Abstract
The pathological hallmark of Parkinson's disease (PD) is a selective and progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc). In the vast majority of cases the appearance of PD is sporadic, and its etiology remains unknown. Several postmortem studies demonstrate reduced levels of brain-derived neurotrophic factor (BDNF) in the SNc of PD patients. Application of BDNF promotes the survival of DA neurons in PD animal models. Here we show that BDNF signaling via its TrkB receptor tyrosine kinase is important for survival of nigrostriatal DA neurons in aging brains. Immunohistochemistry revealed that the TrkB receptor was expressed in DA neurons located in the SNc and ventral tegmental area (VTA). However, a significant loss of DA neurons occurred at 12-24 months of age only in the SNc but not in the VTA of TrkB hypomorphic mice in which the TrkB receptor was expressed at a quarter to a third of the normal amount. The neuronal loss was accompanied by a decrease in dopaminergic axonal terminals in the striatum and by gliosis in both the SNc and striatum. Furthermore, nigrostriatal DA neurons in the TrkB mutant mice were hypersensitive to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a mitochondrial complex I inhibitor that selectively kills DA neurons. These results suggest that BDNF-to-TrkB signaling plays an important role in the long-term maintenance of the nigrostriatal system and that its deficiency may contribute to the progression of PD.
Keywords: TrkB, neuronal survival, dopaminergic neurons, reactive gliosis, substantia nigra pars compacta, ventral tegmental area, MPTP
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by resting tremor, muscular rigidity, postural imbalance, and bradykinesia. The common pathological features of PD include a selective and progressive loss of dopaminergic (DA) neurons and deposition of cytoplasmic protein aggregates, termed Lewy bodies, in the substantia nigra pars compacta (SNc) (Dauer and Przedborski, 2003). Several genes responsible for familial forms of PD have been identified in recent years, including α-synuclein (Kruger, et al., 1998, Polymeropoulos, et al., 1997), parkin (Kitada, et al., 1998), UCH-L1 (Leroy, et al., 1998), DJ-1 (Bonifati, et al., 2003), and LRRK2 (Paisan-Ruiz, et al., 2004, Zimprich, et al., 2004). However, less than 10% of all diagnosed PD cases have a strict familial etiology (Payami and Zareparsi, 1998), and very few sporadic PD patients have mutations in any of these genes (Hu, et al., 1999, Scott, et al., 1999). Thus, the etiology of sporadic PD remains unknown.
Neurotrophins are a family of highly related, small, secreted proteins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5). They exert their effects on neurons mainly through Trk receptor tyrosine kinases. NGF specifically activates TrkA; NT-3 activates TrkC; and BDNF and NT-4/5 activate TrkB. Among them, BDNF and its TrkB receptor are most abundantly and widely expressed in the brain (Conner, et al., 1997, Yan, et al., 1997). BDNF promotes neuronal survival and differentiation and modulates synaptic plasticity by activating the TrkB receptor tyrosine kinase (Huang and Reichardt, 2001). Recently, viable conditional knockouts have been employed to demonstrate that deletion of the TrkB gene or the Bdnf gene in excitatory neurons of the dorsal forebrain causes dendritic degeneration and loss of neurons (Gorski, et al., 2003, Xu, et al., 2000b). These results suggest that neurotrophin deficiencies may cause neurodegeneration in some brain regions. Several post-mortem studies show that BDNF expression is reduced in the surviving DA neurons in the SNc of PD patients (Howells, et al., 2000, Mogi, et al., 1999, Parain, et al., 1999). In addition, application of BDNF protects SNc DA neurons from the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine in animals (Levivier, et al., 1995, Shults, et al., 1995, Tsukahara, et al., 1995). These observations suggest that diminished TrkB signaling in the SNc may contribute directly to the death of nigrostriatal DA neurons and the development of PD. Although ablation of the Bdnf gene impairs the survival and/or maturation of SNc DA neurons during development (Baquet, et al., 2005), it remains unknown if TrkB signaling is required for the long-term survival of these neurons in adults.
Here we report the development of a TrkB hypomorphic mutant, fBneo/fBneo, which expresses TrkB at 1/4-1/3 of the normal amount. Using this mouse strain we have found that a chronic reduction in TrkB signaling causes age-dependent and selective degeneration of SNc DA neurons and increases the vulnerability of these neurons to neurotoxins.
