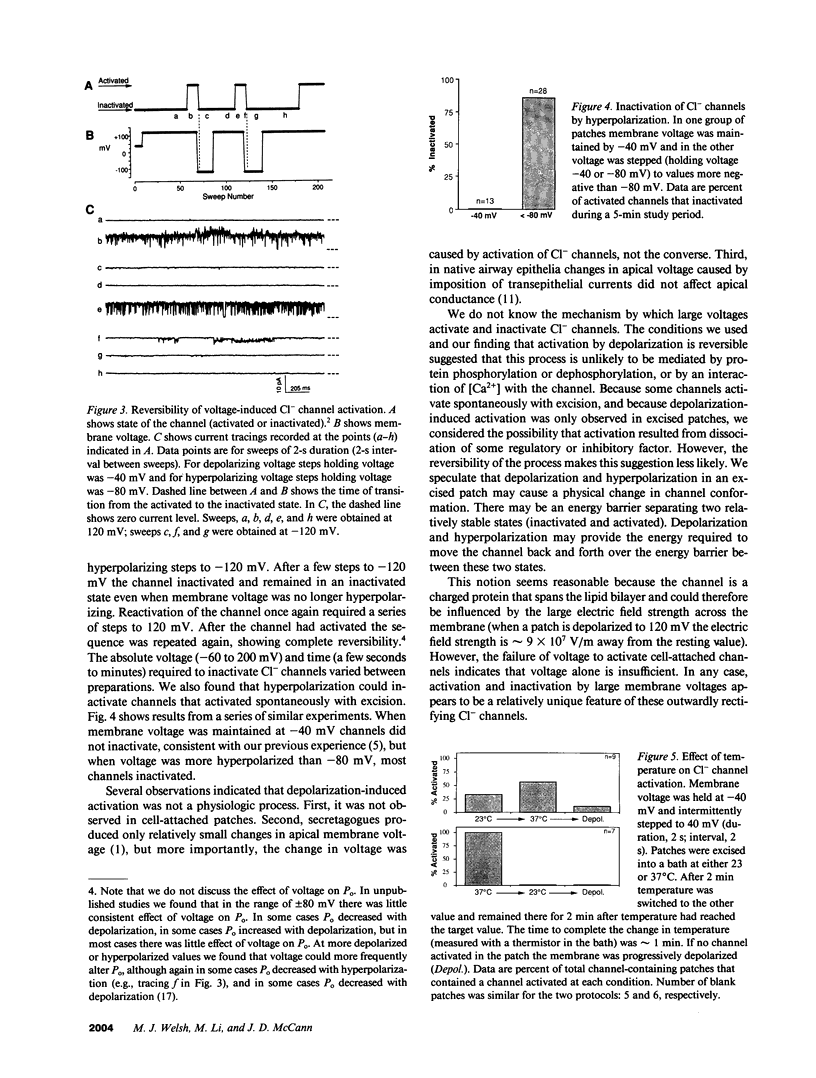
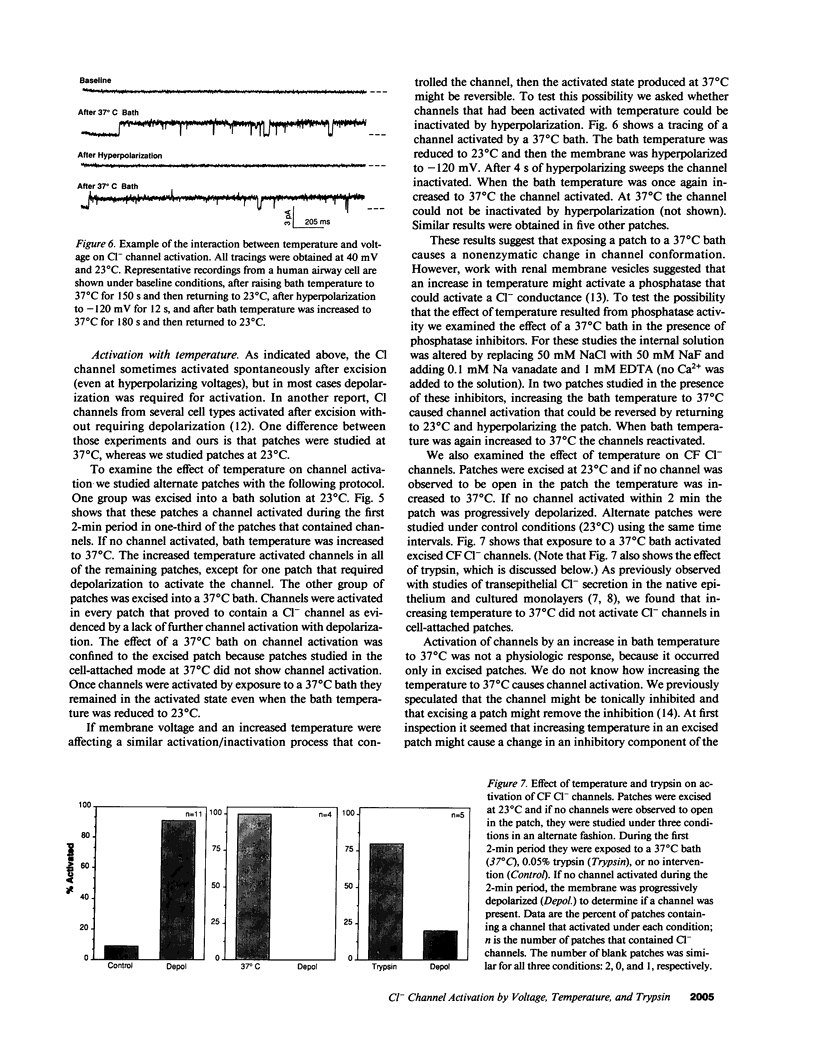
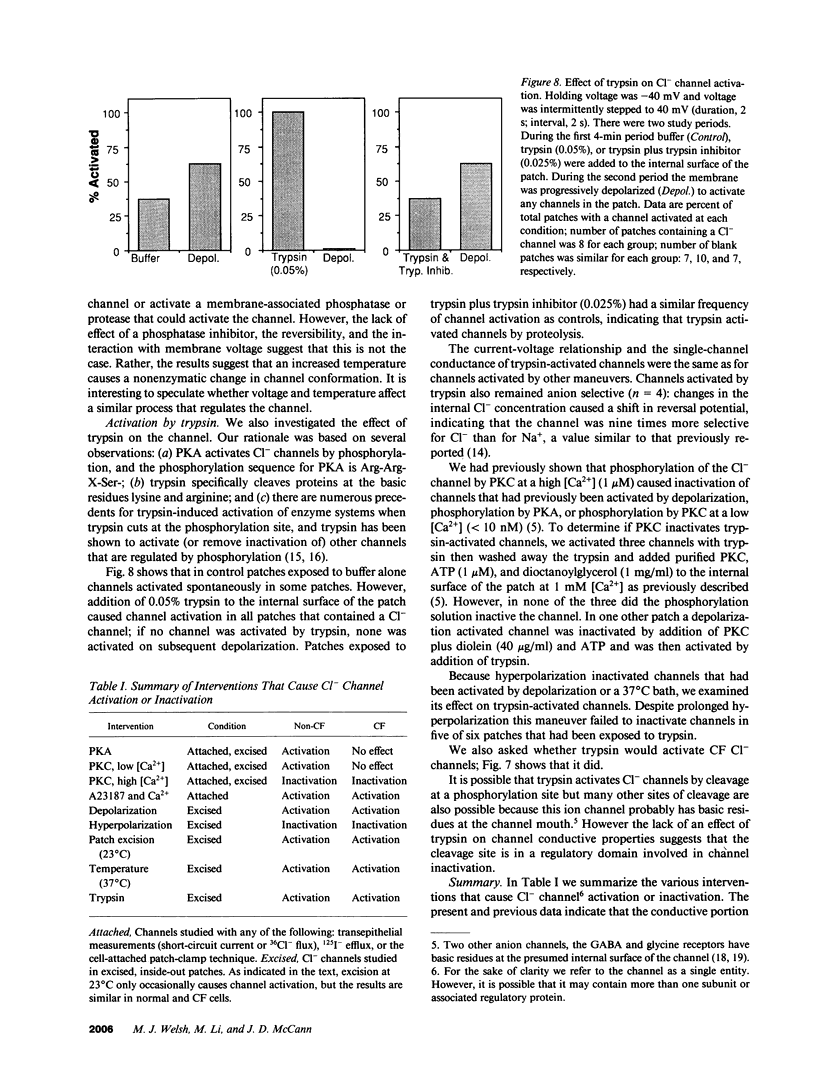
Abstract
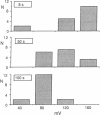
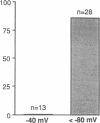
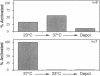
In cystic fibrosis (CF) phosphorylation-dependent activation of outwardly rectifying apical membrane Cl- channels is defective. To further understand regulation of this channel we examined several other mechanisms of channel activation in normal and CF cells. Previous studies have shown that strong membrane depolarization can activate channels in excised cell-free membrane patches. Here we show that such activation is dependent on both the absolute membrane voltage and the duration of depolarization. Moreover, activation was reversible by membrane hyperpolarization. In some cases, excising patches of membrane from the cell caused channel activation, even in the absence of depolarization. However, the frequency of channel activation with patch excision increased when bath temperature was increased from 23 to 37 degrees C. Although the channel remained in the activated state when temperature was reduced to 23 degrees C, subsequent hyperpolarization inactivated the channel. In cell-attached patches, neither depolarization nor increasing bath temperature to 37 degrees C activated channels, suggesting that neither is physiologically important in regulation of the channel. Thus changes in membrane voltage and bath temperature appear to cause a nonenzymatic change in the channel's conformation; the interactions between voltage and temperature suggest that they may affect the same process. To determine if a proteolytic