Abstract
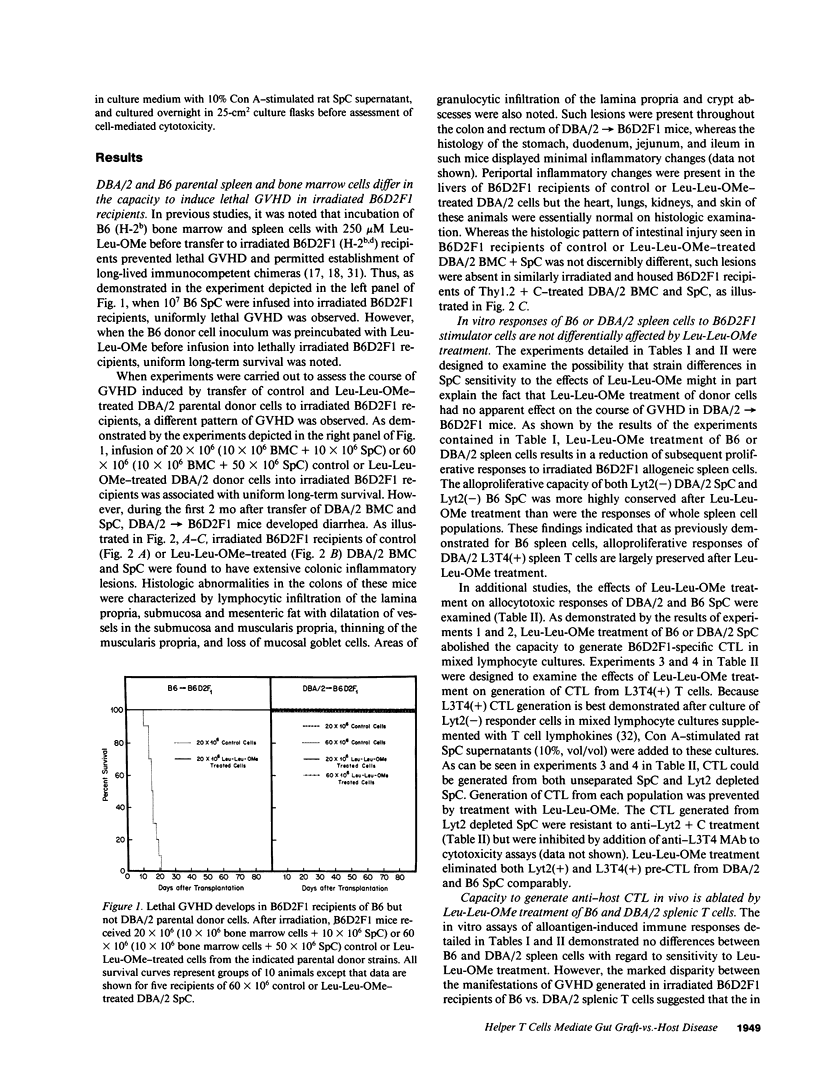
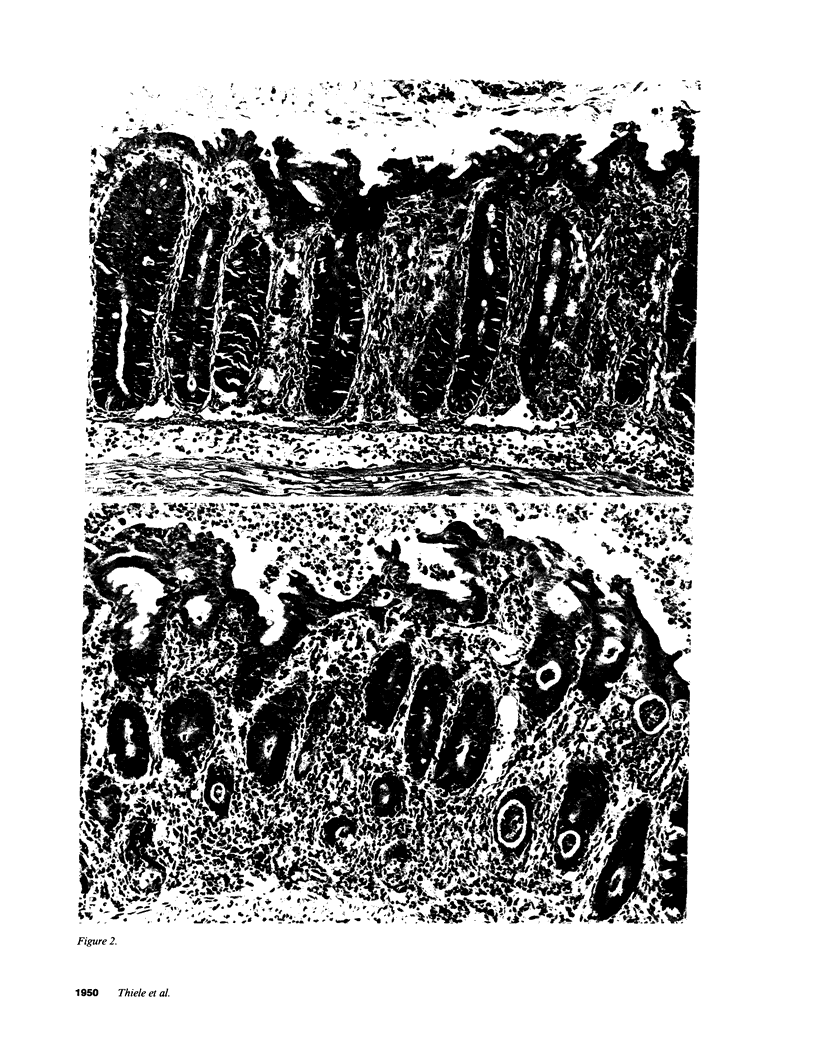
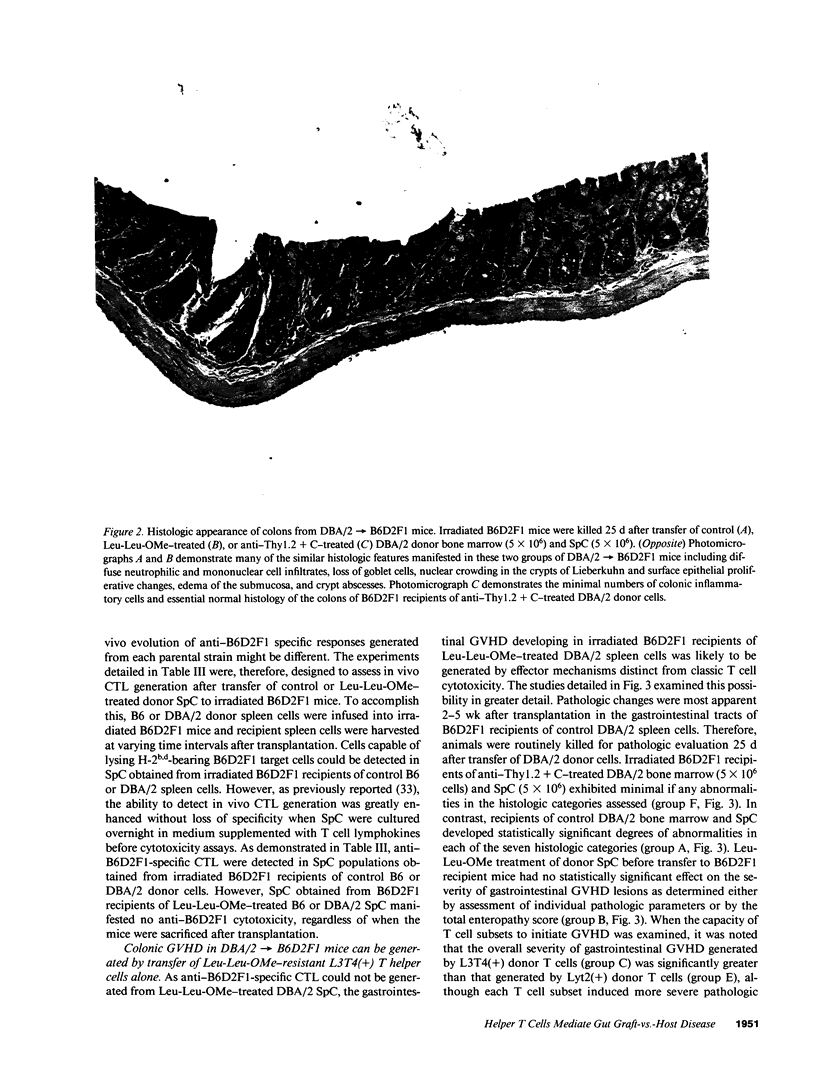
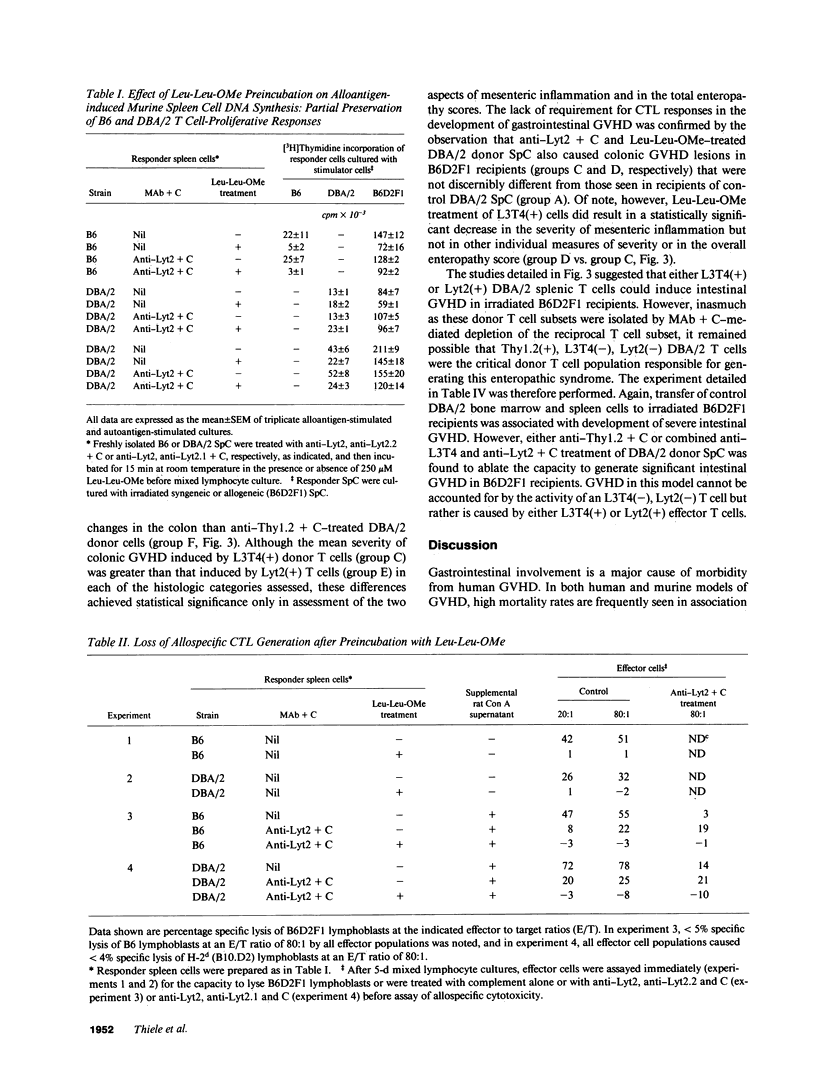
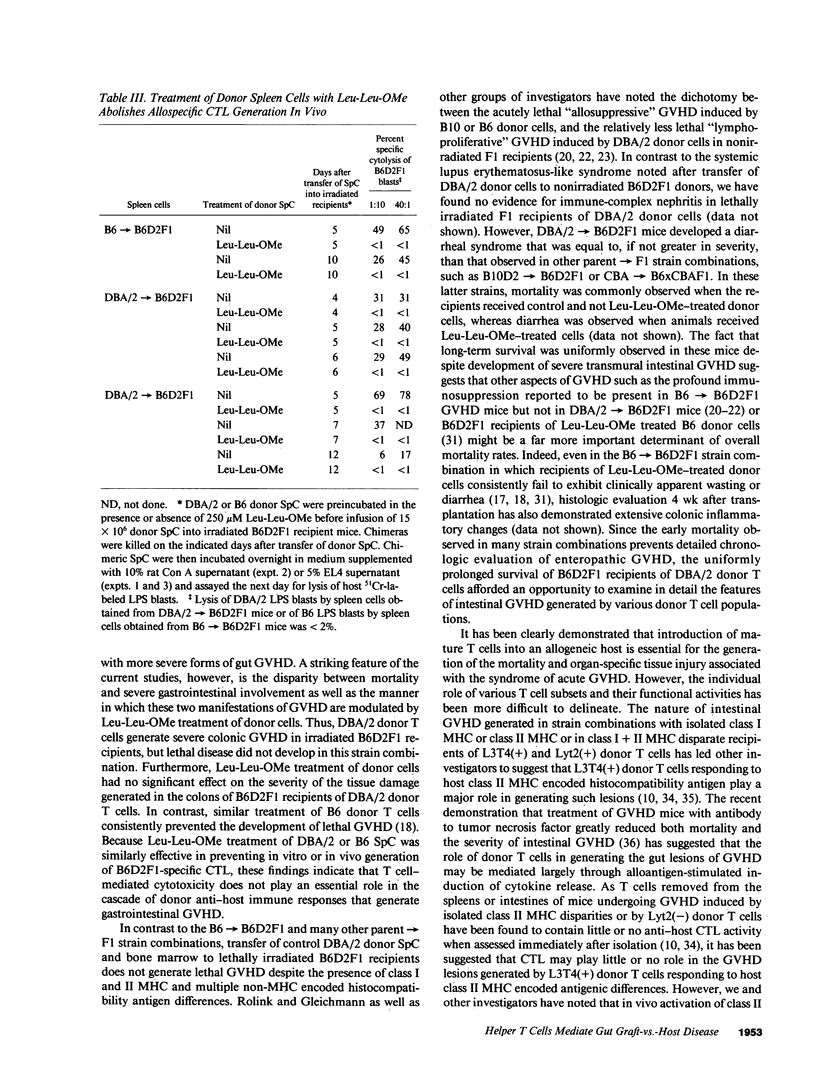
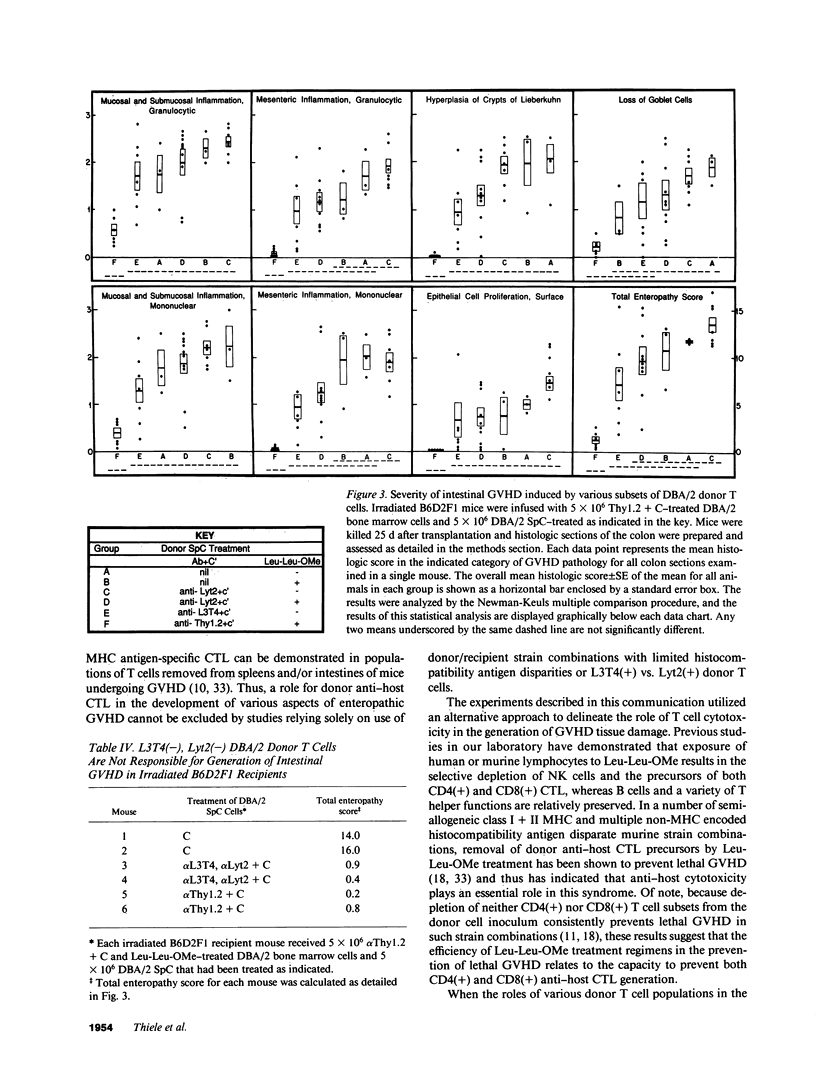
In these studies, the role of T helper and T cytotoxic cells in generating intestinal graft-vs.-host disease (GVHD) was examined. Treatment of C57BL/6J (B6) splenocytes with L-leucyl-L-leucine methyl ester (Leu-Leu-OMe) selectively removes natural killer cells, cytotoxic T lymphocyte (CTL) precursors, and the capacity to cause lethal GVHD in irradiated B6xDBA/2 F1 (B6D2F1) mice while preserving T helper cell function. Neither control nor Leu-Leu-OMe-treated DBA/2 donor spleen and bone marrow cells were found to induce lethal GVHD in B6D2F1 recipients. However, extensive colonic GVHD developed in B6D2F1 recipients of DBA/2 bone marrow and spleen cells. Enteropathic GVHD in DBA/2----B6D2F1 mice was reduced in severity after anti-L3T4 + C treatment of donor cells, and was eliminated by anti-Thy1.2 + C or the combination of anti-L3T4 and anti-Lyt2 + C treatment of the donor cell inoculum. However, neither anti-Lyt2 + C, Leu-Leu-OMe, nor anti-Lyt2 + C and Leu-Leu-OMe treatment of donor cells significantly decreased severity of gut GVHD. Leu-Leu-OMe treatment of DBA/2 or B6 SpC was comparably effective in preventing in vitro or in vivo generation of B6D2F1-specific CTL. These