Materials and Methods
Animals
Mice were maintained on a 12 h/12 h light/dark cycle with food and water ad libitum. TrkB hypomorphic mutant mice were maintained on a mixed 129 and C57BL/6 background, and housed together with their respective control mice (either wild type or heterozygous littermates). We used polymerase chain reaction to genotype mice. The following PCR primers were used for the presence of the fBneo allele: 5'-AGCCCTGAGGTGCGCACCGATATCG-3' and 5'-CTCCAGAAGCCCAAGACCAGCAGGC-3'. All animal procedures were approved by the Georgetown University Animal Care and Use Committee.
Histology and Immunohistochemistry
Mice were anaesthetized with avertin and transcardially perfused with phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA). Brains were removed from the skull, post-fixed overnight, transferred to a 30% sucrose solution for 3-5 days, sectioned at 50 μm using a sliding microtome, and collected in PBS. Immunohistochemistry was performed as described previously (An, et al., 2008). Double-labeled immunohistochemistry was performed with the following modifications. For Nissl and tyrosine hydroxylase (TH) double-staining, sections were processed first for TH immunohistochemistry. Mounted sections were then stained with cresyl violet, dehydrated, and coverslipped with dibutyl phthalate-xylene mixture. For glial fibrillary acidic protein (GFAP) and TH double-labeling, sections were processed first for GFAP immunohistochemistry, developed in 0.04% 3,39- diaminobenzidine tetrahydrochloride (DAB; Sigma, Saint Louis, MO), 0.02% cobalt chloride and nickel sulfate (Fisher Scientific, Pittsburgh, PA), and 0.01% hydrogen peroxide dissolved in PBS for enhanced dark staining. After GFAP immunohistochemistry, sections were processed for TH immunohistochemistry, resulting in light brown staining. TH staining was used to outline boundaries of the SNc. Sources and dilutions of primary antibodies were as follows: TH (Chemicon, Temecula, CA, 1:1000; Sigma, Saint Louis, MO, 1:30000), β-galactosidase (Promega, Madison, WI, 1:300; Cappel, Aurora, OH, 1:2000), and GFAP (Sigma, Saint Louis, MO, 1:400). The same dilutions of primary antibodies were used for both immunoperoxidase and immunofluorescent detection procedures. Biotinylated and fluorescent dye-conjugated secondary antibodies were obtained from the Vector Laboratories, Inc (Burlingame, CA) or the Jackson ImmunoResearch Laboratories (West Grove, PA) and used according to the manufacturer's instructions. Sections were processed and developed simultaneously.
Analysis of TH+ terminals
Confocal images of TH immunoreactivity were obtained from the dorsal lateral striatum and nucleus accumbens using the same setting (i.e. laser intensity, gain, offset). Images were converted to gray-scale using Image J software (NIH). The threshold of the gray-scale images was adjusted, and the same parameters were used for all images. Number and size of TH+ puncta larger than 0.1 μm2 were measured using the particle analysis function of Image J. Four to six images per animal and 3-4 animals per genotype were analyzed.
Immunoblotting
Immunoblotting was performed as described previously (Gharami, et al., 2008) with the following modifications. Tissue samples were homogenized in the LW buffer (50 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, protease inhibitors, pH 7.4), and centrifuged at 13,000 rpm for 20 min at 4 °C. Protein extracts were denatured for 5 min at 100 °C, separated on SDS-PAGE gels, and transferred onto PVDF membranes. The membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 hour and then incubated with primary antibodies overnight at 4 °C. Sources and dilutions of primary antibodies were as follows: TrkB (Cell Signaling, Danvers, MA, 1:500), α tubulin (Sigma, Saint Louis, MO, 1:5000). After three washes in TBST (10 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.5% Tween-20), the membranes were incubated with an appropriate secondary antibody (IR-Dye 680 and/or IR-Dye 800, 1:10,000, LI-COR Biosciences, Lincoln, NE) for 1 hour at room temperature. The membranes were then washed three times with TBST, and proteins were visualized and quantified using the Odyssey® Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). The amount of TrkB protein was normalized to α tubulin.
Stereology
The total number of TH-immunoreactive neurons in the SNc and VTA was determined by unbiased stereological cell counting method (West, 1993). Using Stereo Investigator software (MicroBrightField, Williston, VT), measurements were performed on every third coronal section immunostained for TH, extending from the most rostral to the most caudal parts (5-10 histological sections per brain). The two hemispheres of each brain were analyzed separately. The boundary between the SNc and the VTA was defined by the medial lemniscus. For each stereological probe (10-12 probes for each brain) TH+ neurons were counted within a counting frame of 50 μm × 50 μm (a sampling site) and 10-15 sampling sites were randomly picked by the software within the outlined area. The counts were then extrapolated to estimate the total number of neurons in the SNc and the VTA. For Nissl+ and GFAP+ cell count, light TH staining was used to outline the SNc. For all probe runs the coefficient of error (CE Scheaffer) was less than 1.
