Abstract
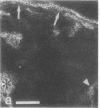
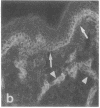
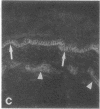
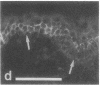
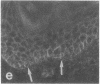
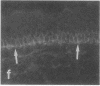
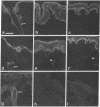
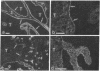
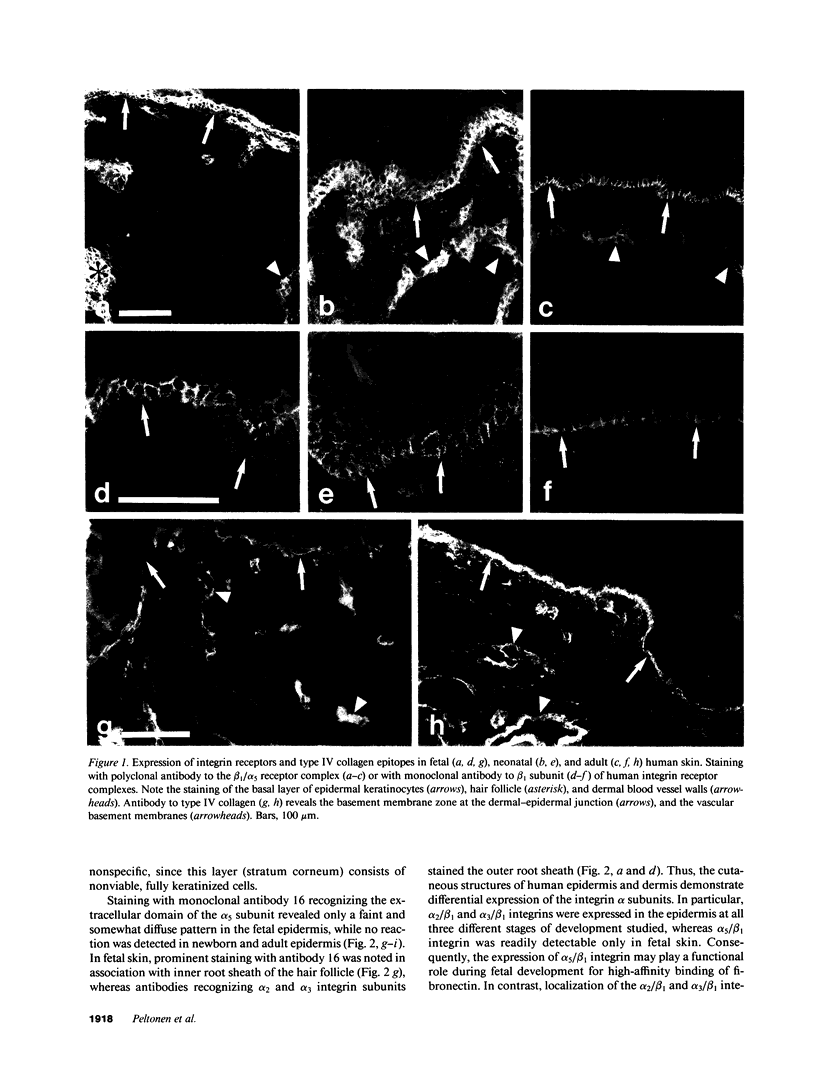
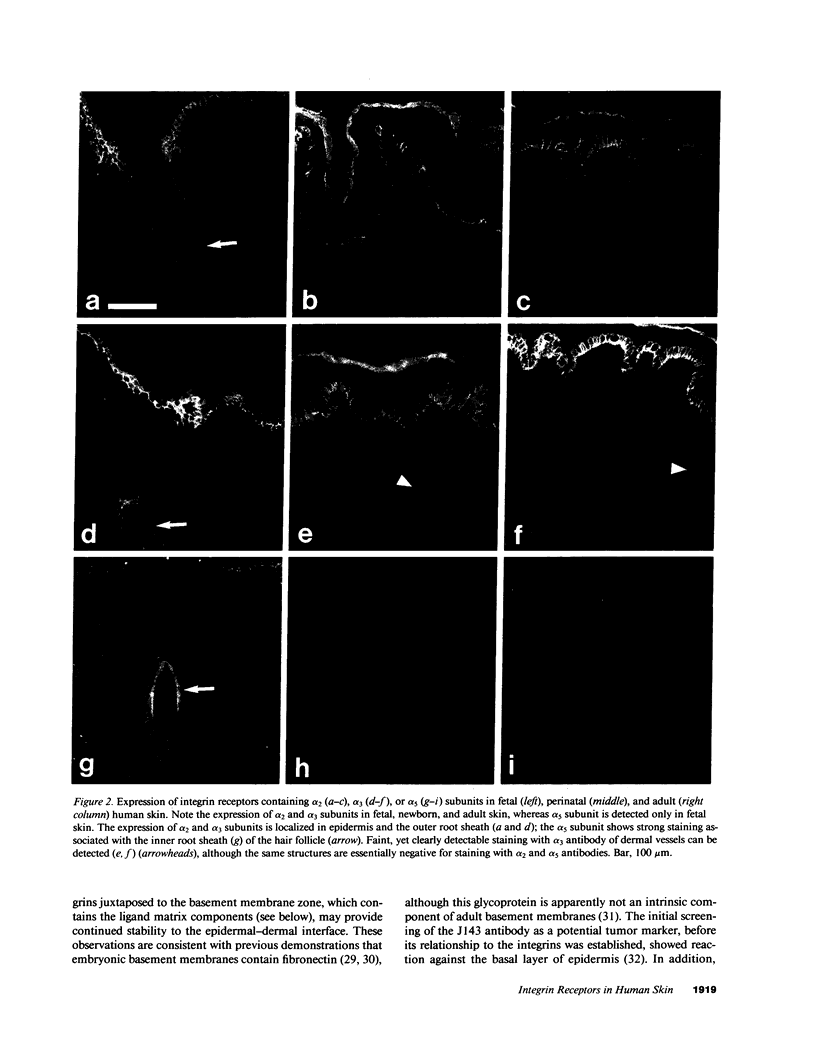
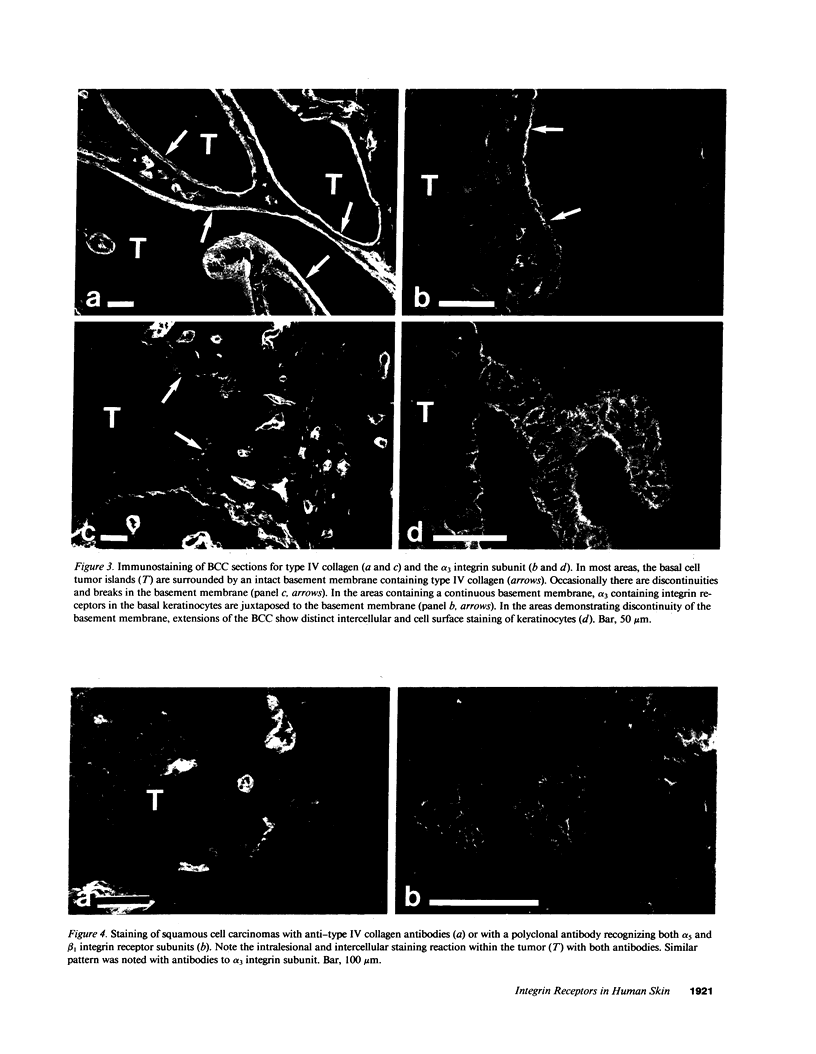
VLA integrins in human skin were examined by indirect immunofluorescence utilizing antibodies recognizing the beta 1, alpha 2, alpha 3, or alpha 5 subunits. Staining of fetal, newborn, or adult skin with antibodies to beta 1, alpha 2, or alpha 3 subunits gave essentially similar staining patterns: intense staining was associated with the basal layer of the epidermis, hair follicles, and blood vessel walls. The alpha 5 subunit could be detected only in epidermis and the inner root sheath of hair follicles in fetal skin. In epidermis, the staining reaction for the beta 1 subunit was not only found in sites interfacing with the basement membrane zone, but also around the entire periphery of these cells. We speculate that these receptors might have previously unrecognized functions in cell-cell interactions or that these findings may suggest the presence of previously unrecognized ligands in the intercellular spaces of keratinocytes. Examination of nine nodular basal cell carcinomas revealed a prominent staining reaction with anti-beta 1 and anti-alpha 3 antibodies at the periphery of the tumor islands. In contrast, staining of five squamous cell carcinomas revealed either the absence of integrins or altered and variable expression. Thus, matrix components and their receptors may participate in modulation of growth, development, and organization of human skin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Wertz R. L. Fibronectin, as well as other extracellular matrix proteins, mediate human keratinocyte adherence. J Invest Dermatol. 