findings, therefore, demonstrate that histologically severe enteropathic GVHD does not require participation of CTL and is not always associated with high mortality rates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charley M., Thiele D. L., Bennett M., Lipsky P. E. Prevention of lethal murine graft versus host disease by treatment of donor cells with L-leucyl-L-leucine methyl ester. J Clin Invest. 1986 Nov;78(5):1415–1420. doi: 10.1172/JCI112730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Elkins W. L. Cellular immunology and the pathogenesis of graft versus host reactions. Prog Allergy. 1971;15:78–187. [PubMed] [Google Scholar]
- Golding H., Singer A. Specificity, phenotype, and precursor frequency of primary cytolytic T lymphocytes specific for class II major histocompatibility antigens. J Immunol. 1985 Sep;135(3):1610–1615. [PubMed] [Google Scholar]
- Golding H., Singer A. Specificity, phenotype, and precursor frequency of primary cytolytic T lymphocytes specific for class II major histocompatibility antigens. J Immunol. 1985 Sep;135(3):1610–1615. [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngold R., Sprent J. Lethal GVHD across minor histocompatibility barriers: nature of the effector cells and role of the H-2 complex. Immunol Rev. 1983;71:5–29. doi: 10.1111/j.1600-065x.1983.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Korngold R., Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J Exp Med. 1978 Dec 1;148(6):1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngold R., Sprent J. Surface markers of T cells causing lethal graft-vs-host disease to class I vs class II H-2 differences. J Immunol. 1985 Nov;135(5):3004–3010. [PubMed] [Google Scholar]
- Korngold R., Sprent J. T cell subsets and graft-versus-host disease. Transplantation. 1987 Sep;44(3):335–339. doi: 10.1097/00007890-198709000-00002. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Buckner C. D., Sanders J. E., Deeg H. J., Stewart P., Appelbaum F. R., Clift R., Fefer A., Witherspoon R. P. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985 Sep;66(3):664–672. [PubMed] [Google Scholar]
- Mitsuyasu R. T., Champlin R. E., Gale R. P., Ho W. G., Lenarsky C., Winston D., Selch M., Elashoff R., Giorgi J. V., Wells J. Treatment of donor bone marrow with monoclonal anti-T-cell antibody and complement for the prevention of graft-versus-host disease. A prospective, randomized, double-blind trial. Ann Intern Med. 1986 Jul;105(1):20–26. doi: 10.7326/0003-4819-105-1-20. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Golding H., Rosenberg A. S., Glimcher L. H., Malek T. R., Singer A. Both L3T4+ and Lyt-2+ helper T cells initiate cytotoxic T lymphocyte responses against allogenic major histocompatibility antigens but not against trinitrophenyl-modified self. J Exp Med. 1985 Aug 1;162(2):427–443. doi: 10.1084/jem.162.