MPTP treatment
TrkB hypomorphic mutant (fBneo/fBneo) and control mice (either wild type or heterozygous) at 8-10 weeks of age were used for MPTP injections. For 5 consecutive days, fBneo/fBneo and control mice received one i.p. injection per day of either MPTP/HCl (Sigma, Saint Louis, MO; 25 mg of free base per kg of body weight) or an equivalent volume of saline. 14 days after the last injection, mice were transcardially perfused with PBS and 4% PFA; brains were removed and processed for immunohistochemistry.
Statistical analysis
All data are expressed as mean ± SEM. All measurements were analyzed by either Student's t test using Exel software or two-way ANOVA using GraphPad Prism software. Post hoc comparisons were assessed with the Student's t test.
Results
The expression of the TrkB receptor in midbrain DA neurons
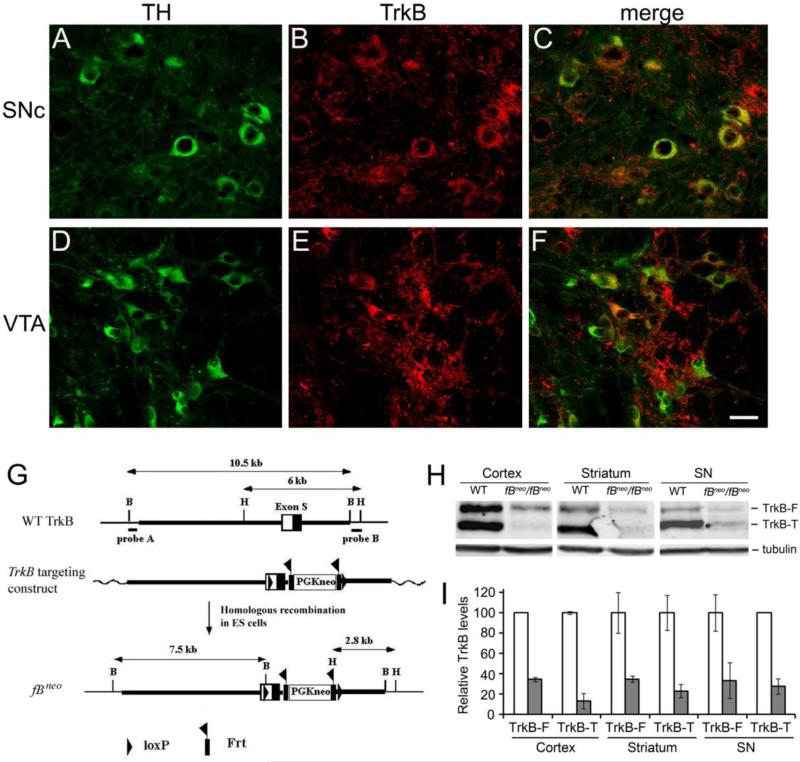
The TrkB receptor has been shown to be expressed in the SNc and VTA (Yan, et al., 1997), the midbrain regions that form the nigrostriatal pathway and the mesocorticolimbic pathway, respectively. To determine the extent of TrkB expression in DA neurons of these two brain regions, we took advantage of TrkBLacZ/+ knockin mice, where expression of β-galactosidase is under the control of the TrkB promoter and thus recapitulates the expression pattern of the TrkB gene (Xu, et al., 2000a). Fluorescent immunohistochemistry against tyrosine hydroxylase (TH) and β-galactosidase revealed that 60% (48/81) of DA neurons in the SNc (Figs. 1A-C) and 47% (57/121) in the VTA (Figs.1D-F) expressed the TrkB receptor.
Figure 1.
