Abstract
Glioblastoma, the most frequent and aggressive malignant brain tumor, has a very poor prognosis of approximately 1-year. The associated aggressive phenotype and therapeutic resistance of glioblastoma is postulated to be due to putative brain tumor stem-like cells (BTSC). The best hope for improved therapy lies in the ability to understand the molecular biology that controls BTSC behavior. The tumor vascular microenvironment of brain tumors has emerged as important regulators of BTSC behavior. Emerging data have identified the vascular microenvironment as home to a multitude of cell types engaged in various signaling that work collectively to foster a supportive environment for BTSCs. Characterization of the signaling pathways and intercellular communication between resident cell types in the microvascular niche of brain tumors is critical to the identification of potential BTSC-specific targets for therapy.
Key words: glioblastoma, perivascular niche, brain tumor, cancer stem-like cells, microenvironment
Introduction
Progress in the treatment of malignant brain tumors has been disappointing, particularly in regards to the treatment of high-grade gliomas (WHO grades III and IV). With current standard therapy, which includes surgical resection when feasible, radiation and temozolomide chemotherapy, patient survival is a mere 2–5 years for anaplastic glioma patients and only 12–15 months for patients with glioblastoma.1 Studies suggest that the development and prevalent resistance of brain tumors is attributed to a small population of tumor cells within the heterogeneous tumor mass with stem-like character. These cells form tumors more readily upon transplantation into mice and are called brain tumor stem-like cells (BTSCs) also called brain tumor-initiating cells. These BTSCs have been shown to utilize a variety of mechanisms to preferentially survive therapy.
It is believed that our best hope for improved treatment lies in the development of therapies that selectively target this population. Successful therapeutic targeting of BTSCs requires improvements in our understanding of the signaling mechanisms that regulate their stem-like behavior. Similar to their normal stem cell counterparts, BTSCs are not randomly distributed throughout the tumor mass, but are concentrated in specific microenvironments. The microenvironments in which BTSCs reside, is gradually beginning to be understood to heavily influence the behavior of resident BTSCs. It follows that better characterization of the signaling events within these niches is central to understanding BTSC function.
Within certain brain tumors, particularly PDGF-induced gliomas, these BTSC microenvironments are observed in pseudopalisading necrotic regions and microvascular proliferating zones (also called perivascular niches).2–4 Our current knowledge of the molecular mechanisms active within these niches in brain tumors is best understood in perivascular niche locations and will subsequently be the focus of this review. In the present work we review the current understanding of the various signaling pathways activated in the brain tumor perivascular niche (PVN) as well as recent evidence highlighting various inter-cellular crosstalk between the multitude of cell types located in the brain tumor PVN. Finally, we outline a model, based on the available evidence, to describe the potential signaling mechanisms operating in the PVN of brain tumors. Investigations of the cellular interactions and signaling pathways activated in the brain tumor PVN that potentially maintain BTSC properties may lead to the design of novel therapeutic strategies that can efficiently target the BTSC population.
The Tumor Perivascular Niche as a Haven for Brain Tumor Stem Cells
Adult neural stem cells (NSCs) are not distributed in random locations throughout the brain but are instead specifically concentrated in close proximity to blood vessels.5 Indeed, the vasculature is an integral component of the major stem cell niches in the brain [the subventricular zone (SVZ) and subgranular zone (SGZ)], with a critical function of supporting stem cell proliferation and regeneration.6,7 The importance of the vasculature to neural stem cells has been identified most clearly in the SVZ where in vivo, NSCs and their progenitors directly contact the vasculature. The vasculature appears critical for supporting NSCs and their progeny6,7 by regulating the capacity of these cells to proliferate and differentiate. Emerging data suggest that this relationship extends to the microenvironment of brain tumors.
A characteristic feature of high-grade character in gliomas is the presence of microvascular proliferating structures.1 These angiogenic entities represent regions of hyper-proliferative tumor stromal and endothelial cells.8 The density of microvascular proliferation in human astrocytic brain tumors strongly correlates with patient survival.9 In fact, the existence of microvascular proliferating structures is recognized as one of the early indicators of malignant progression and is currently a component that defines high-grade gliomas by the WHO.
The brain tumor PVN, defined as the area that borders angiogenic/tumor microvascular structures, is a prime location for BTSCs.2–4 Recent data suggest that establishment of the brain tumor PVN facilitates expansion and differentiation of BTSCs. Indeed, the density of these PVN regions appeared to correlate with the number of BTSCs across several brain tumor subtypes including oligodendrogliomas and glioblastomas.2
A similar study identified PVN regions in medulloblastomas as prime locations for BTSCs that express both nestin and notch.3 These nestin-expressing BTSCs preferentially survived radiotherapy relative to the tumor bulk to allow repopulation and regeneration of the tumor after therapy. Several reports identify a variety of mechanisms that mediate these radioresistant effects. A discussion of the mechanisms that mediate BTSC resistance to therapy is beyond the scope of this review, the interested reader is directed to the following reviews.162,163 However, these studies highlight the growing recognition of the PVN as a critical participant in BTSC regulation.
Other Non-Tumor Perivascular Niche-Associated Cells
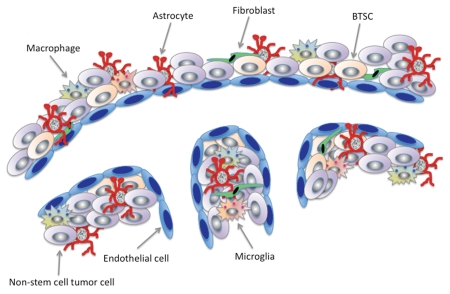
Currently, very little is understood about the structural organization of the PVN in brain tumors. In terms of the architectural make-up of cell types located in the PVN, we are only at the beginning stages of our understanding of their associated functions as they might relate to supporting resident BTSC populations localized to the niche. In addition to the BTSCs discussed above, the major cell types known to reside in the brain tumor PVN, are pericytes; immune cells including lymphocytes, macrophages and microglia; astrocytes, fibroblasts and endothelial cells that line the vasculature (Fig. 1). Each of these individual cell types makes distinct contributions to tumor progression by either contributing to the formation and stability of the microenvironment that supports the BTSC population or by promoting conditions within the niche that facilitates tumor progression.
