Abstract
Pulmonary fibrosis complicates a number of disease processes and leads to substantial morbidity and mortality. Idiopathic pulmonary fibrosis (IPF) is perhaps the most pernicious and enigmatic form of the greater problem of lung fibrogenesis with a median survival of three years from diagnosis in affected patients. In this review, we will focus on the pathology of IPF as a model of pulmonary fibrotic processes, review possible cellular mechanisms, review current treatment approaches and review two transgenic mouse models of lung fibrosis to provide insight into processes that cause lung fibrosis. We will also summarize the potential utility of signaling pathway inhibitors as a future treatment in pulmonary fibrosis. Finally, we will present data demonstrating a minimal contribution of epithelial-mesenchymal transition in the development of fibrotic lesions in the transforming growth factor-alpha transgenic model of lung fibrosis.
Key words: epithelial mesenchymal transition, epidermal growth factor receptor, transforming growth factor alpha
Pulmonary Fibrosis the Extent of Disease
Pulmonary fibrosis represents a heterogeneous group of diseases where progressive parenchymal fibrosis disrupts the structure and function of gas-exchanging regions of the lung. These changes result in functional limitations and considerable morbidity and mortality. Aberrant lung fibrosis is a pathologic hallmark of relatively common and uncommon diseases including chronic obstructive pulmonary disease, chronic asthma, cystic fibrosis and bronchopulmonary dysplasia (reviewed in ref. 1–4). Lung fibrosis can also occur in connective tissue diseases, granulomatous disorders such as sarcoidosis and following acute and chronic toxic inhalations such as asbestosis, silicosis and other forms of pneumoconiosis (reviewed in ref. 5). Idiopathic pulmonary fibrosis (IPF) is perhaps the most pernicious and enigmatic form of the greater problem of lung fibrogenesis with a median survival of three years from diagnosis in affected patients. Recent studies indicate that the prevalence and mortality of IPF are growing in the US and elsewhere.6 The development of fibrotic lesions in such heterogeneous diseases suggests that causes are multifactorial. Currently, there are no effective therapies for pulmonary fibrosis indicating a need to develop further understanding of pathophysiologic mechanisms. In this review, we will focus on the pathology of IPF as a model of pulmonary fibrotic processes, review possible cellular mechanisms, review current treatment approaches and review two transgenic mouse models of lung fibrosis to provide insight into processes that cause lung fibrosis.
Pathology of IPF
The pathological form of IPF, sub-classified as usual interstitial pneumonia (UIP), is temporally heterogeneous. In the lung regions of normal histological appearance are intermixed with mature, collagenized fibrosis and small, focal areas of less mature matrix. Within the immature matrix are fibroblastic foci consisting of aggregates of proliferating and collagen-producing myofibroblasts.7 Fibroblastic foci appear in the transition zone between normal lung and the abnormal fibrotic lung. There is a direct correlation between the numbers of foci on surgical lung biopsy with progressive physiologic deterioration and length of survival.8 Fibroblastic foci were initially thought to represent discrete lesions caused by recurrent acute lung injury. More recent studies demonstrate the fibroblastic foci to be the leading edge of a highly interconnected reticulum extending from the pleural into the underlying parenchyma.9 Clonality analysis shows a polyclonal fibroblast proliferation, supporting the idea that the primary pathologic lesion is a type of neoplasm which, rather than a malignant lesion, is composed of multiple sources of fibroblasts proliferating in a reactive process.9
Origin of Fibroblasts
Central to the formation of fibrotic lesions are the accumulation of fibroblasts and extracellular matrix. The origin of fibroblasts in lung fibrosis has been the subject of numerous studies. Current evidence suggests multiple sources which may vary with the type of injury or inciting events including proliferation of resident lung fibroblasts, differentiation of progenitor cells from the bone marrow, and transition of epithelial cells to a fibroblast phenotype, termed epithelial-mesenchymal transition (EMT). Bone marrow derived circulating fibroblast precursors are called fibrocytes.10 Fibrocytes have been shown to be a component of hypertrophic scars and keloids,11 scleroderma, kidney lesions,12 thickened airways caused by asthma13,14 and lung fibrosis15 in certain experimental models. Fibrocytes, defined by co-expression of CXCR4, a fibrocyte-associated chemokine receptor, procollagen I, α-SMA and prolyl-4-hydroxylase, were identified in lung tissue of 8/9 pulmonary fibrosis patients16 supporting a role of fibrocytes in human disease. A positive correlation of fibroblastic foci and lung fibrocytes (r = 0.79; p < 0.02) was shown but the extent of fibrocyte recruitment in multiple forms of pulmonary fibrosis is not known. The precise role of fibrocytes remains controversial because although cultured fibrocytes can be induced to differentiate into myofibroblasts in vitro, it is not clear that fibrocytes contribute to pathologic fibrosis in vivo.15,17,18 In the setting of fibrogenic injury, bone-marrow derived mesenchymal stem cells may actually be promoting repair and ameliorating fibrosis rather that causing persistent fibrotic lesions.19 Conflicting results using different stem cell pools and different animal models (reviewed in ref. 20) indicate that the role of fibrocytes is not resolved.
