S. Immunopathogenesis of the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):704–709. doi: 10.7326/0003-4819-103-5-704. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Prince H., Weaver M., Groopman J., Visscher B., Schwartz K., Detels R. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984 Jan;76(1):95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. AIDS: immunopathogenic mechanisms and research strategies. Clin Res. 1987 Oct;35(6):503–510. [PubMed] [Google Scholar]
- Folks T., Kelly J., Benn S., Kinter A., Justement J., Gold J., Redfield R., Sell K. W., Fauci A. S. Susceptibility of normal human lymphocytes to infection with HTLV-III/LAV. J Immunol. 1986 Jun 1;136(11):4049–4053. [PubMed] [Google Scholar]
- Garbrecht F. C., Siskind G. W., Weksler M. E. Lymphocyte transformation induced by autologous cells: XVIII. Impaired autologous mixed lymphocyte reaction in subjects with AIDS-related complex. Clin Exp Immunol. 1987 Feb;67(2):245–251. [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Fahey J. L., Smith D. C., Hultin L. E., Cheng H. L., Mitsuyasu R. T., Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987 Jun 1;138(11):3725–3730. [PubMed] [Google Scholar]
- Goedert J. J., Biggar R. J., Weiss S. H., Eyster M. E., Melbye M., Wilson S., Ginzburg H. M., Grossman R. J., DiGioia R. A., Sanchez W. C. Three-year incidence of AIDS in five cohorts of HTLV-III-infected risk group members. Science. 1986 Feb 28;231(4741):992–995. doi: 10.1126/science.3003917. [DOI] [PubMed] [Google Scholar]
- Golding H., Robey F. A., Gates F. T., 3rd, Linder W., Beining P. R., Hoffman T., Golding B. Identification of homologous regions in human immunodeficiency virus I gp41 and human MHC class II beta 1 domain. I. Monoclonal antibodies against the gp41-derived peptide and patients' sera react with native HLA class II antigens, suggesting a role for autoimmunity in the pathogenesis of acquired immune deficiency syndrome. J Exp Med. 1988 Mar 1;167(3):914–923. doi: 10.1084/jem.167.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding H., Shearer G. M., Hillman K., Lucas P., Manischewitz J., Zajac R. A., Clerici M., Gress R. E., Boswell R. N., Golding B. Common epitope in human immunodeficiency virus (HIV) I-GP41 and HLA class II elicits immunosuppressive autoantibodies capable of contributing to immune dysfunction in HIV I-infected individuals. J Clin Invest. 1989 Apr;83(4):1430–1435. doi: 10.1172/JCI114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Gelmann E. P., Longo D. L., Steis R. G., Chused T., Whalen G., Edgar L. C., Fauci A. S. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985 Mar;78(3):417–422. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- Mathur-Wagh U., Mildvan D., Senie R. T. Follow-up at 41/2 years on homosexual men with generalized lymphadenopathy. N Engl J Med. 1985 Dec 12;313(24):1542–1543. doi: 10.1056/NEJM198512123132412. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Melbye M., Biggar R. J., Ebbesen P., Neuland C., Goedert J. J., Faber V., Lorenzen I., Skinhøj P., Gallo R. C., Blattner W. A. Long-term seropositivity for human T-lymphotropic virus type III in homosexual men without the acquired immunodeficiency syndrome: development of immunologic and clinical abnormalities. A longitudinal study. Ann Intern Med. 1986 Apr;104(4):496–500. doi: 10.7326/0003-4819-104-4-496. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Penta A. C., Fitzgerald K. A., Stadler B. M., Schlossman S. F., Reinherz E. L. Cellular origin of interleukin 2 (IL 2) in man: evidence for stimulus-restricted IL 2 production by T4+ and T8+ T lymphocytes. J Immunol. 1982 Sep;129(3):1076–1079. [PubMed] [Google Scholar]