Figure 1.
The brain tumor PVN is a heterogenous composition of cell types. Some of these include astrocytes, endothelial cells, macrophages, microglia, non-tumor initiating cells and brain tumor stem-like cells (BTSCs). BTSCs are intimately connected with endothelial cells, which are a source of niche-driven signals that help maintain BTSC properties. Astrocytes are closely aligned with endothelial cells but are also found widely dispersed throughout the tumor and signal to endothelial cells as ell as other stromal cells within the tumor microenvironment to support tumor progression. Therapies targeted to the PVN and individual contributions provided by stromal cells within the tumor microenvironment. More importantly, understanding the molecular signals that mediate interactions between multiple cell types is key to successfully finding therapies targeted to the PVN.
Pericytes.
Pericytes are reportedly derived from mesenchymal stem cells or neural crest cells.11 They surround, stabilize and maintain the integrity of the walls of newly developed vasculature.12,13 In the brain, pericytes have also been reported to be involved in immunological functions.11 They tend to be largely localized to microvessels where they are tightly associated with endothelial cells, separated only by a shared basement membrane. In addition, a codependent relationship appears to exist between pericytes and endothelial cells where they can each influence the mitotic activities of one another.14 Pericytes express various markers some of which include, nerve/glial antigen 2 proteoglycan (NG2),15,16 α-smooth muscle actin (α-SMA),17 desmin18 and platelet-derived growth factor receptor β (PDGFRβ).19 However, none of these markers are absolutely specific for pericytes, their expression varies within specific tissues and developmental stages. Previous studies suggested a role for pericytes in microvascular proliferation in tumors. Pericyte recruitment has been identified to contribute to tumor vessel stabilization and survival of the vascular niche in pancreatic,18 melanoma20 and brain tumors.10,21 In addition, in gliomas and other malignant human brain tumors, perivascular localized pericytes are believed to play a critical role in shaping the angio-architecture of the tumor vascular niche.10,16
Immune cells (macrophages).
Immune cells are an additional component of the non-malignant cell populations located in the brain tumor microenvironment and include macrophages, lymphocytes and natural killer cells. As macrophages are the predominant immune-cell population in gliomas,22,23 the discussion will focus on this population. In general, macrophages in the brain are referred to as microglia. Macrophages have been shown to reside in perivascular niche locations of the brain.24 Historically, the presence of these immune cells in gliomas was postulated as a host defense mechanism to suppress dividing neoplastic cells25 however, recent studies have identified glioma cells to play a direct role in recruitment of immune cells into tumors to support tumor growth.
Macrophages originate from CD34+ bone marrow progenitors, which later mature into monocytes.26 Monocytes subsequently differentiate into several cell types including macrophages, which constitute a significant proportion (5–20%) of the total cell population in brain tumors,27 and can be as much as 50% in some tumors.28 It has been suggested that BTSCs within the tumor microenvironment help facilitate tumorigenesis by driving an inflammatory environment in the BTSC vascular niche.29 Macrophages can be localized to distinct locations following their recruitment into the tumor. They can be localized to the advancing tip of the tumor where they are involved in mediating tumor cell motility, they can be localized to stromal and PVN locations where they have been shown to be involved in promoting metastasis, or macrophages can be localized to perinecrotic areas where they promote angiogenesis.30
Macrophage recruitment to brain tumors is suggested to be a primary source of cytokines and other mediators that promote glioma proliferation and migration.27 Monocyte chemoattraction protein-1 (MCP-1) has been implicated in driving macrophage recruitment to gliomas to promote glioma cell proliferation31 and a recent report also suggests a similar role for MCP-3.32 In addition to promoting glioma cell proliferation, macrophages have also been implicated in glioma tumor invasion, expansion and angiogenesis. For instance, upregulation of the membrane bound metalloprotease MMP-1, by tumor macrophages was required for glioma expansion.33 Tumor infiltration of macrophages was strongly correlated with vascular density in human gliomas.34,35 In a similar report, Tie-2 expressing monocytes (from which macrophages originate) were identified to function critically in the induction of angiogenesis in several glioma mouse models. Genetic deletion of Tie-2 expressing cells in vivo was found to substantially suppress angiogenesis and initiated significant tumor regression.36 In a more recent study that utilized an orthotopic glioblastoma mouse model, macrophage recruitment was shown to play a role in the induction of brain tumor angiogenesis and tumor cell invasiveness.37 Recruitment of macrophages in this study was dependent on the activity of HIF-1α. Some reports have also identified tumor suppressor effects of macrophages in brain tumors. For example bone marrow derived macrophages were demonstrated to bind and phagocytose T9 glioma tumor cells that expressed the membrane bound form of macrophage-CSF. Macrophage induced cytolysis of T9 glioma cells was shown to mediate survival of mice.38 Tumor infiltrating macrophages were recently found to express the immune-modulatory nonclassical molecules HLA-G and HLA-E in a majorty of human GBMs analyzed. The expression of these molecules was suggested to function in tumor suppressor effects mediated by the macrophage population.39
Astrocytes.
In the normal brain, astrocytes function as support cells as well as bonafide stem cells that express glial fibrillary acidic protein (GFAP).40 Perivascular astrocytes are closely associated with the endothelium. This close association is tied to its critical function in the induction and maintenance of the blood brain barrier (BBB).41 Astrocytes are known to regulate the activity of neural stem cells through contact as well as by secretion of diffusible signals.42–44 At the molecular level, the specific role of reactive astrocytes in the perivascular niche of brain tumors is poorly understood. One study identified the localization of sonic hedge-hog (SHH) expressing reactive astrocytes in the perivascular niche of gliomas.45 In this study, the levels of reactive astrocytes and Gli signaling were found to directly correlate with increasing grade of these gliomas. In addition, Gli signaling corresponded to the expression of SHH in the PVN of these tumors and the SHH expressing astrocytes were observed to be closely apposed to the perivascular nestin expressing stem cell-like population. Moreover, SHH/Gli signaling has been shown to be critical for BTSC self-renewal and is required for sustained growth and survival of gliomas.46,47 In addition, tumor associated astrocytes play a role in glioblastoma cell invasion via activation of metalloproteinases.48 Astrocyte elevated gene-1 (AEG-1) initially isolated from human fetal astrocytes,49 was identified to be highly expressed in malignant gliomas and was demonstrated to mediate glioma invasion.50 Furthermore, glioma stem cell-like/progenitor cells are known to express the SHH receptor Patched.51 It is therefore plausible that perivascular reactive astrocytes identified in these giomas can mediate Gli activation in adjacent BTSCs. This would imply that reactive astrocytes in the glioma PVN may help maintain the stem cell properties of BTSC populations in these gliomas.