TrkB expression in DA neurons and generation of a hypomorphic TrkB allele, fBneo. (A-F) Expression of the TrkB receptor in DA neurons. Brain sections from TrkBLacZ/+ mice were stained with antibodies against tyrosine hydroxylase (TH) and β-galactosidase. In TrkBLacZ/+ mice β-galactosidase recapitulates the expression pattern of TrkB. Scale bar, 25 μm. (G) Schematic diagrams of the TrkB gene, targeting construct, and fBneo locus. The homology arms are represented in thick lines. B, BamHI; H, HindIII. (H) Representative immunoblots of full-length TrkB (TrkB-F) and truncated TrkB (TrkB-T). Protein extracts were prepared from the cortex, striatum, and substantia nigra of wild-type (WT) and fBneo/fBneo homozygous mice at 9 months of age, and probed with antibodies against the TrkB extracellular domain and α-tubulin. (I) Relative levels of TrkB-F and TrkB-T. TrkB levels were normalized to α-tubulin levels in the same samples (n=2 pairs of WT and mutant mice for the cortical samples and n=3 pairs of mice for other samples). The open bars represent WT mice and the filled bars fBneo/fBneo mice.
Generation of a TrkB hypomorphic mutant
A TrkB hypomorphic mutant was created during the process of generating a TrkB lox allele. A targeting construct was created to include one loxP site in the 5’untranslated region of exon S that encodes the signal peptide for the TrkB receptor, a PGKneo expression unit flanked by two frt sites in the intron downstream of exon S, and the second loxP site immediately downstream of the PGKneo expression unit (Fig. 1G). Through homologous recombination in mouse embryonic stem cells, we used this targeting construct to generate an fBneo allele (Fig. 1G). Because the PGKneo expression unit contains a polyadenylation signal sequence and multiple cryptic 3’ splice sites, its insertion in the TrkB locus likely interferes with the expression of the TrkB receptor. This prediction was confirmed by immunoblotting analyses of brain protein extracts (Fig. 1H). The TrkB gene produces two proteins: a full-length TrkB receptor (TrkB-F) and a truncated TrkB receptor (TrkB-T) that lacks the tyrosine kinase domain and thus is not able to activate receptor tyrosine kinase-mediated signaling cascades in neurons. Mice homozygous for the fBneo allele expressed TrkB-F at approximately 1/3 of the normal amount and TrkB-T at approximately 25% of the normal amount (Figs. 1H and 1I). This result indicates that fBneo is a hypomorphic TrkB allele. These hypomorphic mice survived well, which is different from another TrkB hypomorphic strain, fBZ/fBZ (Xu, et al., 2000b).
Selective degeneration of nigrostriatal DA neurons in aged fBneo/fBneo mice
To determine if DA neurons in the SNc are dependent on TrkB signaling for survival, we sectioned brains from fBneo/fBneo mice and littermate controls (either +/+ or fBneo/+) at 9, 12, 18, and 24 months of age, stained every third coronal section with antibodies to TH, and counted DA neurons in the SNc bilaterally using an unbiased stereological method (Fig. 2). The number of nigrostriatal DA neurons in fBneo/fBneo mice was similar to that in control mice at 9 months of age (Fig. 2C, P=0.699, n=3 pairs of mice). However, the number was significantly reduced by 22% at 12 months of age (P=0.017, n=3 pairs of mice), by 29% at 18 months of age (P=0.011, 4 control mice and 3 mutant mice), and by 26% at 24 months of age (P=0.004, n=3 pairs of mice), compared to age-matched control mice (Fig. 2C). Since TH immunoreactivity in surviving nigral DA neurons in fBneo/fBneo mice was similar to that in control mice at 18 months of age (Figs. 2A and 2B), the reduction in the number of TH-immunopositive (TH+) neurons in the SNc of fBneo/fBneo mice unlikely results from reduced TH expression. We confirmed cell loss by counting all Nissl stained neurons in the SNc at 18-24 months of age (Fig. 2D, 21% reduction, P=0.02, n=6 pairs of mice). To determine if loss of DA neurons is specific to the SNc, we counted DA neurons in the VTA bilaterally. Counts of DA neurons in the VTA were similar between fBneo/fBneo and control mice at 18 months of age (Fig. 2C, P=0.593, 4 control mice and 3 mutant mice). These results indicate that chronic reduction in TrkB signaling leads to late-onset and selective nigrostriatal degeneration.
Figure 2.
Degeneration of nigrostriatal DA neurons in fBneo/fBneo mice. (A and B) TH immunoreactivity in the SNc and VTA of fBneo/fBneo and control mice at 18 months of age. Scale bar, 500 μm. (C) The number of DA neurons in the SNc and VTA. The number of DA neurons in control mice is set at 100%. At each time point 3-4 mice per genotype were used. (D) Nissl+ neuronal counts in the SNc of mice at 18-24 months of age (6 mice per genotype). Student t test, *, P<0.05; **, P<0.01.