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Borland A., Parrott D. M. Hypersensitivity reactions in the small intestine. VII. Induction of the intestinal phase of murine graft-versus-host-reaction by Lyt 2- T cells activated by I-A alloantigens. Transplantation. 1986 Feb;41(2):192–198. [PubMed] [Google Scholar]
- Pals S. T., Gleichmann H., Gleichmann E. Allosuppressor and allohelper T cells in acute and chronic graft-vs-host disease. V. F1 mice with secondary chronic GVHD contain F1-reactive allohelper but no allosuppressor T cells. J Exp Med. 1984 Feb 1;159(2):508–523. doi: 10.1084/jem.159.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F. GVHR elicited by products of class I or class II loci of the MHC: analysis of the response of mouse T lymphocytes to products of class I and class II loci of the MHC in correlation with GVHR-induced mortality, medullary aplasia, and enteropathy. J Immunol. 1985 Sep;135(3):1637–1643. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport H., Khalil A., Halle-Pannenko O., Pritchard L., Dantchev D., Mathé G. Histopathologic sequence of events in adult mice undergoing lethal graft-versus-host reaction developed across H-2 and/or non-H-2 histocompatibility barriers. Am J Pathol. 1979 Jul;96(1):121–142. [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Gottlieb P. D., Bevan M. J. Fractionation of lymphocyte populations with monoclonal antibodies specific for LYT-2.2 and LYT-3.1. J Immunol. 1980 Sep;125(3):1136–1143. [PubMed] [Google Scholar]