Endothelial cells.
Endothelial cells are a core component of the BBB. They protect the brain from potentially harmful substances and also serve the nutritional demands of the CNS. Endothelial cells originate from hematopoetic stem cells and some markers used to identify endothelial cells in gliomas include CD31, CD34 and CD36.52
Emerging data suggest that in addition to providing oxygen and nutrients to NSCs, endothelial cells are also important sources of diffusible niche factors, which regulate neural stem cell self-renewal and neurogenesis.53 This mechanism appears to be conserved in brain tumors where endothelial derived factors appear to drive glioma cell proliferation54 and self-renewal characteristics of perivascular BTSCs.2,4 Signaling between endothelial cells and stem cells of the PVN is described below.
Fibroblasts.
Fibroblasts have been shown to play an important role in tumor progression in other cancers.55–57 Fibroblasts localized to the brain tumor microenvironment have been identified to promote the invasion of brain tumor cells. The expression of matrix metalloproteases is critical for breakdown of the ECM for tumor invasion. Fibroblasts were shown to produce and mediate activation of proMMP-2,58 a metalloproteinase associated with increased invasiveness and malignant progression of gliomas.59–61
Intercellular Cross-Talk within the Tumor Perivascuar Niche
Communication between the multitude of cell types that exist in neurogenic niches of the normal brain is coordinated to help maintain neural stem cells and to preserve their potential to proliferate and differentiate. This logic may be extended to brain tumors as well.
Communication between endothelial cells and astrocytes.
In the normal brain, astrocytes have traditionally been considered as support cells. The close association between endothelial cells and astrocytes enables effective communication between the two cell types to foster induction and maintenance of the BBB.41 Crosstalk between perivascular astrocytes and endothelial cells can upregulate the expression of tight junction proteins,62 GLUT1 and Pgp transporters63,64 and BBB-associated enzyme activities of endothelial cells. These interactions ultimately enhance the stability of the vascular niche to support NSCs. Some studies suggest that endothelial cells may mediate growth and differentiation of perivascular astrocytes.65 Whether similar intercellular communication between endothelial cells and perivascular astrocytes exists in the context of brain tumors is unclear. However, what we do know is that tumor development is associated with impaired BBB function and correlates with loss of astrocytic regulation of endothelial activity.41 The extent to which it may contribute to tumor progression is not known.
Communication between endothelial cells and pericytes.
Pericytes can communicate with endothelial cells through direct contact and also via pathways involved in paracrine signaling.11 Pericyte-endothelial interactions have been reported in the regulation of blood flow and blood brain barrier functions.14 During angiogeneis, pericytes can directly communicate with endothelial cells through direct interaction or through paracrine signals to affect the rate of endothelial cells differentiation and growth arrest.14,66,67
Communication between endothelial cells and BTSCs.
Endothelial cells and NSCs are involved in molecular crosstalk that is mediated through direct interactions of NSCs with the vasculature6 as well as through diffusible signals released from the vasculature.53,68–70 Co-culture experiments of endothelial cells with embryonic or SVZ NSCs, spurred the self-renewal and proliferation of NSCs, which was mediated by diffusible signals from endothelial cells.53 Pigment epithelium derived factor (PEDF), another diffusible signal identified to be secreted by endothelial cells has been proposed to regulate SVZ stem cell self-renewal.70 Other factors include leukemia inhibitory factor (LIF)65 and brain derived neurotrophic factor (BDNF).68,71 Both factors are known to promote proliferation and/or differentiation in neurogenic niches of adults.
This intercellular communication appears to be conserved in the context of the brain tumor microenvironment. BTSCs residing in the tumor perivscular niche, like their normal stem cell counterparts, can engage in cell-to-cell communication with tumor endothelial cells. BTSCs were observed to home towards the vasculature where they initiated contact, aligning themselves along the entire length of the vasculataure.2 The localization of BTSCs to the tumor vasculature puts them in an ideal position to respond to signaling cues from tumor endothelia. Soluble factors released from the endothelium have been shown to promote proliferation and self-renewal of BTSCs.2 Moreover, increasing the quantity of endothelial cells and therefore the endothelial-derived factors enhanced the number of BTSCs, subsequently accelerating the initiation and growth of brain tumors in vivo.2 More recently, nitric oxide has been identified as an endothelial-derived factor within the tumor perivascular niche of the PDGF-subset of gliomas that plays an important role in promoting BTSC properties. This endothelium-derived factor was shown to activate Notch signaling in a population of stem-like cells in a brain tumor mouse model. Activation of Notch signaling by NO accelerated the self-renewing capacity of BTSCs in vitro and enhanced the tumor initiating capacity of gliomas of the PDGF subtype in vivo.4 These studies underscore the importance of the cross communication between the tumor vasculature and BTSCs, to support BTSC function.
However, the crosstalk between the vasculature and neural stem cells is by no means unidirectional. In fact, neural stem cells have been demonstrated to communicate to the vascular endothelium where they drive robust vascular tube formation by stimulating nearby endothelial cells to produce vascular endothelial growth factor (VEGF) and BDNF.72 Like their normal stem cell counterparts, BTSCs can also modulate the activity of the tumor vasculature, which helps sustain them. Bao and coworkers demonstrated that human glioblastoma stem-like cells expressing elevated levels of VEGF could induce tumor endothelial cells to undergo angiogenesis.73 In this study tumors derived from brain tumor stem cell-like populations showed greater levels of angiogenesis, necrosis and hemorrhage in comparison to their non stem cell-like counterparts expressing significantly lower levels of VEGF. This therefore indicates the bi-directionality of crosstalk between BTSCs and the vascular niche.