Another possible pathological source of lung fibroblasts is EMT. EMT is known to occur during embryogenesis and organogenesis. In some forms of metastatic epithelial-based malignancies, epithelial and mesenchymal cells undergo EMT.21 Primary human and rat alveolar epithelial cells and human bronchial and distal airspace epithelial cell lines can be induced to undergo EMT.22,23 Bleomycin lung injury and transgenic mice with TGFβ overexpression in the lung cause pulmonary fibrosis. In both models upregulation of mesenchymal markers, α-SMA and vimentin, were detected in cells co-expressing E-cadherin and surfactant protein-C (SP-C), which are markers of epithelial cells.23,24 Other studies have demonstrated colocalization of epithelial markers, such as thyroid transcription factor-1 or pro-surfactant protein B or C, with mesenchymal markers, α-SMA or N-cadherin in cells overlying fibroblastic foci in IPF.24,25 The precise role of EMT remains controversial because other studies have failed to detect dual expression of epithelial and mesenchymal markers in vivo in either clinical samples or the bleomycin model.26
Considered together, fibrocytes, EMT and activation of resident lung fibroblasts may contribute to lung fibrosis but the precise role for each process in disease burden remains unresolved. Understanding the relative contribution of these processes and the specific forms of fibrosis to which each process contributes is important, as there may be different pathways regulating these processes suggesting different targets for potential therapy.
Epithelial Injury and Pulmonary Fibrosis
A current model of IPF suggests that recurrent injury to pulmonary epithelial cells and ineffective repair leads to aberrant fibroblastic responses. The source of injury may be variable and include microbes and inhaled or systemic toxins. The epithelial injury leads to release of cytokines and growth factors that affect inflammatory cells including macrophages, neutrophils, lymphocytes, eosinophils and structural cells including endothelial cells, muscle cells, myofibroblasts and resident fibroblasts. In turn these other cells also release mediators that may affect migration, differentiation, proliferation and matrix synthesis and deposition of fibroblasts (reviewed in ref. 27 and 28). The alveolar epithelium functions as an adaptive entity that orchestrates the response to inhaled environmental challenges. Alveolar type II cells fine-tune alveolar homeostasis through continuous reciprocal interactions with resident phagocytic cells, mesenchymal and vascular cells by controlling surfactant production, releasing soluble growth factors and cytokines and direct cell-cell interactions. Mutations or injuries that induce cell stress may disrupt epithelial-mesenchymal interactions leading to fibroproliferation.29
While the complex cascade from epithelial cell injury-to-fibrosis is the subject of considerable research, transgenic mouse models that lead to alveolar type II epithelial cell death also lead to pulmonary fibrosis. These data directly demonstrate that injury to the respiratory epithelium is a cause of lung fibrosis.30 Mutations essential for alveolar epithelial cell function and integrity have been recently identified as the cause of discrete forms of pulmonary fibrosis including genetic deficiency of SP-C. Direct microbial injury of the lung epithelium by the Herpesviridae family of viruses has been shown to cause a fibrotic response in an experimental model.
SP-C Deficiency
Pulmonary surfactant is a lipoprotein complex that maintains alveolar stability during respiration and is critical for normal lung function. Surfactant deficiency due to premature birth or inactivation from injury results in respiratory distress. In the respiratory alveolus, SP-C enhances surface activity and innate immunity of the lung. Both of these functions prevent injury to the fragile gas exchanging epithelium suggesting that disruption of production or delivery of SP-C to the airspace could elicit alveolar damage. A variety of mutations in the SP-C gene (SFTPC) have been identified in individuals with extended family histories of lung disease that include various forms of interstitial pneumonia and fibrosis.31 Synthesis and secretion of SP-C is only detected in alveolar type II cells. Thus the consequence of SFTPC mutations would originate from stress to this subset of alveolar cells. More than 40 distinct mutations in the SFTPC gene have been identified. The disease phenotype follows an inheritance pattern of an autosomal dominant trait with variable penetrance and severity (45%) or arises spontaneously as disease caused by a de novo mutation on one allele (55%). Respiratory symptoms in patients with SFTPC mutations are identified from early immediate newborn to adulthood (71 years). 10–15% of affected patients develop respiratory symptoms within the first month of life, while 40% develop symptoms between 1 and 6 months of life. In adults with SFTPC mutations and chronic ILD, the histopathologic diagnosis is mixed forms of pulmonary fibrosis.
Transgenic mice with SP-C deficiency were generated by gene targeting. The SP-C deficient mice developed a strain-specific ILD that progressed with age to remodeling and fibrosis that is similar to the disease in affected humans.32 The severity of bleomycin-induced lung fibrosis was increased in the lungs of SP-C deficient mice, showing that absence of SP-C predisposes the lung to injury and subsequent fibrosis.33 While SFTPC mutations can cause ILD in adults, SFTPC specific mutations are a rare cause. In two recent studies of adults with mixed forms of pulmonary fibrosis including UIP, only one patient out of 124 was identified with a SFTPC mutation.