alteration of the channel could also cause activation, we added trypsin to the cytosolic surface of excised membrane patches. Trypsin activated channels, which could not then be inactivated by either hyperpolarization or phosphorylation with PKC, suggesting that trypsin removed or altered a region of the channel involved in inactivation. All of these interventions activated Cl- channels from both normal and CF cells. Thus many aspects of Cl- channel activation are normal in CF; only phosphorylation-dependent activation is defective.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Trautwein W. Modification of L-type calcium current by intracellularly applied trypsin in guinea-pig ventricular myocytes. J Physiol. 1988 Oct;404:259–274. doi: 10.1113/jphysiol.1988.sp017289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Landry D. W., Reitman M., Cragoe E. J., Jr, Al-Awqati Q. Epithelial chloride channel. Development of inhibitory ligands. J Gen Physiol. 1987 Dec;90(6):779–798. doi: 10.1085/jgp.90.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Basolateral membrane potassium conductance is independent of sodium pump activity and membrane voltage in canine tracheal epithelium. J Membr Biol. 1985;84(1):25–33. doi: 10.1007/BF01871645. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Ion transport by primary cultures of canine tracheal epithelium: methodology, morphology, and electrophysiology. J Membr Biol. 1985;88(2):149–163. doi: 10.1007/BF01868429. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol. 1986 Oct;251(4 Pt 2):R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989 Feb;256(2 Pt 1):C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]