Signaling Pathways in the Brain Tumor Perivascular Niche
Our current knowledge of molecular pathways that are active in brain tumors are mainly from studies that describe these signaling pathways in the collective tumor microenvironment rather than in designated locations such as the PVN. Therefore, specific references will be made to those pathways that have specifically been identified, by reports, to be activated in the PVN and others will be described within the context of the overall tumor microenvironment.
The various cell types localized to the brain tumor PVN are involved in a multitude of signaling that may be coordinated to support the activity of BTSCs. These signaling pathways which include the SHH, PI3K/Akt, Wnt and the Notch signaling pathways have all been demonstrated to be activated in the microenvironemnt of brain tumors. As the aforementioned signaling pathways are known to regulate the self-renewal and proliferative properties of normal stem cells,74 much research is focused on their potential involvement in regulating the stem-cell properties of BTSCs.
Sonic hedgehog signaling in the brain tumor PVN.
Sonic hedgehog (SHH) is a secreted protein that plays a critical role in controlling fate of ventral cell types in the CNS during embryogenesis by mediating both cell proliferation and differentiation.75–77 The binding of SHH to its receptor Patched releases tonic inhibition of Smoothened (SMO) by Patched. Smoothened release triggers activation of the Gli (named for their discovery in gliomas) family of transcription factors. These transcription factors subsequently induce translation of genes that function in maintaining the “stemness” of NSCs in the adult CNS.78 SHH regulates the proliferation of astrocytic cells (type B cells) and transit amplifying cells (type C cells) in the adult SVZ.36,79 Although GLI was first described in the context of gliomas,80 its role in tumor formation has been studied in more detail in medulloblastoma, a primary brain tumor that arises in children.81,82 Recent reports have helped to elucidate the mechanisms that mediate SHH induced proliferation and transformation of medulloblastomas.83,84 A recent report has identified the transcription factor Yap1 expression in the PVN of medulloblatomas, which co-localized with stem-like markers and was identified to mediate SHH driven medulloblastoma formation.83
There have been several isolated studies exploring the importance of GLI and the broader SHH/PTCH/SMO/GLI cascade in gliomas. These studies have described the activity of SHH signaling within the collective tumor microenvironment. For example, GLI amplification occurs in a small subset of gliomas85–87 and SMO inhibition by the drug cyclopamine reduces glioma cell proliferation.46,51,88,89 Recent reports have suggested potential roles of the SHH pathway in glioma progression. Gli1 expression was closely correlated with pathological grades of human gliomas and RNAi knock-down of SHH/Gli signaling significantly suppressed glioma migration and invasion.90 Using the RCAS/tva system in Gli reporter mice, Becher and colleagues demonstrated that sonic signaling was activated in mouse gliomas induced by PDGF overexpression. Immunohistochemical analysis of gliomas demonstrated elevated SHH protein expression localized to the glioma PVN regions and its expression was strongly correlated with increasing glioma grade.45 These findings highlight the importance of the hedge-hog pathway in the microenvironment of brain tumors, however a specific role of the pathway in the PVN and potential effects on resident BTSC populations within the niche is yet to be described.
PI3K/Akt signaling in the brain tumor PVN.
The PI3K/Akt pathway is frequently activated in gliomas ∼70%.91–93 Activation of this pathway is advantageous to the tumor as Akt promotes glioma proliferation, invasion and inhibits apoptosis and is thus critical for survival and growth of these tumors. Activation of the PI3K/Akt pathway and the expression of stem cell markers have been associated with aggressive behavior and tumor resistance. Studies in human gliomas demonstrate that alterations in components of Akt signaling at the DNA, RNA and protein levels correlates with poor prognoses in patients.94 A number of other investigations that utilize genetic engineered mouse models of gliomas have shown that Akt activity contributes to glioma tumor formation and growth.95–98 In addition, the expression of nestin and CD133 were shown to be strong prognostic factors for glioma malignancy.99–101 Coincidentally, nestin-expressing BTSCs in mouse models of GBM are known to have high-levels of Akt pathway activity.102
In a recent study, BTSCs were demonstrated to be more dependent on Akt signaling than their matched non stem-cell counterparts. Subsequent inhibition of Akt by pharmacologic agents diminished BTSC capacity for neural stem cell formation, enhanced apoptosis and delayed tumor formation.103 The significance of the role of PI3K/Akt signaling in facilitating resistance of PVN BTSCs to therapy, is highlighted by Hambardzumyan and coworkers, using mouse models of medulloblastomas. In this study BTSCs (identified by the expression of nestin and Notch) were found to preferentially survive radiation therapy relative to the non-stem cell populations within the tumor by activating the PI3K/Akt signaling pathway in response to radiation. These BTSCs preferentially survived by enhancing their expression of nestin and arresting instead of undergoing apoptosis as the bulk of tumor cells did. Following a temporary period of arrest (72 hrs), these BTSCs reentered the cell cycle to proliferate and reconstitute the tumor following therapy.3 The importance of AKT signaling in PDGF-induced gliomas has also been demonstrated in preclinical trials in glioma-bearing mice by blocking the activity of mTOR using the rapamycin analog temsirolimus.104
Notch signaling in the brain tumor PVN.