Loss of nigrosriatal DA projections in fBneo/fBneo mice
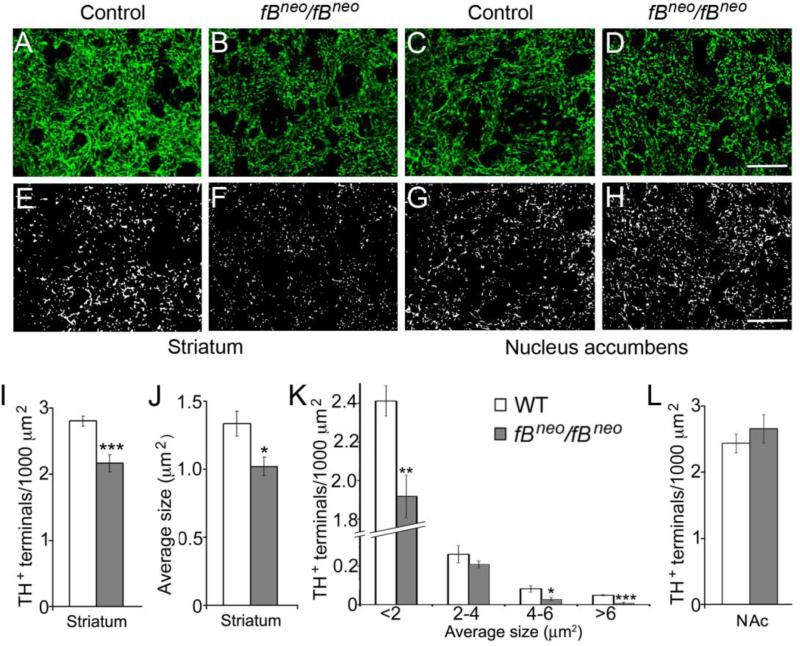
To determine whether loss of DA neurons in the SNc of fBneo/fBneo mice was accompanied by reduced number of nigrostriatal projections, we quantified TH+ nerve terminals in the dorsal striatum (Fig. 3). Confocal images revealed reduction in TH+ fiber density in the striatum of the fBneo/fBneo mice (Figs. 3A and 3B). To quantify TH+ nerve terminals, we adjusted the threshold to unmask TH+ puncta representing axonal endings (Figs. 3E and 3F). The total number and average size of TH+ nerve terminals per unit of striatal area were significantly reduced in fBneo/fBneo mice compared to controls at 18 months of age (Figs. 3I and 3J; 23% reduction in number, P=0.0007; 24% reduction in size, P=0.01). A further analysis based on the cross area of TH+ terminals showed significant reduction in most size bins (Fig. 3K). We obtained similar results using dopamine transporter (DAT) as a selective marker for DA terminals (data not shown). To determine whether the loss of nigrostriatal projections is selective, we analyzed TH+ projections from the VTA to the nucleus accumbens and found no difference between control and fBneo/fBneo mice (Figs. 3C, 3D, 3G, 3H, and 3L). Thus, loss of DA neurons in the SNc of fBneo/fBneo mice correlates with reduction in nigrostriatal innervation.
Figure 3.
Loss of nigrostriatal projections in fBneo/fBneo mice. (A-D) Representative confocal images of TH+ nerve terminals in the dorsal striatum and nucleus accumbens of control and fBneo/fBneo mice at 18 months of age. Scale bar, 25 μm. (E-H) The same images as panels A-D with increased threshold to visualize TH+ puncta. Scale bar, 25 μm. (I-K) Quantification of total number, average size, and size distribution of TH+ nerve terminals reveals a significant reduction of nigrostriatal projections in fBneo/fBneo mice. (L) No difference was found in the TH projections from the VTA to the nucleus accumbens (NAc). Student t test, *, P<0.05; **, P<0.01; ***, P<0.001.