Viral Infection
Viral infection causes injury to the pulmonary epithelium. However unresolved infection or viral latency is recognized as a source of ongoing stress to the recovering epithelium that could eventually result in fibrotic injury. Attention has focused to the Herpesvirus family that can establish latency after acute infection. Ebstein-Barr virus (EBV) was found in lung tissue of almost half of patients with IPF, and in a separate study either EBV, cytomegalovirus, human herpesvirus 7, human herpesvirus 8 (also known as Kaposi's sarcoma herpesvirus) were detected.34–36 Herpesviruses were detected in the lungs of non-IPF patients at a lower prevalence. Herpesvirus infection was also detected in the lungs of 15/23 individuals with IPF, with similar prevalence in sporadic, non-SFTPC-associated familial and SFTPC-associated cases, but none was detected in controls. The latter findings suggest that combinations of viral and mutational causes may underlie or exacerbate severity of pulmonary fibrosis. In studies of genome-wide expression patterns, patients with IPF undergoing acute exacerbations had expression patterns consistent with epithelial injury and proliferation as key molecular and genetic events. However expression of overwhelming inflammatory response associated genes, that would indicate a response to viral or bacterial infections, was not detected.37
Lack of Effective Fibrosis Therapy
Currently there is no clear or consistent pathological explanation for the initiation of IPF. However the current specific examples of rare mutations or unresolved infection linked to IPF support the concept that events (genetic or otherwise) that compromise epithelial cell integrity initiate the eventual fibroblast expansion and pathology. Thus the term unexplained pulmonary fibrosis rather than idiopathic pulmonary fibrosis may be more appropriate as it is likely that additional diverse causes of fibrosis will be discovered as either the initiating and/or promoting process in this disease. Together, these uncertainties have contributed to the lack of effective therapy for lung fibrotic disease. Until recently, pulmonary fibrosis was thought to be initiated and propagated by inflammatory stimuli. Therefore, treatments were directed toward reducing inflammation using immunosuppressive agents including corticosteroids, cytotoxic and/or immunomodulatory agents. However, there is no evidence to date to support that immune suppression or modulation improves mortality in IPF, and in fact, may be harmful as patients suffer significant side effects from therapy.38 To date, there are no approved medical antifibrotic therapies for progressive pulmonary fibrosis; lung transplantation is the only measure shown to prolong survival. Recent animal and clinical studies have shown that fibrosis can occur without inflammation suggesting that other approaches to therapy are needed.
As the primary pathologic process in lung fibrosis is the expansion of fibroblasts and myofibroblasts into the gas-exchanging regions of the lung, an alternative approach is centered on developing therapeutics that directly target fibroblast and myofibroblast proliferation and matrix gene expression. Advances in molecular biology have defined specific, targetable signaling pathways mediating pathologic proliferation in a number of diseases such as malignancies. While fibroblasts activation is polyclonal and thus not malignant, a number of cellular processes in malignancy are also seen in fibroproliferation. Therefore fibrotic disease may prove sensitive to therapeutics successful in malignancy.
Epidermal Growth Factor Receptor (EGFR) and Pulmonary Fibrosis
EGFR (HER1) is a member of a receptor tyrosine kinase family including HER2/neu, HER3 and HER4. EGFR and its ligands, transforming growth factor-alpha (TGFα) and five other ligands have been identified in lungs or lung cells39–45 including the alveolar and airway epithelium, fibroblasts and macrophages.46–51 EGFR is activated directly or indirectly by inflammatory mediators including cytomegalovirus, endotoxin, TNFα and IL-13.47,52–54 EGFR activation regulates diverse cellular functions, many of which are associated with malignancy and fibrogenesis, including cell growth, proliferation, differentiation, migration, survival and transformation among others.55,56 TGFα was detected in the lung lavage fluid of all 10 patients with IPF, but not in lavage of 13 normal volunteers.57 Baughman demonstrated an increase in TGFα and EGFR by immunohistochemistry in IPF lung sections with TGFα identified in type II epithelial cells, fibroblasts and the vascular endothelium.51
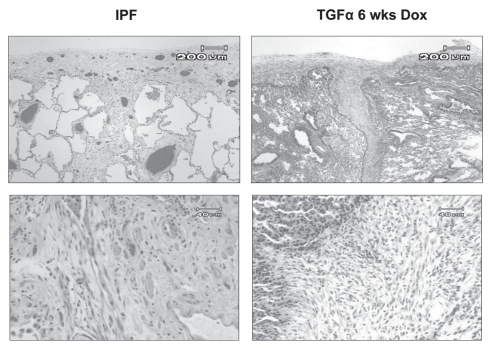
To further determine roles of EGFR-mediated lung remodeling transgenic mice expressing TGFα in the lung epithelium using the conditional Clara Cell Secretory Protein (CCSP) promoter were generated (hereafter referred to as CCSP/TGFα mice). TGFα was only expressed in bronchoalveolar and type II epithelial cells when mice are administered doxycycline (Dox). The transgenic TGFα expression in the respiratory epithelium of adult CCSP/TGFα mice caused progressive and extensive adventitial, interstitial and pleural fibrosis58 without inducing inflammatory cell influx. Proinflammatory cytokines were not induced as measured from lung homogenates. TGFβ1 protein levels or TGFβ activity were not increased.58 Several histological features of fibrosis in the TGFα model are similar to pathologic lesions of UIP including type II cell hyperplasia, pleural