Notch signaling is highly conserved across evolution and is critically important for directing patterning during development and plays a key role in stem cell proliferation and self-renewal.105 Notch ligands and receptors are single-pass transmembrane proteins that mediate Notch signaling.106 Notch signaling involves the binding of the Notch ligands (Delta-like 1, 3 & 4 and Jagged 1 & 2) to Notch receptors (Notch 1–4) in a juxtacrine signaling mechanism. Ligand-receptor interaction induces a series of cleavages the final of which involves gamma secretase cleavage of Notch intracellular domain (NICD). NICD translocates to the nucleus where the binding to CSL transcription factors culminates in neural stem cell proliferation and suppression of their differentiation107,108 among many other functions. Notch is required for neural progenitors both in vitro and in vivo109 and it is essential for the maintenance of neural stem cells.110,111
With regards to cancer, a substantial amount of data has linked activation of the Notch pathway to tumor development in many types of cancers112 including brain tumors. The expression of Hey1 (a downstream target of Notch) was correlated with tumor grade and survival of human GBM patients.113 Notch-1, along with its receptors Jagged-1 and delta-like-1, play an integral role in high-grade glioma and medulloblastoma formation.114–117 Notch was identified to drive BTSC activity in medulloblastomas.118 Pharmacological blockade of the notch pathway diminished nestin- and CD133-positive populations as well as the side population (SP) within medulloblastoma cells and decreased their tumor initiating capacity in vivo. Notch activation was also shown to enhance the expression of nestin in these medulloblatoma cells.118 The link between Notch signaling and BTSC function in gliomas was established in a related study that identified CSL binding sites for Notch in the promoter region of human nestin, implying regulation of nestin by the Notch pathway. In this investigation Notch signaling was shown to increase the expression of nestin and in combination with Kras, induced proliferative lesions in the NSC enriched periventricular regions in a glioblastoma mouse model.119 In addition, activated Notch signaling in a human glioma cell line enhanced the formation of neurosphere-like colonies120 and Notch signaling was required for Id-4 induced neural stem-like properties of Ink4aArf−/− astrocytes.117
We have recently identified a critical role of the notch pathway in driving the stem cell-like characteristics of BTCSs which reside in the glioma PVN.4 Perivascular nestin-expressing BTSCs co-expressed Notch-1 and activation of Notch mediated through nitric oxide signaling was shown to enhance the tumor initiating capacity of PDGF-induced glioma primary cultures.4
Notch signaling within the tumor vascular microenvironment has also been shown to regulate tumor angiogenesis in several cancers.121–123 Delta-like4 (DLL4) expression was shown to be upregulated in tumor cells and tumor endothelial cells of human glioblastoma. Using a mouse model of glioblastoma it was demonstrated that DLL4 expression by tumor cells activated notch signaling in endothelial cells to drive tumor angiogenesis.124 In addition, anti-DDL4 treatment of tumor cells in a glioblastoma model inhibited growth of glioblastomas by paradoxically enhancing growth of non-functional tumor vasculature.125 Collectively, these studies have highlighted the importance of the Notch pathway in tumor development, and suggest that selective targeting of the Notch pathway may be an effective way to target the brain tumor microenvironment.
Nitric oxide (NO)/cGMP signaling in the brain tumor perivascular niche.
NO is a highly reactive free radicle with a half-life of approximately 2–5 secs.126 It is a highly lipophilic and diffusible molecule, which can readily disperse from its site of synthesis to traverse multiple cell membranes en route to its final target. NO is generated from a family of NADPH-dependent enzymes called nitric oxide synthases (NOS) from the terminal guanido nitrogen atom of L-arginine. The three major NOS isoforms are neuronal (nNOS), inducible (iNOS) and endothelial NOS (eNOS). In general nNOS and eNOS are expressed constitutively in neurons and endothelial cells respectively, but can be expressed by other cells. Their activity depends on the levels of intracellular calcium to produce approximately nanomolar amounts of NO. Expression of iNOS in contrast, is induced, and its expression is generally not affected by levels of intracellular calcium.127 The major NO signaling pathway involves production of cyclic guanosine-3′,5′-monophosphate (cGMP) by way of activation of soluble guanylyl cyclase, the major receptor for NO and its downstream effector, protein kinase G (PKG).
We have recently demonstrated signaling by the NO/cGMP pathway in the perivascular niche of the PDGF subset of gliomas.4 We show that NO activates Notch signaling in a population of perivasular localized brain tumor stem-like cells that express nestin and notch. This is consistent with previous reports where NO was identified to drive activation of the Notch pathway in cholangiocarcinomas.128 Our data demonstrates that activation of Notch signaling in perivascular BTSCs by NO released from the tumor endothelium enhanced BTSC-like characteristics and accelerated tumor formation in mice. This data identifies components of the NO-cGMP pathway as potential targets for therapy and suggests that changes of brain tumor cells towards BTSC-like features can be induced by the microenvironment.
Other brain tumor stem cell signaling pathways that may or may not contribute to perivascular niche biology.
In addition to the pathways mentioned above, there are several other signaling pathways known to be important regulators of BTSC activity. These include the Bone morphogenetic protein/Smad (BMP/Smad) signaling and Wnt signaling pathways. Nonetheless, although the assertion can be made that these pathways are likely active in the brain tumor PVN specifically, this remains to be demonstrated.
BMP/Smad signaling. Bone morphogenetic proteins (BMPs) are members of the TGF-β/Smad family of growth factors that regulate a variety of biological processes including bone and cartilage formation and embryonic stem cell self-renewal and differentiation.129 Binding of BMPs to their cognate receptors BMPRs elicits the downstream effects of BMPs, which involves activation of Smad proteins. BMPs play a critical role in the regulation of NSC proliferation and apoptosis, and usually promotes NSC differentiation.130 BTSCs were identified to express BMPRs and therefore retained the capacity for regulation by BMPs. Treatment of BTSCs with BMPs inhibited proliferation of BTSCs and induced their differentiation to astroglial and neuron-like cell fates, which diminished the capacity of BTSCs to initiate tumors in vivo.131 A similar report by Lee and co workers demonstrated differentiation of BTSCs following BMP treatment. However, a subset of BTSCs that did not express the appropriate BMPR1B receptor showed enhanced proliferation in response to BMP treatment instead of differentiation. Forced expression of BMPR1B sensitized this subset of BTSCs to BMP-induced differentiation132 suggesting that the expression pattern of BMPR may dictate the response of BTSCs to BMP treatment.