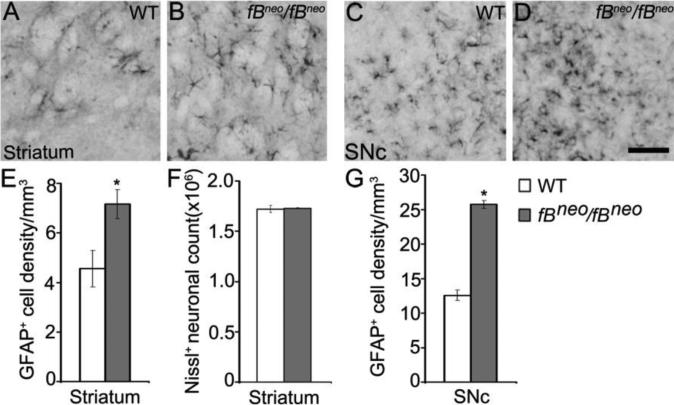
Reactive gliosis in the striatum of fBneo/fBneo mice
Reactive gliosis, the cellular manifestation of neuroinflammation, is a pathological hallmark of neurodegenerative diseases including PD (Forno, et al., 1992, Hirsch, et al., 1998). To evaluate levels of gliosis, we counted reactive astrocytes that expressed glial fibrillary acidic protein (GFAP) in the striatum (Figs. 4A and 4B) and SNc (Figs. 4C and 4D) of fBneo/fBneo and control mice. GFAP+ cell density was significantly increased in the striatum and the SNc of fBneo/fBneo mice (Figs. 4E and 4G), indicating increased inflammation that has been shown to accompany neuronal degeneration. In the striatum of fBneo/fBneo mice, reactive gliosis was most likely caused by axonal degeneration of nigrostriatal DA fibers, since we observed a reduction in the number and size of TH+ terminals (Fig. 3), but not in the total number of striatal neurons assessed by stereological count of Nissl+ cells (Fig. 4F).
Figure 4.
Reactive gliosis in the striatum of fBneo/fBneo mice. (A-D) Representative images of GFAP immunoreactivity in the striatum and SNc of control and fBneo/fBneo mice. Scale bar, 100 μm. (E) Increased GFAP+ cell density was observed in the striatum of fBneo/fBneo mice at 18months of age. (F) No changes were detected in the total number of striatal neurons. (G) Increased GFAP+ cell density was observed in the SNc of fBneo/fBneo mice at 18months of age. Student t test, *, P<0.05.
Nigrostriatal DA neurons in fBneo/fBneo mice are more vulnerable to MPTP
Administration of MPTP in mice results in pathology similar to PD, with high doses of MPTP leading to massive loss of nigrostriatal DA neurons (Dauer and Przedborski, 2003). Upon systemic administration, MPTP crosses the blood brain barrier and is converted to its active metabolite (MPP+), which is selectively taken up by DA neurons within the SNc but not the VTA (Muthane, et al., 1994, Seniuk, et al., 1990). The mechanisms of MPTP induced nigrostriatal DA cell death include both apoptosis and necrosis depending on the administration regimen (Jackson-Lewis, et al., 1995). It has been shown that a sub-acute dosing of MPTP daily over five days induces cell death by apoptosis (Tatton and Kish, 1997), the mechanism of cell death that leads to nigral degeneration in PD patients (Hartmann, et al., 2000, Tatton, et al., 1998). In order to determine whether DA neurons in fBneo/fBneo mice at 8-10 weeks of age are hypersensitive to insults, we chose a sub-chronic, low-dose treatment that consisted of one daily i.p. injection of either MPTP (25 mg/kg) or vehicle for five consecutive days. Animals were sacrificed 14 days after the last injection to ensure that apoptosis was complete and that neuronal count of TH+ cells was not affected by decreased TH expression. Stereological cell count of TH+ neurons in the SNc showed no difference between vehicle treated control and fBneo/fBneo mice (Figs. 5A, 5C, and 5E). However, in fBneo/fBneo mice MPTP caused a significantly greater reduction in the number of TH+ neurons in the SNc compared to control mice: 43% vs 28%, respectively (Figs. 5B, 5D, and 5E; P=0.0009). Furthermore, there was a strong interaction between genotype and MPTP treatment (F(1, 9) = 11.25, P=0.0085). These results suggest that a reduction in TrkB signaling renders nigrostriatal DA neurons more vulnerable to neurotoxin-induced cell death.
Figure 5.
Downregulation of TrkB expression increases vulnerability of nigrostriatal DA neurons to MPTP. (A-D) TH immunoreactivity in the brains of mice treated with MPTP. Mice at 8-10 weeks of age were injected i.p. once a day with MPTP 25 mg/kg for 5 days. Two weeks later, mice were killed and their brains were processed for immunohistochemistry. Scale bar, 250 μm. (E) Counts of nigrostriatal DA neurons. Student t test, ***, P<0.001.