based fibrosis migrating into the interstitium, differentiation of myofibroblasts and areas of fibroblastic foci (Fig. 1).7,59–61 CCSP/TGFα mice on Dox develop progressive cachexia, changes in pulmonary function (increased airway resistance and elastance and decreased lung compliance) and they develop pulmonary hypertension.62,63 Gene expression profiles in Dox induced CCSP/TGFα mice were similar to expression profiles in IPF samples.64 These data support the following: (1) although EGFR activation can be initiated by inflammatory mediators, activation of EGFR induces fibrosis without inflammation showing that EGFR signaling is a downstream effecter in the cascade causing fibrosis; (2) progressive fibrosis without inflammation may model processes in clinical diseases such as IPF, which is not treatable with current anti-inflammatory therapies; (3) the initiation and progression of EGFR-mediated fibrosis in the absence of TGFβ activation shows that EGFR activation is a novel cause of pulmonary fibrosis. Therefore, the CCSP/TGFα mouse is a useful model to identify signaling pathways mediating pulmonary fibrosis and to test therapeutic approaches to treat progressive pulmonary fibrosis.
Figure 1.
Comparison of pathologic features on H&E staining of lung biopsy from patient with IPF with TGFα transgenic mice. Upper part (4X) reveals extension of pleural fibrosis into lung parenchyma. Lower part (20X) demonstrates focal areas of fibroblastic expansion within lung parenchyma.
Pharmacologic inhibition of phosphoinositide 3-kinase activity ameliorates TGFα-induced fibrosis.
As reviewed in previous sections, fibrosis is likely heterogeneous in etiology and molecular pathophysiology. Therefore, attempts to block or counteract single upstream pathways may not be sufficient to inhibit cellular processes associated with fibrosis. Ongoing studies have identified potential points of confluence, where multiple inputs eventually converge to elicit the cellular response of mesenchymal proliferation and matrix deposition. Phosphoinositide 3-kinase (PI3K) is activated by several fibrogenic growth factors including EGFR, platelet derived growth factor and non-canonical TGFβ.65 PI3K catalyzes the phosphorylation of phosphatidylinositol (4,5)-biphosphate to form phosphatidylinositol (3,4,5)-triphosphate. The tumor suppressor phosphatase and tensin homolog (PTEN) is a negative regulator of the PI3K-Akt pathway. In normal tissues, PTEN is thought to be constitutively high. Genomic studies of human tumor samples showed detection of mutations in Akt or in the Akt regulatory proteins PI3K and PTEN in one third to one half of all cancers.66 Inhibiting the PI3K pathway and its downstream effectors is being evaluated as a potential therapeutic goal in a number of malignancies. As activation of PI3K is essential to a number of cellular processes associated with fibrosis including cell growth, proliferation, migration, survival and collagen gene expression,65 PI3K may also prove an effective target in lung fibrosis.
Data supporting PI3K signaling in pulmonary fibrosis are demonstrated in several animal models of fibrosis including CCSP/TGFα. PI3K inhibition at the time of TGFα induction in CCSP/TGFα mice completely prevented fibrosis and physiologic changes. PI3K inhibition 4 weeks after development of fibrosis prevented further progression; further evidence for the PI3K pathway in fibrosis is demonstrated in other fibrosis models. Both cell proliferation and collagen production are upregulated in lung fibroblasts deficient in PTEN.67 In PTEN haploinsufficient mice, using both cutaneous wound healing and bleomycin-induced lung injury models, deficiency in PTEN resulted in a more durable fibroproliferative response.68 In a regulatable TGFβ1 transgenic mouse model fibrosis was significantly attenuated when mice were treated with an Akt inhibitor.69 Together these data strongly support PI3K signaling as a common pathway where multiple fibrogenic cytokines and growth factors converge or synergistically function.
Inhibition of the mTOR pathways reduces TGFα-induced pulmonary fibrosis.
The mammalian target of rapamycin (mTOR) is a highly conserved intracellular serine/threonine kinase and a major downstream component of the PI3K pathway.70 mTOR isoforms (C1 and C2) regulate the rate of cell growth, proliferation and protein synthesis in a number of cell types including fibroblasts, vascular smooth muscle and epithelial cells.71,72 Inhibitors of mTOR, such as rapamycin, bind to an intracellular cytoplasmic receptor, the FK506-binding protein-12.70 The complex formed then interacts and disrupts mTOR function causing cell cycle arrest in the G1 phase. In addition to blocking cell proliferation, mTOR inhibitors have been identified with anti-inflammatory, anti-tumor and anti-fibrotic properties. In rodent models of renal fibrosis and cirrhosis, rapamycin treatment has either reversed or prevented fibrosis.73,74 In pulmonary fibrosis models the rapamycin analog SDZ RAD prevented bleomycin-induced pulmonary fibrosis although it was unclear whether changes in lung inflammation may have contributed to these improvements.75 Rapamycin administered to CCSP/TGFα mice at the time of TGFα induction prevented the development of lung fibrosis and prevented changes in pulmonary function.76 Rapamycin administered as a rescue therapy was effective in preventing progression of fibrosis but only partially corrected endpoints including lung collagen content, lung mechanics or changes in body weight.