1985 May;84(5):378–383. doi: 10.1111/1523-1747.ep12265466. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Van der Schueren B., Jaspers M., Saison M., Spaepen M., Van Leuven F., Van den Berghe H., Cassiman J. J. Distribution of the beta 1 subgroup of the integrins in human cells and tissues. J Histochem Cytochem. 1989 Mar;37(3):299–307. doi: 10.1177/37.3.2645360. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Nuckolls G. H., Ishihara A., Hasegawa T., Yamada K. M., Thiery J. P., Jacobson K. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988 Oct;107(4):1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradet Y., Cordon-Cardo C., Thomson T., Daly M. E., Whitmore W. F., Jr, Lloyd K. O., Melamed M. R., Old L. J. Cell surface antigens of human bladder cancer defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Jan;81(1):224–228. doi: 10.1073/pnas.81.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood R. E., Ferris C. F., Nielsen L. D., Huff J. C., Clark R. A. Basal cell carcinomas grown in nude mice produce and deposit fibronectin in the extracellular matrix. J Invest Dermatol. 1986 Jul;87(1):42–46. doi: 10.1111/1523-1747.ep12523552. [DOI] [PubMed] [Google Scholar]
- Grimwood R. E., Huff J. C., Harbell J. W., Clark R. A. Fibronectin in basal cell epithelioma: sources and significance. J Invest Dermatol. 1984 Feb;82(2):145–149. doi: 10.1111/1523-1747.ep12259701. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- McNutt N. S. Ultrastructural comparison of the interface between epithelium and stroma in basal cell carcinoma and control human skin. Lab Invest. 1976 Aug;35(2):132–142. [PubMed] [Google Scholar]
- Murray J. C., Stingl G., Kleinman H. K., Martin G. R., Katz S. I. Epidermal cells adhere preferentially to type IV (basement membrane) collagen. J Cell Biol. 1979 Jan;80(1):197–202. doi: 10.1083/jcb.80.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Little C. D., Balian G. Distribution of fibronectin and laminin in basal cell epitheliomas. J Invest Dermatol. 1983 May;80(5):446–452. doi: 10.1111/1523-1747.ep12555545. [DOI] [PubMed] [Google Scholar]