Discussion
In this study we provide evidence that TrkB signaling is important for the survival of DA neurons in the SNc during aging and neurotoxin-induced insult. Using a newly developed TrkB hypomorphic mutant, we demonstrate that chronic deprivation of TrkB signaling leads to late-onset DA neuronal loss that does not appear until 12 months of age. Moreover, a reduction in DA cell number is selective for the SNc, since we did not find any changes in the VTA, which is a dopaminergic area shown to be spared in PD (Dymecki, et al., 1996). In addition, we show loss of nigrostriatal projections and reactive gliosis in the striatum and SNc of TrkB hypomophic mutant, neuropathological changes also observed in PD (Hartmann, et al., 2003). As the majority of DA neurons in the SNc express TrkB, it is likely that the observed nigrostriatal degeneration in TrkB hypomorphic mice is due to the loss of an autonomous TrkB function. However, we could not exclude the possibility that the TrkB deficiency is primarily affecting a non-SNc neuronal population that secondarily causes degeneration of SNc neurons. The late onset and selective DA neuronal loss in the TrkB mutant resemble the pathological process of PD. Deletion of the gene for the receptor of glial cell line-derived neurotrophic factor (GDNF), Ret, has also been shown to cause late onset and selective degeneration of nigrostriatal DA neurons (Kramer, et al., 2007). Thus, a reduction in levels of neurotrophic factors such as BDNF and GDNF or their downstream signaling may contribute to the pathogenesis of PD.
Kramer and colleagues used the DAT-Cre transgene to delete the TrkB gene in dopaminergic neurons and found a negligible role for TrkB in the development and maintenance of nigrostriatal DA neurons (Kramer, et al., 2007), which is contradictory with our observations. The discrepancy likely results from inefficient deletion of their TrkB lox allele in the SNc by DAT-Cre for unknown reasons, as evidenced by the presence of a normal amount of the TrkB receptor in the SNc of mutant mice on the basis of immunoblotting analysis (Kramer, et al., 2007).
In PD patients and neurotoxin-based animal models of PD, DA neurons in the SNc are more affected than in the VTA (Dauer and Przedborski, 2003). The mechanisms behind this selectivity are not understood. It has been suggested that possible differences in expression of cell death molecules or survival factors may contribute to hypersensitivity of the SNc to chronic and acute insults (Krieglstein, 2004). We found that the TrkB receptor was not a differentiating factor between the SNc and the VTA, since it was expressed in both brain regions at similar levels. Future studies might identify other trophic factors or their receptors responsible for the survival of DA neurons in the VTA.
Several post-mortem studies demonstrate that BDNF expression is reduced in the surviving DA neurons in the SNc of PD patients (Howells, et al., 2000, Mogi, et al., 1999, Parain, et al., 1999). These observations suggest that diminished TrkB signaling in the SNc may contribute directly to the death of nigrostriatal DA neurons and the development of PD. In fact, a recent study shows that deficiencies in TrkB signaling cause a loss of some nigrostriatal DA neurons (Zaman, et al., 2004) by using a TrkB hypomorphic mutant, fBZ/fBZ, that expresses the full-length TrkB at ~25% of the normal amount (Xu, et al., 2000a, Xu, et al., 2000b). However, as fBZ/fBZ mice do not survive for more than three months (Xu, et al., 2000a), they could not be used to examine age-dependent and progressive nigrostriatal degeneration.
MPTP reproduces PD pathology in both humans and mice by selectively affecting DA neurons in the SNc (Przedborski and Vila, 2003). A sub-acute chronic MPTP treatment has been shown to induce cell death by apoptosis (Tatton and Kish, 1997), a mechanism that contributes to PD neuronal loss (Vila and Przedborski, 2003). Low doses of MPTP do not cause massive neuronal loss but rather uncover if cells are hypersensitive to neurotoxic insult, as it has been shown before in other animal models (Kim, et al., 2005). We show that TrkB signaling protects DA neurons in the SNc from MPTP-induced cell death.
Currently available animal models of PD have greatly contributed to our understanding of pathogenesis of PD; however, there is still no optimal model available that recapitulates all cardinal features of PD. Although not displaying the more drastic aspects of PD, TrkB hypomorphic mutant still shows several important neuropathological characteristics of this disease. More detailed studies are necessary to further determine the mechanisms that protect vulnerable neurons against damaging processes of aging and insults, which may lead to the development of novel therapeutic strategies to treat neurodegenerative disorders like PD.
