Abstract
BACKGROUND AND PURPOSE
The PPAR-γ agonist 15d-PGJ2 is a potent anti-inflammatory agent but only at high doses. To improve the efficiency of 15d-PGJ2, we used poly(D,L-lactide-co-glycolide) nanocapsules to encapsulate it, and function as a drug carrier system. The effects of these loaded nanocapsules (15d-PGJ2-NC) on inflammation induced by different stimuli were compared with those of free 15d-PGJ2.
EXPERIMENTAL APPROACH
Mice were pretreated (s.c.) with either 15d-PGJ2-NC or unloaded 15d-PGJ2 (3, 10 or 30 µg·kg−1), before induction of an inflammatory response by i.p. injection of either endotoxin (LPS), carrageenan (Cg) or mBSA (immune response).
KEY RESULTS
The 15d-PGJ2-NC complex did not display changes in physico-chemical parameters or drug association efficiency over time, and was stable for up to 60 days of storage. Neutrophil migration induced by i.p. administration of LPS, Cg or mBSA was inhibited by 15d-PGJ2-NC, but not by unloaded 15d-PGJ2. In the Cg model, 15d-PGJ2-NC markedly inhibited serum levels of the pro-inflammatory cytokines TNF-α, IL-1β and IL-12p70. Importantly, 15d-PGJ2-NC released high amounts of 15d-PGJ2, reaching a peak between 2 and 8 h after administration. 15d-PGJ2 was detected in mouse serum after 24 h, indicating sustained release from the carrier. When the same concentration of unloaded 15d-PGJ2 was administered, only small amounts of 15d-PGJ2 were found in the serum after a few hours.
CONCLUSIONS AND IMPLICATIONS
The present findings clearly indicate the potential of the novel anti-inflammatory 15d-PGJ2 carrier formulation, administered systemically. The formulation enables the use of a much smaller drug dose, and is significantly more effective compared with unloaded 15d-PGJ2.
Keywords: 15d-PGJ2, nanocapsules, PLGA, inflammation, PPAR-γ
Introduction
Inflammatory diseases involve the infiltration and activation of inflammatory and immune cells, releasing multiple inflammatory mediators that interact and activate structural cells at the sites of inflammation. Although they have a protective role in inflammation, tissue damage is a consequence of intense leucocyte migration, as observed in immune inflammatory diseases (Jones et al., 1991). The challenge in the development of new anti-inflammatory drugs has been to find appropriate targets that are essential in the inflammatory process but are mostly dispensable in terms of the host's defence against pathogens (Mackay, 2008).
Peroxisome proliferator-activated receptor (PPAR)-γ is a ligand-dependent transcription factor and member of the nuclear hormone receptor family, which has been characterized primarily for its role in regulating insulin and glucose metabolism (Brun and Spiegelman, 1997; Chinetti et al., 2000). A diverse set of natural and synthetic molecules have been classified as ligands that can induce activation and expression of PPAR-γ, such as thiazolidinediones and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (Lehrke and Lazar, 2005). We and others (Padilla et al., 2000; Napimoga et al., 2008a; Fukui et al., 2009; Hassumi et al., 2009; Housley et al., 2009; Pena-dos-Santos et al., 2009) have demonstrated that PPAR-γ ligands are capable of down-regulating most cells of the innate and adaptive immune system, with the agonists of PPAR-γ emerging as promising drugs for the treatment of inflammatory diseases. It has been reported that 15d-PGJ2 also interferes with nuclear factor κB (NF-κB) signalling, by inhibiting the phosphorylation of IκB kinase (which is mediated by the IKK complex), a negative regulator of the NF-κB pathway (Rossi et al., 2000; Straus et al., 2000). In addition, it has been demonstrated that whereas 15d-PGJ2 inhibits NF-κB transcription activity and NO production in primary microglial cells, the PPAR-γ agonist troglitazone has no significant effect, indicating that 15d-PGJ2 may be more effective than other PPAR-γ agonists (Petrova et al., 1999).
The development of new drugs and/or new formulations to treat chronic inflammatory diseases continues to be of considerable research interest. The pharmacological response to a drug is directly associated with its concentration at the required site of action. A non-specific distribution leads to high drug concentrations in healthy organs, tissues and cells, resulting in toxicity (Soppimath et al., 2001). To address these concerns, nanotechnology has been increasingly employed in drug delivery systems.
Polymeric nanoparticles are carrier systems that are classified as spheres or capsules, composed of natural or artificial polymers that must be biocompatible and biodegradable for drug delivery purposes. Nanocapsules consist of polymeric involucres around a nucleus, and are usually oily, while nanospheres are composed of a polymeric matrix alone (Fessi et al., 1989). It has been found previously that nanoencapsulation of drugs greatly prolongs their pharmacological activity and decreases their toxicity (Bernardi et al., 2009; Elron-Gross et al., 2009).