- Nishida T., Nakagawa S., Awata T., Ohashi Y., Watanabe K., Manabe R. Fibronectin promotes epithelial migration of cultured rabbit cornea in situ. J Cell Biol. 1983 Nov;97(5 Pt 1):1653–1657. doi: 10.1083/jcb.97.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Payne R. E., Jr, Russell N., Woodley D. T. Spreading and enhanced motility of human keratinocytes on fibronectin. J Invest Dermatol. 1985 Aug;85(2):125–130. doi: 10.1111/1523-1747.ep12276531. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Jaakkola S., Lask G., Virtanen I., Uitto J. Fibronectin gene expression by epithelial tumor cells in basal cell carcinoma: an immunocytochemical and in situ hybridization study. J Invest Dermatol. 1988 Oct;91(4):289–293. doi: 10.1111/1523-1747.ep12475415. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Jaakkola S., Lebwohl M., Renvall S., Risteli L., Virtanen I., Uitto J. Cellular differentiation and expression of matrix genes in type 1 neurofibromatosis. Lab Invest. 1988 Dec;59(6):760–771. [PubMed] [Google Scholar]
- Rettig W. J., Dracopoli N. C., Goetzger T. A., Spengler B. A., Biedler J. L., Oettgen H. F., Old L. J. Somatic cell genetic analysis of human cell surface antigens: chromosomal assignments and regulation of expression in rodent-human hybrid cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6437–6441. doi: 10.1073/pnas.81.20.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Murty V. V., Mattes M. J., Chaganti R. S., Old L. J. Extracellular matrix-modulated expression of human cell surface glycoproteins A42 and J143. Intrinsic and extrinsic signals determine antigenic phenotype. J Exp Med. 1986 Nov 1;164(5):1581–1599. doi: 10.1084/jem.164.