Role of EMT in CCSP/TGFα Fibrosis
Comparison between PI3K and mTORC1 inhibition studies in CCSP/TGFα mice demonstrated that inhibition of either pathway was effective in preventing the initiation of fibrosis and blocking progression of established fibrosis when administered as a rescue therapy. However, fibrosis endpoints persisted despite inhibitor treatment demonstrating that prevention of fibrosis does not ensure reversal once the fibrotic process is initiated. Together these studies show the PI3K-mTORC1 pathways are contributory, but not exclusive, pathways mediating fibrosis maintenance and support investigation of other fibrogenic pathways. A greater understanding of the cellular origin of fibroblast accumulation after TGFα expression may be valuable if the regulation of cellular transdifferentiation into a fibroblasts phenotype differs from the regulation of resident fibroblasts proliferation.
To more definitively test the capacity of alveolar epithelial cells for EMT, mice expressing β-galactosidase (β-gal) exclusively in lung epithelial cells were generated and their fate followed in the CCSP/TGFα model. Triple transgenic mice containing one copy of the rat CCSP, tetO-CMV-Cre and floxed ROSA26 (CCSP/Cre/ROSA) were generated by crossing. Floxed ROSA26 is a silent form of the B-gal gene that permits tracking cell fate. Dox administration to CCSP/Cre/ROSA mice would induce the lung epithelial-specific Cre-recombinase enzyme that in turn mediated activation of the silent Bgal gene resulting in genetically tagged lung epithelial cells that permanently express β-gal. CCSP/Cre/ROSA mice were then placed on Dox and sacrificed. Lung epithelial cell-specific expression of the β-gal marker was confirmed by several techniques. Frozen lung sections were stained with B-gal reactive substrate-x-gal. Further confirmation of β-gal was performed by staining paraffin-embedded lung sections with anti β-gal antibodies. CCSP/Cre/ROSA mice on Dox demonstrated localized x-gal staining as well as anti β-gal antibody reactivity of alveolar and airway epithelial cells on Dox with minimal to no epithelial staining off Dox and no mesenchymal staining on and off Dox. These essential control experiments indicate specific epithelial cell staining is achieved for subsequent lineage tracing during experimentally induced fibrosis.
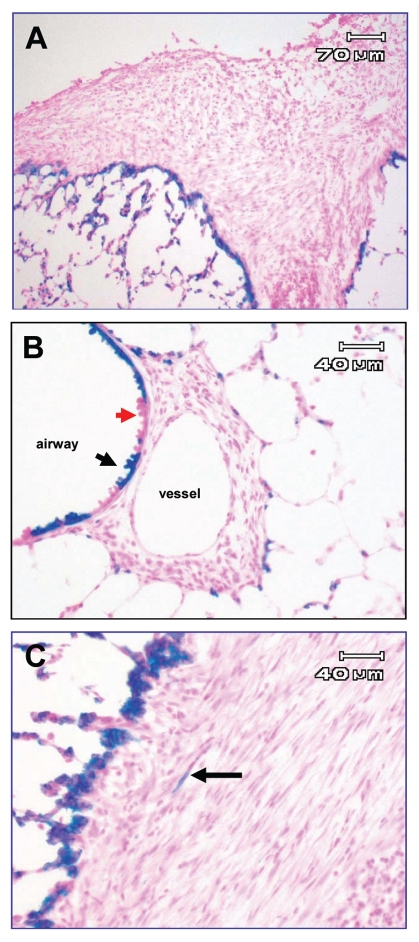
To determine if TGFα-induced fibrosis involved EMT, TGFα transgenic mice were bred to previously mentioned triple transgenic mice, generating quadruple transgenic mice (CCSP/Cre/ROSA/TGFα) thereby allowing evaluation of epithelial contribution in the progression of TGFα-induced fibrosis. A shift of B-gal reactivity from epithelial to mesenchymalderived cells would identify the degree of EMT that occurred. Controls included CCSP/Cre/ROSA/TGFα mice off Dox, and triple transgenic mice lacking ROSA expression (CCSP/TGFα/Cre). CCSP/Cre/ROSA/TGFα mice off Dox control for leak, (non-induced recombination) or β-gal expression in the absence of Dox induction; CCSP/TGFα/Cre on Dox control for non-specific x-gal staining in fibrotic regions indicate the lack of non-ROSA26 (i.e., background) epithelial tagging. All groups of transgenic mice were placed on Dox and sacrificed at weekly interval between 3 to 8 weeks of Dox and lungs examined to determine if developing or mature fibrotic regions revealed β-gal expression with x-gal and anti β-gal antibodies. In the time intervals studied, quadruple transgenic mice on Dox exhibited progressive fibrotic lesion in airway and pleural regions as previously described (Fig. 2A and B). However there was minimal β-gal expression in either developing or mature fibrotic lesions despite robust staining in adjacent epithelial cells (Fig. 2C) indicative of a minor epithelial cell conversion (EMT) into cells of the fibrotic lesions. Controls did not exhibit β-gal expression when not on Dox or in the fibrotic regions on Dox in the absence of ROSA expression (not shown). The Dox induced Cre activation of the B-gal reporter is incomplete in some areas of the airway epithelium indicating recombination has not occurred in all epithelial cells (Fig. 2B, red versus black arrow). Thus the in vivo genetic recombination-activation of ROSA26-B-gal marker in these experiments may slightly underestimate the very modest EMT detected in TGFα induced fibrosis. In addition, we have previously demonstrated that alveolar epithelial cells are entrapped within the fibrotic regions of the TGFα transgenic mice.77 Thus, epithelial cells may become embedded in lung fibrotic regions without EMT.