A more recent study demonstrated that knockdown of TRRAP—an adaptor protein with homology to PIKK kinases—could suppress BTSC proliferation and induce their differentiation to an astroglial lineage. These effects of TRRAP were mediated by suppression of cyclin A2 gene expression and enhanced BMP/Smad signaling.133,134
Wnt signaling. The Wnt pathway is involved in several key developmental processes such as proliferation, stem cell maintenance, pattern formation and differentiation.135 Wnt signaling has been shown to play a role in SVZ progenitor cell proliferation and neurogenesis in neonatal and adult mice136,137 and to regulate stem cell fate and differentiation in vivo.138 Wnt signaling was initially implicated in contributing to medulloblastomas.139,140 Mutations in downstream mediators of Wnt signaling, such as Axin, β-catenin and APC were found in sporadic medulloblastomas,141–143 and nuclear localization of β-catenin, which is indicative of constitutive Wnt signaling, has also been found.144
Moreover, DKK-1, an endogenous antagonist of the Wnt pathway was reported to be epigenetically silenced in medulloblastomas and its reactivation was found to enhance apoptosis and induce a 60% reduction in tumor growth.145
Negative regulators of the Wnt pathway are frequently hypermethylated in glioblastomas.146 Ectopic expression of the Wnt negative regulator, DKK-1 in the human glioma U87MG cell line sensitized these cells to chemotherapy-induced apoptosis.147 In addition, several components of the Wnt pathway were found to be upregulated in gliomas, including Wnt 5A and Frizzled 9, a receptor of the canonical Wnt pathway148–150 The Wnt pathway has also been demonstrated to mediate cell proliferation and the invasive capacity of gliomas. Immunohistochemical analysis of 45 human astrocytomas demonstrated an upregulation of core components of the Wnt pathway. Suppression of these components significantly decreased cell proliferation and invasion and delayed tumor formation in mice. This effect of Wnt suppression on gliomas was associated with disruption of the PI3K/Akt pathway151 suggesting cross communication between these two signaling axes.
Therapeutic Targeting of the Brain Tumor Perivascular Niche Complex
According to the cancer stem cell hypothesis, BTSCs are the engines that drive brain tumor progression and recurrence. Therefore, effective targeting of this population is critical to all efforts designed to eradicate brain tumors. Since BTSCs are known to reside in the PVN regions of these tumors, one promising strategy involves targeting intracellular pathways that are active in the brain tumor PVN, pathways which promote BTSCs self-renewal, proliferation and migration. These signaling pathways include SHH, PI3K/AKT, Notch and more recently the NO/cGMP signaling pathway. Targeting these pathways with inhibitors have all been demonstrated to suppress glioma progression and sensitize brain tumors to therapy.3,89,118,152,153 Additional pathways such as DNA damage checkpoint pathways (Chk1&2), Wnt and BMP/Smad that regulate the activity of brain tumor cells are also important, and targeting these pathways have been demonstrated to suppress brain tumor growth in experimental and preclinical models.131,133,154 However, it is unknown whether these pathways are active in the PVN of brain tumors.
Targeting endothelial cells of the tumor vasculature can be an alternative approach used to subvert BTSC function. Studies have shown that disrupting the vascular niche microenvironment in which BTSCs reside, and which is critical for their maintenance might be a viable approach to targeting BTSCs.2,155 One might imagine that destruction of the vascular niche likely eliminates the supportive environment the vasculature provides to BTSCs. Indeed, Bevacizumab (Avastin), a monoclonal antibody targeted against VEGF-A and Cediranib (AZD2171), a small molecule inhibitor of VEGF, have been used in the clinic with some success.156–158 In experimental models, targeting the brain tumor vascular niche with the use of anti-angiogenics has shown some promise. The anti-angiogenic monoclonal antibody against VEFGR-2, DC101, markedly suppressed experimental malignant glioma growth.159 The suppression of tumor volumes and microvessel density of tumor-bearing mice, relative to control animals, corresponded with decreased tumor cell proliferation and increased apoptosis. The authors of the study observed an increase in tumor invasive features from DC101 monotherapy, which was suppressed with combined EGFR blockade.160 Another study that utilized PTK787, an inhibitor of VEGFR and PDGFR tyrosine kinases, led to significant reductions in tumor volumes and vessel density.161
Although suppression of the tumor-associated effects of many of these studies is ascribed to suppression of angiogenesis, it is conceivable that adverse effects on the BTSC populations might have also contributed to these overall effects of tumor suppression. Examination of the BTSC populations before and after treatment in these tumors may shed some light on this possibility. Indeed, work by Calabrese and co workers demonstrated that treatment of mice bearing orthotopic xenografts of human glioblastomas cells with bevacizumab, significantly diminished the population of perivascular localized BTSCs (with little impact on the actual tumor cells) and suppressed tumor growth rate.2 In addition, Folkins et al. showed, using a C6 rat glioma model, that anti-angiogenic treatment in combination with cytotoxic compounds, diminished the BTSC populations as well as their neurosphere forming capacities.155 These studies strongly suggest that current anti-angiogenic treatments may already be targeting the BTSC populations in brain tumors.