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Saga S., Chen W. T., Yamada K. M. Enhanced fibronectin receptor expression in Rous sarcoma virus-induced tumors. Cancer Res. 1988 Oct 1;48(19):5510–5513. [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Stanley J. R., Foidart J. M., Murray J. C., Martin G. R., Katz S. I. The epidermal cell which selectively adheres to a collagen substrate is the basal cell. J Invest Dermatol. 1980 Jan;74(1):54–58. doi: 10.1111/1523-1747.ep12514618. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Strominger J. L., Hemler M. E. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci U S A. 1987 May;84(10):3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Takashima A., Billingham R. E., Grinnell F. Activation of rabbit keratinocyte fibronectin receptor function in vivo during wound healing. J Invest Dermatol. 1986 May;86(5):585–590. doi: 10.1111/1523-1747.ep12355243. [DOI] [PubMed] [Google Scholar]
- Takashima A., Grinnell F. Human keratinocyte adhesion and phagocytosis promoted by fibronectin. J Invest Dermatol. 1984 Nov;83(5):352–358. doi: 10.1111/1523-1747.ep12264522. [DOI] [PubMed] [Google Scholar]
- Timpl R., Dziadek M. Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol. 1986;29:1–112. [PubMed] [Google Scholar]
- Toda K., Tuan T. L., Brown P. J., Grinnell F. Fibronectin receptors of human keratinocytes and their expression during cell culture. J Cell Biol. 1987 Dec;105(6 Pt 2):3097–3104. doi: 10.1083/jcb.105.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberge D., Pierard G. E., Foidart J. M., Lapiere C. M. Immunohistochemical localization of laminin, type IV and type V collagen in basal cell carcinoma. Br J Dermatol. 1983 Feb;108(2):163–170. doi: 10.1111/j.1365-2133.1983.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Leivo I., Vaheri A. Expression of the cell surface-associated glycoprotein, fibronectin, in the early mouse embryo. Dev Biol. 1979 Mar;69(1):247–257. doi: 10.1016/0012-1606(79)90289-6. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]