Acknowledgments
This work was supported by grants from the Michael J. Fox Foundation for Parkinson's Research, the American Parkinson Disease Association, and the National Institutes of Health (R01 NS050596) to BX and a grant from the National Institutes of Health to MB (F31 NS060523).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- The study created a TrkB hypomorphic mouse mutant with a normal lifespan
- This mutant displays selective and late nigrostriatal dopaminergic degeneration
- The degeneration accompanies reactive gliosis in substantia nigra and striatum
- Nigrostriatal dopaminergic neurons in the mutant are more vulnerable to MPTP
References
- 1.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 4.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 6.Dymecki J, Lechowicz W, Bertrand E, Szpak GM. Changes in dopaminergic neurons of the mesocorticolimbic system in Parkinson's disease. Folia Neuropathol. 1996;34:102–106. [PubMed] [Google Scholar]
- 7.Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW. Astrocytes and Parkinson's disease. Prog Brain Res. 1992;94:429–436. doi: 10.1016/s0079-6123(08)61770-7. [DOI] [PubMed] [Google Scholar]
- 8.Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice. J Neurochem. 2008;105:369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann A, Hunot S, Hirsch EC. Inflammation and dopaminergic neuronal loss in Parkinson's disease: a complex matter. Exp Neurol. 2003;184:561–564. doi: 10.1016/j.expneurol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 13.Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 14.Hu CJ, Sung SM, Liu H, Chang JG. No mutation of G209A in the alpha-synuclein gene in sporadic Parkinson's disease among Taiwan Chinese. Eur Neurol. 1999;41:85–87. doi: 10.1159/000008008. [DOI] [PubMed] [Google Scholar]
- 15.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 19.Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieglstein K. Factors promoting survival of mesencephalic dopaminergic neurons. Cell Tissue Res. 2004;318:73–80. doi: 10.1007/s00441-004-0920-8. [DOI] [PubMed] [Google Scholar]
- 21.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 22.Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson's disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 23.Levivier M, Przedborski S, Bencsics C, Kang UJ. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson's disease. J Neurosci. 1995;15:7810–7820. doi: 10.1523/JNEUROSCI.15-12-07810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson's disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- 25.Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994;126:195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- 26.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, de Munain AL, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R. Reduced expression of brain-derived neurotrophic factor protein in Parkinson's disease substantia nigra. Neuroreport. 1999;10:557–561. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- 28.Payami H, Zareparsi S. Genetic epidemiology of Parkinson's disease. J Geriatr Psychiatry Neurol. 1998;11:98–106. doi: 10.1177/089198879801100207. [DOI] [PubMed] [Google Scholar]
- 29.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 30.Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson's disease. Ann N Y Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- 31.Scott WK, Yamaoka LH, Stajich JM, Scott BL, Vance JM, Roses AD, Pericak-Vance MA, Watts RL, Nance M, Hubble J, Koller W, Stern MB, Colcher A, Allen FH, Jr., Hiner BC, Jankovic J, Ondo W, Laing NG, Mastaglia F, Goetz C, Pappert E, Small GW, Masterman D, Haines JL, Davies TL. The alpha-synuclein gene is not a major risk factor in familial Parkinson disease. Neurogenetics. 1999;2:191–192. doi: 10.1007/s100480050083. [DOI] [PubMed] [Google Scholar]
- 32.Seniuk NA, Tatton WG, Greenwood CE. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990;527:7–20. doi: 10.1016/0006-8993(90)91055-l. [DOI] [PubMed] [Google Scholar]
- 33.Shults CW, Kimber T, Altar CA. BDNF attenuates the effects of intrastriatal injection of 6-hydroxydopamine. Neuroreport. 1995;6:1109–1112. doi: 10.1097/00001756-199505300-00009. [DOI] [PubMed] [Google Scholar]
- 34.Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 35.Tatton NA, Maclean-Fraser A, Tatton WG, Perl DP, Olanow CW. A fluorescent double-labeling method to detect and confirm apoptotic nuclei in Parkinson's disease. Ann Neurol. 1998;44:S142–148. doi: 10.1002/ana.410440721. [DOI] [PubMed] [Google Scholar]
- 36.Tsukahara T, Takeda M, Shimohama S, Ohara O, Hashimoto N. Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery. 1995;37:733–739. doi: 10.1227/00006123-199510000-00018. discussion 739-741. [DOI] [PubMed] [Google Scholar]
- 37.Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 38.West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 39.Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000a;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000b;26:233–245. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- 41.Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]
- 42.Zaman V, Nelson ME, Gerhardt GA, Rohrer B. Neurodegenerative alterations in the nigrostriatal system of trkB hypomorphic mice. Exp Neurol. 2004;190:337–346. doi: 10.1016/j.expneurol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]