- Rolink A. G., Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J Exp Med. 1983 Aug 1;158(2):546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Lo D., Korngold R. Properties of purified T cell subsets. II. In vivo responses to class I vs. class II H-2 differences. J Exp Med. 1986 Apr 1;163(4):998–1011. doi: 10.1084/jem.163.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. L., Bryde S. E., Lipsky P. E. Lethal graft-vs-host disease induced by a class II MHC antigen only disparity is not mediated by cytotoxic T cells. J Immunol. 1988 Nov 15;141(10):3377–3382. [PubMed] [Google Scholar]
- Thiele D. L., Calomeni J. A., Lipsky P. E. Leucyl-leucine methyl ester treatment of donor cells permits establishment of immunocompetent parent----F1 chimeras that are selectively tolerant of host alloantigens. J Immunol. 1987 Oct 1;139(7):2137–2142. [PubMed] [Google Scholar]
- Thiele D. L., Charley M. R., Calomeni J. A., Lipsky P. E. Lethal graft-vs-host disease across major histocompatibility barriers: requirement for leucyl-leucine methyl ester sensitive cytotoxic T cells. J Immunol. 1987 Jan 1;138(1):51–57. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Modulation of human natural killer cell function by L-leucine methyl ester: monocyte-dependent depletion from human peripheral blood mononuclear cells. J Immunol. 1985 Feb;134(2):786–793. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Regulation of cellular function by products of lysosomal enzyme activity: elimination of human natural killer cells by a dipeptide methyl ester generated from L-leucine methyl ester by monocytes or polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2468–2472. doi: 10.1073/pnas.82.8.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. The immunosuppressive activity of L-leucyl-L-leucine methyl ester: selective ablation of cytotoxic lymphocytes and monocytes. J Immunol. 1986 Feb 1;136(3):1038–1048. [PubMed] [Google Scholar]
- Thomas E. D., Storb R., Clift R. A., Fefer A., Johnson L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (second of two parts). N Engl J Med. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- Vallera D. A., Soderling C. C., Carlson G. J., Kersey J. H. Bone marrow transplantation across major histocompatibility barriers in mice. Effect of elimination of T cells from donor grafts by treatment with monoclonal Thy-1.2 plus complement or antibody alone. Transplantation. 1981 Mar;31(3):218–222. doi: 10.1097/00007890-198103000-00015. [DOI] [PubMed] [Google Scholar]
- Via C. S., Sharrow S. O., Shearer G. M. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987 Sep 15;139(6):1840–1849. [PubMed] [Google Scholar]
- von Boehmer H., Kisielow P., Leiserson W., Haas W. Lyt-2- T cell-independent functions of Lyt-2+ cells stimulated with antigen or concanavalin A. J Immunol. 1984 Jul;133(1):59–64. [PubMed] [Google Scholar]