- Moser M., Mizuochi T., Sharrow S. O., Singer A., Shearer G. M. Graft-vs-host reaction limited to a class II MHC difference results in a selective deficiency in L3T4+ but not in Lyt-2+ T helper cell function. J Immunol. 1987 Mar 1;138(5):1355–1362. [PubMed] [Google Scholar]
- Prince H. E., Moody D. J., Shubin B. I., Fahey J. L. Defective monocyte function in acquired immune deficiency syndrome (AIDS): evidence from a monocyte-dependent T-cell proliferative system. J Clin Immunol. 1985 Jan;5(1):21–25. doi: 10.1007/BF00915164. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., Tramont E. C. The Walter Reed staging classification for HTLV-III/LAV infection. N Engl J Med. 1986 Jan 9;314(2):131–132. doi: 10.1056/NEJM198601093140232. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Masur H., Lane H. C., Frederick W., Kasahara T., Macher A. M., Djeu J. Y., Manischewitz J. F., Jackson L., Fauci A. S. Interleukin-2 enhances the depressed natural killer and cytomegalovirus-specific cytotoxic activities of lymphocytes from patients with the acquired immune deficiency syndrome. J Clin Invest. 1983 Jul;72(1):398–403. doi: 10.1172/JCI110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Landes R., Lockshin C., Richman D., McCutchan A., Kennedy C., Oldstone M. B., Nelson J. A. T cell recognition of HIV synthetic peptides in a natural infection. J Immunol. 1989 Feb 15;142(4):1166–1176. [PubMed] [Google Scholar]
- Shearer G. M., Bernstein D. C., Tung K. S., Via C. S., Redfield R., Salahuddin S. Z., Gallo R. C. A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS). J Immunol. 1986 Oct 15;137(8):2514–2521. [PubMed] [Google Scholar]
- Smolen J. S., Bettelheim P., Köller U., McDougal S., Graninger W., Luger T. A., Knapp W., Lechner K. Deficiency of the autologous mixed lymphocyte reaction in patients with classic hemophilia treated with commercial factor VIII concentrate. Correlation with T cell subset distribution, antibodies to lymphadenopathy-associated or human T lymphotropic virus, and analysis of the cellular basis of the deficiency. J Clin Invest. 1985 Jun;75(6):1828–1834. doi: 10.1172/JCI111896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Schwartz K., Detels R. The time from infection with human immunodeficiency virus (HIV) to the onset of AIDS. J Infect Dis. 1986 Oct;154(4):694–697. doi: 10.1093/infdis/154.4.694. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Via C. S., Shearer G. M. Functional heterogeneity of L3T4+ T cells in MRL-lpr/lpr mice. L3T4+ T cells suppress major histocompatibility complex-self-restricted L3T4+ T helper cell function in association with autoimmunity. J Exp Med. 1988 Dec 1;168(6):2165–2181. doi: 10.1084/jem.168.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via C. S., Shearer G. M. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol Today. 1988 Jul-Aug;9(7-8):207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- de Wolf F., Lange J. M., Houweling J. T., Coutinho R. A., Schellekens P. T., van der Noordaa J., Goudsmit J. Numbers of CD4+ cells and the levels of core antigens of and antibodies to the human immunodeficiency virus as predictors of AIDS among seropositive homosexual men. J Infect Dis. 1988 Sep;158(3):615–622. doi: 10.1093/infdis/158.3.615. [DOI] [PubMed] [Google Scholar]
- el-Sadr W., Marmor M., Zolla-Pazner S., Stahl R. E., Lyden R., William D., D'Onofrio S., Weiss S. H., Saxinger W. C. Four-year prospective study of homosexual men: correlation of immunologic abnormalities, clinical status, and serology to human immunodeficiency virus. J Infect Dis. 1987 Apr;155(4):789–793. doi: 10.1093/infdis/155.4.789. [DOI] [PubMed] [Google Scholar]