Figure 2.
X-gal staining of frozen lung sections of CCSP/Cre/ROSA/TGFα mice following 4 weeks of Dox. Blue staining represents β-gal expression from epithelial ROSA-tagged cells. (A and B) are representative areas of pleural and adventitial fibrosis. Intense β-gal expression is detected on the leading edge of fibrotic regions but no staining in fibrotic regions. Red arrow in (B) demonstrates cells without ROSA recombination adjacent to cells with recombination (black arrow). (C) is higher power of separate fibrotic region with one β-gal-expressing cell (arrow) with morphologic appearance suggesting fibroblast.
TGFβ and FGF family member have been shown to activate specific transcription factors during EMT. These include evolutionarily conserved zinc-finger family of transcription factors Snail1 (Snail), Snail2 (Slug) and Smad-interacting protein (SIP1) or Zeb2, which is a hallmark of EMT. These factors act as transcriptional repressors of adheren junction and tight junction proteins leading to a progressive loss in epithelial characteristics and gain in expression of mesodermal markers associated with fibroblast phenotype.78–80 Therefore, to independently obtain evidence of TGFα mediated EMT, lung sections of CCSP/TGFα mice on Dox for selected intervals were immunostained for transcription factors Snail, Slug and Zeb2. Analysis did not demonstrate any staining at any time points during progression of TGFα-induced fibrosis (not shown). Together, these findings are consistent with minimal to no contribution by EMT in the development or progression of TGFα/EGFR-induced fibrosis. Our results contrast with recent studies reporting EMT in both bleomycin and adenoviral TGFβ1 models of lung fibrosis.21,81 These differences may reflect the distinct mechanisms of produced by different profibrotic mediators in what appears as a common endpoint disease. Both bleomycin and TGFβ1 models induce lung injury and inflammation to induced fibrosis. EMT may be more prevalent in acute lung injury and wound repair models, while less prominent in the non-inflammatory, proliferative TGFα/EGFR model.
Summary
Causes and treatments of pulmonary fibrosis remain significant research problems and as illustrated by IPF, the pathology is complex. The precise pathology likely involves deficiency of critical epithelial proteins that modulate processes involved in epithelial protection and repair including SP-C and EGFR signaling. Regardless of the etiology for disease initiation and cellular origins, therapy must also be directed to preventing progression or reversing this “fibrotic wave” in the lung interstitium. Studies utilizing the CCSP/TGFα transgenic mice suggest that PI3K/mTORC1 pathway regulation may be a useful target in some forms of fibrosis, possibly where EMT is not a pathologic process. Additionally, assessing levels of SP-C as a marker or potential therapeutic target may prove useful in future approaches to treating pulmonary fibrosis. Since pulmonary fibrosis is heterogeneous in origin and progression future therapeutic strategies will likely involve targeting multiple pathways.
Acknowledgements
The authors thank Dr. Susan Wert for assistance in immunostaining and interpretation of EMT studies. This work was supported by the National Institutes of Health HL086598 (W.D.H.), HL 082818 (J.S.H.), AI58795 (T.R.K.), HL90156 (J.A.W.) and HL61646, HL50046 (S.W.G.).