Conclusion
The tumor PVN microenvironment is composed of a complex array of cell types, each with individual contributing roles to play in the maintenance of the tumor. This reality has far-reaching implications for therapies designed to target the PVN. These therapies will have to consider the complex milieu of cellular elements within the PVN and the signals that facilitate communication among resident cell types. Approaches designed to antagonize the various combinations in which these signals may interact, will be key to successfully targeting the PVN for therapy.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12710
Financial Disclosures
Support was provided by NIH grant U54 CA126518, NIH grant U54 CA143798 and the Litwin Foundation.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riquelme PA, Drapeau E, Doetsch F. Brain microecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesseling P, Vandersteenhoven JJ, Downey BT, Ruiter DJ, Burger PC. Cellular components of microvascular proliferation in human glial and metastatic brain neoplasms. A light microscopic and immunohistochemical study of formalin-fixed, routinely processed material. Acta Neuropathol. 1993;85:508–514. doi: 10.1007/BF00230490. [DOI] [PubMed] [Google Scholar]
- 9.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77:362–372. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL, Tse VC. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 14.Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 15.Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chekenya M, Enger PO, Thorsen F, Tysnes BB, Al-Sarraj S, Read TA, et al. The glial precursor proteoglycan, NG2, is expressed on tumour neovasculature by vascular pericytes in human malignant brain tumours. Neuropathol Appl Neurobiol. 2002;28:367–380. doi: 10.1046/j.1365-2990.2002.00412.x. [DOI] [PubMed] [Google Scholar]
- 17.Verbeek MM, Otte-Holler I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- 18.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74:269–277. doi: 10.1007/BF00688191. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 25.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 26.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 28.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 29.Veeravagu A, Bababeygy SR, Kalani MY, Hou LC, Tse V. The cancer stem cell-vascular niche complex in brain tumor formation. Stem Cells Dev. 2008;17:859–867. doi: 10.1089/scd.2008.0047. [DOI] [PubMed] [Google Scholar]
- 30.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 31.Platten M, Kretz A, Naumann U, Aulwurm S, Egashira K, Isenmann S, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54:388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 32.Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34:1621–1627. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- 33.Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 35.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92:288–293. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- 36.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadus MR, Williams CC, Avina MD, Ly M, Kim S, Liu Y, et al. Macrophages kill T9 glioma tumor cells bearing the membrane isoform of macrophage colony stimulating factor through a phagocytosis-dependent pathway. J Immunol. 1998;160:361–368. [PubMed] [Google Scholar]
- 39.Kren L, Muckova K, Lzicarova E, Sova M, Vybihal V, Svoboda T, et al. Production of immune-modulatory nonclassical molecules HLA-G and HLA-E by tumor infiltrating ameboid microglia/macrophages in glioblastomas: A role in innate immunity? J Neuroimmunol. 220:131–135. doi: 10.1016/j.jneuroim.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39:339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- 42.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 44.Kornyei Z, Szlavik V, Szabo B, Gocza E, Czirok A, Madarasz E. Humoral and contact interactions in astroglia/stem cell co-cultures in the course of glia-induced neurogenesis. Glia. 2005;49:430–444. doi: 10.1002/glia.20123. [DOI] [PubMed] [Google Scholar]
- 45.Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–2249. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- 46.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stecca B, Ruiz i Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64:476–490. doi: 10.1002/neu.20160. [DOI] [PubMed] [Google Scholar]
- 48.Le DM, Besson A, Fogg DK, Choi KS, Waisman DM, Goodyer CG, et al. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci. 2003;23:4034–4043. doi: 10.1523/JNEUROSCI.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 50.Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, et al. Astrocyte elevated gene-1: a novel target for human glioma therapy. Mol Cancer Ther. 9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- 52.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 53.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 54.Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 55.Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R, Politi V, et al. Gene expression profiling reveals crosstalk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–4146. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein LJ, Chen H, Bauer RJ, Bauer SM, Velazquez OC. Normal human fibroblasts enable melanoma cells to induce angiogenesis in type I collagen. Surgery. 2005;138:439–449. doi: 10.1016/j.surg.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 57.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, et al. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177–184. doi: 10.1016/s0304-3835(00)00485-7. [DOI] [PubMed] [Google Scholar]
- 59.Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, et al. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- 60.Uhm JH, Dooley NP, Villemure JG, Yong VW. Glioma invasion in vitro: regulation by matrix metalloprotease-2 and protein kinase C. Clin Exp Metastasis. 1996;14:421–433. doi: 10.1007/BF00128958. [DOI] [PubMed] [Google Scholar]
- 61.Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, et al. Expression and tissue localization of membrane-type 1, 2 and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol. 1999;154:417–428. doi: 10.1016/S0002-9440(10)65288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dehouck MP, Meresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible and mass-production method to study the blood-brain barrier in vitro. J Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 63.McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, Mayberg MR, et al. Mechanisms of glucose transport at the blood-brain barrier: An in vitro study. Brain Res. 2001;904:20–30. doi: 10.1016/s0006-8993(01)02418-0. [DOI] [PubMed] [Google Scholar]
- 64.Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 65.Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D'Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 68.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 69.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 71.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 72.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 73.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 74.Stiles CD, Rowitch DH. Glioma stem cells: A midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 75.Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 76.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 77.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 78.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 79.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 80.Collins VP. Amplified genes in human gliomas. Semin Cancer Biol. 1993;4:27–32. [PubMed] [Google Scholar]
- 81.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, Khafaga Y, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 49:302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 83.Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, et al. YAP1 is amplified and upregulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326:1424–1427. doi: 10.1126/science.1181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bigner SH, Burger PC, Wong AJ, Werner MH, Hamilton SR, Muhlbaier LH, et al. Gene amplification in malignant human gliomas: clinical and histopathologic aspects. J Neuropathol Exp Neurol. 1988;47:191–205. doi: 10.1097/00005072-198805000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Mao X, Hamoudi RA. Molecular and cytogenetic analysis of glioblastoma multiforme. Cancer Genet Cytogenet. 2000;122:87–92. doi: 10.1016/s0165-4608(00)00278-8. [DOI] [PubMed] [Google Scholar]
- 87.Hui AB, Lo KW, Yin XL, Poon WS, Ng HK. Detection of multiple gene amplifications in glioblastoma multiforme using array-based comparative genomic hybridization. Lab Invest. 2001;81:717–723. doi: 10.1038/labinvest.3780280. [DOI] [PubMed] [Google Scholar]
- 88.Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 89.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang K, Pan L, Che X, Cui D, Li C. Sonic Hedgehog/GLI1 signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurol Res. doi: 10.1179/016164110X12681290831360. [DOI] [PubMed] [Google Scholar]