Abbreviations
- IPF
idiopathic pulmonary fibrosis
- UIP
usual interstitial pneumonia
- EMT
epithelial-mesenchymal transition
- SP-C
surfactant protein-C
- SFTPC
SP-C gene
- EBV
Ebstein-Barr virus
- EGFR
epidermal growth factor receptor
- TGF
transforming growth factor-alpha
- CCSP
clara cell secretory protein
- Dox
doxycycline
- PI3K
Phosphoinositide 3-kinase
- PTEN
tumor suppressor phosphatase and tensin homolog
- mTOR
mammalian target of rapamycin
- -gal
-galactosidase
- Snail
snail1
- Slug
snail2
- SIP1
smad-interacting protein
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12268
References
- 1.Ambalavanan N, Carlo WA. Bronchopulmonary dysplasia: new insights. Clin Perinatol. 2004;31:613–628. doi: 10.1016/j.clp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Postma DS, Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:434–439. doi: 10.1513/pats.200601-006AW. [DOI] [PubMed] [Google Scholar]
- 3.Rennard SI. Chronic obstructive pulmonary disease: linking outcomes and pathobiology of disease modification. Proc Am Thorac Soc. 2006;3:276–280. doi: 10.1513/pats.200512-129SF. [DOI] [PubMed] [Google Scholar]
- 4.Weitzenblum E, Chaouat A, Canuet M, Kessler R. Pulmonary hypertension in chronic obstructive pulmonary disease and interstitial lung diseases. Semin Respir Crit Care Med. 2009;30:458–470. doi: 10.1055/s-0029-1233315. [DOI] [PubMed] [Google Scholar]
- 5.Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis. 2008;2:319–338. doi: 10.1177/1753465808096948. [DOI] [PubMed] [Google Scholar]
- 6.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 7.Crystal RG, Gadek JE, Ferrans VJ, Fulmer JD, Line BR, Hunninghake GW. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981;70:542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- 8.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 9.Cook DN, Brass DM, Schwartz DA. A matrix for new ideas in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:122–124. doi: 10.1165/ajrcmb.27.2.f245. [DOI] [PubMed] [Google Scholar]
- 10.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, et al. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 12.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 14.Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 15.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loebinger MR, Aguilar S, Janes SM. Therapeutic potential of stem cells in lung disease: progress and pitfalls. Clin Sci (Lond) 2008;114:99–108. doi: 10.1042/CS20070073. [DOI] [PubMed] [Google Scholar]
- 21.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGFbeta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X, et al. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse. Respir Res. 2007;8:1. doi: 10.1186/1465-9921-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada M, Kuwano K, Maeyama T, Hamada N, Yoshimi M, Nakanishi Y, et al. Dual-immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis. Histochem Cell Biol. 2008;129:453–462. doi: 10.1007/s00418-008-0388-9. [DOI] [PubMed] [Google Scholar]
- 27.Kottmann RM, Hogan CM, Phipps RP, Sime PJ. Determinants of initiation and progression of idiopathic pulmonary fibrosis. Respirology. 2009;14:917–933. doi: 10.1111/j.1440-1843.2009.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent GJ, McAnulty RJ, Hill M, Chambers R. Escape from the matrix: multiple mechanisms for fibroblast activation in pulmonary fibrosis. Proc Am Thorac Soc. 2008;5:311–315. doi: 10.1513/pats.200710-159DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 30.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- 33.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol. 2005;167:1267–1277. doi: 10.1016/S0002-9440(10)61214-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax. 1995;50:1234–1239. doi: 10.1136/thx.50.12.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, et al. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1999;159:1336–1341. doi: 10.1164/ajrccm.159.4.9807077. [DOI] [PubMed] [Google Scholar]
- 36.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41:2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180:167–175. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mapel DW, Samet JM, Coultas DB. Corticosteroids and the treatment of idiopathic pulmonary fibrosis. Past, present and future. Chest. 1996;110:1058–1067. doi: 10.1378/chest.110.4.1058. [DOI] [PubMed] [Google Scholar]
- 39.Kubiak J, Mitra MM, Steve AR, Hunt JD, Davies P, Pitt BR. Transforming growth factor-alpha gene expression in late-gestation fetal rat lung. Pediatr Res. 1992;31:286–290. doi: 10.1203/00006450-199203000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 41.Polosa R, Prosperini G, Leir SH, Holgate ST, Lackie PM, Davies DE. Expression of c-erbB receptors and ligands in human bronchial mucosa. Am J Respir Cell Mol Biol. 1999;20:914–923. doi: 10.1165/ajrcmb.20.5.3308. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes AM, Hamburger AW, Gerwin BI. Production of epidermal growth factor related ligands in tumorigenic and benign human lung epithelial cells. Cancer Lett. 1999;142:55–63. doi: 10.1016/s0304-3835(99)00166-4. [DOI] [PubMed] [Google Scholar]
- 43.Dammann CE, Nielsen HC, Carraway KL. Role of neuregulin-1beta in the developing lung. Am J Respir Crit Care Med. 2003;167:1711–1716. doi: 10.1164/rccm.200205-468OC. [DOI] [PubMed] [Google Scholar]
- 44.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem. 2003;278:26202–26207. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 45.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Rice AB, Adler K, Sannes P, Martin L, Gladwell W, et al. Vanadium stimulates human bronchial epithelial cells to produce heparin-binding epidermal growth factor-like growth factor: a mitogen for lung fibroblasts. Am J Respir Cell Mol Biol. 2001;24:123–131. doi: 10.1165/ajrcmb.24.2.4096. [DOI] [PubMed] [Google Scholar]
- 47.Madtes DK, Raines EW, Sakariassen KS, Assoian RK, Sporn MB, Bell GI, et al. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988;53:285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- 48.Stahlman MT, Orth DN, Gray ME. Immunocytochemical localization of epidermal growth factor in the developing human respiratory system and in acute and chronic lung disease in the neonate. Lab Invest. 1989;60:539–547. [PubMed] [Google Scholar]
- 49.Strandjord TP, Clark JG, Guralnick DE, Madtes DK. Immunolocalization of transforming growth factor-alpha, epidermal growth factor (EGF) and EGF-receptor in normal and injured developing human lung. Pediatr Res. 1995;38:851–856. doi: 10.1203/00006450-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Hardie WD, Bejarano PA, Miller MA, Yankaskas JR, Ritter JH, Whitsett JA, et al. Immunolocalization of transforming growth factor alpha and epidermal growth factor receptor in lungs of patients with cystic fibrosis. Pediatr Dev Pathol. 1999;2:415–423. doi: 10.1007/s100249900144. [DOI] [PubMed] [Google Scholar]
- 51.Baughman RP, Lower EE, Miller MA, Bejarano PA, Heffelfinger SC. Overexpression of transforming growth factor-alpha and epidermal growth factor-receptor in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:57–61. [PubMed] [Google Scholar]
- 52.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 53.Gallucci RM, Simeonova PP, Toriumi W, Luster MI. TNFalpha regulates transforming growth factor-alpha expression in regenerating murine liver and isolated hepatocytes. J Immunol. 2000;164:872–878. doi: 10.4049/jimmunol.164.2.872. [DOI] [PubMed] [Google Scholar]
- 54.Booth BW, Adler KB, Bonner JC, Tournier F, Martin LD. Interleukin-13 induces proliferation of human airway epithelial cells in vitro via a mechanism mediated by transforming growth factor-alpha. Am J Respir Cell Mol Biol. 2001;25:739–743. doi: 10.1165/ajrcmb.25.6.4659. [DOI] [PubMed] [Google Scholar]
- 55.Bogdan S, Klambt C. Epidermal growth factor receptor signaling. Curr Biol. 2001;1:292–295. doi: 10.1016/s0960-9822(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 56.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 57.Madtes DK, Rubenfeld G, Klima LD, Milberg JA, Steinberg KP, Martin TR, et al. Elevated transforming growth factor-alpha levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:424–430. doi: 10.1164/ajrccm.158.2.9711112. [DOI] [PubMed] [Google Scholar]
- 58.Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:741–749. doi: 10.1152/ajplung.00208.2003. [DOI] [PubMed] [Google Scholar]
- 59.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, et al. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung and Blood Institute working group. Am J Respir Crit Care Med. 2002;166:236–246. doi: 10.1164/rccm.2201069. [DOI] [PubMed] [Google Scholar]
- 60.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 61.Selman M, Pardo A. Idiopathic pulmonary fibrosis: misunderstandings between epithelial cells and fibroblasts? Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:165–172. [PubMed] [Google Scholar]
- 62.Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, et al. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-alpha-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:1217–1225. doi: 10.1152/ajplung.00020.2008. [DOI] [PubMed] [Google Scholar]
- 63.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, et al. Genomic profile of matrix and vasculature remodeling in TGFαlpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:309–321. doi: 10.1165/rcmb.2006-0455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, et al. Genomic Profile of Matrix and Vasculature Remodeling in TGF{alpha} Induced Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2007;37:309–321. doi: 10.1165/rcmb.2006-0455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 66.Sawyers CL. Will kinase inhibitors have a dark side? N Engl J Med. 2006;355:313–315. doi: 10.1056/NEJMcibr062354. [DOI] [PubMed] [Google Scholar]
- 67.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, et al. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, et al. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGFbeta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 71.Kramer S, Wang-Rosenke Y, Scholl V, Binder E, Loof T, Khadzhynov D, et al. Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. Am J Physiol Renal Physiol. 2008;294:440–449. doi: 10.1152/ajprenal.00379.2007. [DOI] [PubMed] [Google Scholar]
- 72.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol. 2005;98:722–731. doi: 10.1152/japplphysiol.00715.2004. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, et al. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol. 2007;27:495–502. doi: 10.1159/000106782. [DOI] [PubMed] [Google Scholar]
- 74.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22:79–84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 75.Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, et al. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J. 2002;19:1124–1127. doi: 10.1183/09031936.02.00281602. [DOI] [PubMed] [Google Scholar]
- 76.Korfhagen TR, Le Cras TD, Davidson CR, Schmidt SM, Ikegami M, Whitsett JA, et al. Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;41:562–572. doi: 10.1165/rcmb.2008-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikegami M, Le Cras TD, Hardie WD, Stahlman MT, Whitsett JA, Korfhagen TR. TGFalpha perturbs surfactant homeostasis in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;289:34–43. doi: 10.1152/ajplung.00407.2004. [DOI] [PubMed] [Google Scholar]
- 78.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. 2008;68:9574–9577. doi: 10.1158/0008-5472.CAN-08-2316. [DOI] [PubMed] [Google Scholar]
- 81.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]