- 91.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 92.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 93.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 94.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 95.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 96.Rajasekhar VK, Viale A, Socci ND, Wiedmann M, Hu X, Holland EC. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell. 2003;12:889–901. doi: 10.1016/s1097-2765(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 97.Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62:5551–5558. [PubMed] [Google Scholar]
- 98.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strojnik T, Rosland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. discussion 43–4. [DOI] [PubMed] [Google Scholar]
- 100.Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, et al. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 2008;18:370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 102.Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells and akt signaling. Stem Cell Rev. 2008;4:203–210. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 103.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uhrbom L, Nerio E, Holland EC. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nat Med. 2004;10:1257–1260. doi: 10.1038/nm1120. [DOI] [PubMed] [Google Scholar]
- 105.Ehebauer M, Hayward P, Arias AM. Notch, a universal arbiter of cell fate decisions. Science. 2006;314:1414–1415. doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- 106.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 107.Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 108.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 109.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 110.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 112.Sjolund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer. 2005;41:2620–2629. doi: 10.1016/j.ejca.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 113.Hulleman E, Quarto M, Vernell R, Masserdotti G, Colli E, Kros JM, et al. A role for the transcription factor HEY1 in glioblastoma. J Cell Mol Med. 2009;13:136–146. doi: 10.1111/j.1582-4934.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 115.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 116.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 117.Jeon HM, Jin X, Lee JS, Oh SY, Sohn YW, Park HJ, et al. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008;22:2028–2033. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 119.Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang XP, Zheng G, Zou L, Liu HL, Hou LH, Zhou P, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008;307:101–108. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- 121.Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 123.Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth and angiogenesis. Cancer Res. 2008;68:4727–4735. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 125.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 126.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int. 2004;45:813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 127.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci. 2009;14:1–18. doi: 10.2741/3228. [DOI] [PubMed] [Google Scholar]
- 128.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase upregulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 129.Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- 130.Panchision DM, McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12:478–487. doi: 10.1016/s0959-437x(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 131.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 132.Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wurdak H, Zhu S, Romero A, Lorger M, Watson J, Chiang CY, et al. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 6:37–47. doi: 10.1016/j.stem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 134.Charles NA, Holland EC. TRRAP and the maintenance of stemness in gliomas. Cell Stem Cell. 6:6–7. doi: 10.1016/j.stem.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 135.Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 137.Hirsch C, Campano LM, Wohrle S, Hecht A. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res. 2007;313:572–587. doi: 10.1016/j.yexcr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 138.Prakash N, Wurst W. A Wnt signal regulates stem cell fate and differentiation in vivo. Neurodegener Dis. 2007;4:333–338. doi: 10.1159/000101891. [DOI] [PubMed] [Google Scholar]
- 139.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, et al. The molecular basis of Turcot's syndrome. N Engl J Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 140.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 141.Koch A, Waha A, Tonn JC, Sorensen N, Berthold F, Wolter M, et al. Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int J Cancer. 2001;93:445–449. doi: 10.1002/ijc.1342. [DOI] [PubMed] [Google Scholar]
- 142.Baeza N, Masuoka J, Kleihues P, Ohgaki H. AXIN1 mutations but not deletions in cerebellar medulloblastomas. Oncogene. 2003;22:632–636. doi: 10.1038/sj.onc.1206156. [DOI] [PubMed] [Google Scholar]
- 143.Yokota N, Nishizawa S, Ohta S, Date H, Sugimura H, Namba H, et al. Role of Wnt pathway in medulloblastoma oncogenesis. Int J Cancer. 2002;101:198–201. doi: 10.1002/ijc.10559. [DOI] [PubMed] [Google Scholar]
- 144.Eberhart CG, Tihan T, Burger PC. Nuclear localization and mutation of beta-catenin in medulloblastomas. J Neuropathol Exp Neurol. 2000;59:333–337. doi: 10.1093/jnen/59.4.333. [DOI] [PubMed] [Google Scholar]
- 145.Vibhakar R, Foltz G, Yoon JG, Field L, Lee H, Ryu GY, et al. Dickkopf-1 is an epigenetically silenced candidate tumor suppressor gene in medulloblastoma. Neuro Oncol. 2007;9:135–144. doi: 10.1215/15228517-2006-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gotze S, Wolter M, Reifenberger G, Muller O, Sievers S. Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer. 126:2584–2593. doi: 10.1002/ijc.24981. [DOI] [PubMed] [Google Scholar]
- 147.Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002;21:878–889. doi: 10.1038/sj.onc.1205138. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Z, Schittenhelm J, Guo K, Buhring HJ, Trautmann K, Meyermann R, et al. Upregulation of frizzled 9 in astrocytomas. Neuropathol Appl Neurobiol. 2006;32:615–624. doi: 10.1111/j.1365-2990.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 149.Yu JM, Jun ES, Jung JS, Suh SY, Han JY, Kim JY, et al. Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Lett. 2007;257:172–181. doi: 10.1016/j.canlet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 150.Liu X, Wang L, Zhao S, Ji X, Luo Y, Ling F. beta-Catenin overexpression in malignant glioma and its role in proliferation and apoptosis in glioblastma cells. Med Oncol. 2010 doi: 10.1007/s12032-010-9476-5. In press. [DOI] [PubMed] [Google Scholar]
- 151.Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, et al. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16:351–361. doi: 10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- 152.Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 153.Wachsberger PR, Burd R, Marero N, Daskalakis C, Ryan A, McCue P, et al. Effect of the tumor vascular-damaging agent, ZD6126, on the radioresponse of U87 glioblastoma. Clin Cancer Res. 2005;11:835–842. [PubMed] [Google Scholar]
- 154.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 155.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 156.Iwamoto FM, Fine HA. Bevacizumab for malignant gliomas. Arch Neurol. 67:285–288. doi: 10.1001/archneurol.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, et al. Phase II Study of Cediranib, an Oral Pan-Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor, in Patients With Recurrent Glioblastoma. J Clin Oncol. 28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fillbrandt R, Stavrou D, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 160.Lamszus K, Brockmann MA, Eckerich C, Bohlen P, May C, Mangold U, et al. Inhibition of glioblastoma angiogenesis and invasion by combined treatments directed against vascular endothelial growth factor receptor-2, epidermal growth factor receptor and vascular endothelial-cadherin. Clin Cancer Res. 2005;11:4934–4940. doi: 10.1158/1078-0432.CCR-04-2270. [DOI] [PubMed] [Google Scholar]
- 161.Goldbrunner RH, Bendszus M, Wood J, Kiderlen M, Sasaki M, Tonn JC. PTK787/ZK222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases, decreases glioma growth and vascularization. Neurosurgery. 2004;55:426–432. doi: 10.1227/01.neu.0000129551.64651.74. discussion 32. [DOI] [PubMed] [Google Scholar]
- 162.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 163.Lu C, Shervington A. Chemoresistance in gliomas. Mol Cell Biochem. 2008;312:71–80. doi: 10.1007/s11010-008-9722-8. [DOI] [PubMed] [Google Scholar]