We previously demonstrated that a concentration of 1 mg·kg−1 of unloaded 15d-PGJ2 was necessary to reduce neutrophil migration to the peritoneal cavity by inhibiting inter-cellular adhesion molecule 1 expression (Napimoga et al., 2008a). Because conjugates can extend release pharmacokinetics and result in improved biodistribution, we designed this study to provide evidence on the effects of alternative delivery systems using lower doses of 15d-PGJ2, at levels that were previously had no effect on inflammation. To this end, the effects of systemic treatment with 15d-PGJ2-loaded nanocapsules (15d-PGJ2-NC) were studied using different inflammation models, and the cytotoxicity induced by 15d-PGJ2-NC or unloaded 15d-PGJ2 was explored. In addition, the interaction of the systems with erythrocytes was evaluated, in order to compare the anti-inflammatory effects of low doses of 15d-PGJ2-NC with those displayed by free 15d-PGJ2 in solution.
Methods
All drug and molecular target nomenclatures used in the manuscript are in accordance with the BJP's Guide to Receptors and Channels (Alexander et al., 2009).
Drugs and reagents
The materials used were: 15d-PGJ2 (Sigma Aldrich Chem. Co., USA), poly(D,L-lactide-co-glycolide) (PLGA, 50:50, m.w. 40 000–70 000 g·mol−1) (Sigma), sorbitan monostereate (Span60®) (Sigma), polysorbate 80 (Tween80®) (LabSynth, São Paulo, Brazil), caprylic/capric acid triglyceride (Miglyol 810®) (Hüls, Germany) and analytical grade acetone (LabSynth). The solvents used for chromatographic analyses were high performance liquid chromatography (HPLC) grade acetonitrile (JT Baker®, Phillipsburg, NJ, USA) and Milli-Q water.
Preparation of the PLGA nanocapsules with 15d-PGJ2
The PLGA nanocapsules were prepared by the nanoprecipitation method (Fessi et al., 1989), which involves mixing an organic phase into an aqueous phase. The organic phase consisted of PLGA polymer (100 mg), acetone (30 mL), 15d-PGJ2 (100 µg), sorbitan monostereate (40 mg) and caprylic/capric acid triglyceride (200 mg). The aqueous phase was composed of polysorbate 80 (60 mg) and deionized water (30 mL). After dissolution of the components of both phases, the organic phase was gradually added to the aqueous phase, and the suspension agitated for 10 min. The suspension was concentrated to a volume of 10 mL under low pressure, using a rotary evaporator, in order to obtain a suspension of 15d-PGJ2 with a final concentration of 10 µg·mL−1. A control formulation (without 15d-PGJ2) was also prepared, following the methodology described above.
Size and polydispersion measurements
The technique of dynamic light scattering was used to determine the average particle size (as the hydrodynamic diameter) and polydispersion. The nanocapsule suspensions (with or without 15d-PGJ2) were diluted ×100 (v·v−1) and measured using a ZetaPlus® particle analyser (Malvern, London, UK), at 25°C, with the detector at a fixed angle of 90°C. Size distributions and polydispersions were determined as the average of five measurements (Riley et al., 1999; Jie et al., 2005).
Zeta potential measurements
The value of the zeta potential, expressed in mV, was determined using a ZetaPlus potential analyser. The analyses were carried out by diluting the nanocapsule suspensions (with and without 15d-PGJ2) ×100 (v·v−1) in Milli-Q water, and the results are expressed as the means of eight measurements.
Efficiency of association of 15d-PGJ2 in the PLGA nanocapsules
The efficiency of association of 15d-PGJ2 with the nanocapsules was determined by the ultrafiltration/centrifugation method. Samples of nanocapsules containing 15d-PGJ2 were centrifuged in 30 kDa molecular size exclusion pore size regenerated cellulose ultrafiltration filters (Microcon, Millipore®, Billerica, MA, USA), and the filtrate was quantified by HPLC. The concentration of 15d-PGJ2 was determined using an analytical curve (concentration range 0.5–10 µg·mL−1, peak area = 7.68[15d-PGJ2]− 0.19, r = 0.9995, n = 6). The specificity was tested in the presence of the colloidal suspension components, and it was demonstrated that these factors did not affect 15d-PGJ2 quantification (data not shown).
The 15d-PGJ2 association efficiency was determined from the difference between the drug concentration measured in the filtrate and its total concentration (100%) in the nanocapsule suspension.
The chromatographic system consisted of a Varian ProStar HPLC, a PS 325 UV-Vis detector, a PS 210 solvent delivery module and a manual injector. Galaxy Workstation software was used for data collection and calculation. The chromatographic conditions were optimized using a C18 column (Phenomenex Gemini reversed phase, 5 µm, 110 A, 150 × 4.60 mm). The mobile phase consisted of 42:58 (v·v−1) acetonitrile/phosphate buffer (pH 3.5, 10 mmol·mL−1), at a flow rate of 1.0 mL·min−1. The mobile phase was filtered through a 0.22 µm Millipore nylon membrane filter. The wavelength monitored was 205 nm, and the injection volume was 20 µL. Peak areas were measured, and the analyses conducted at room temperature. The total concentration of 15d-PGJ2 (100%) in the PLGA nanocapsule suspension was determined after the suspension had been diluted in acetonitrile.
Animals
A total of 221 male Balb/c mice (20–25 g) were used in the present study. The animals were kept in appropriate cages in a temperature-controlled room, with a 12 h dark/light cycle. Free access to water and food was provided, and an acclimatization period of about 7 days in the laboratory was used before the experiment. All animals were manipulated in accordance with the Guiding Principles in The Care and Use of Animals, approved by the Council of the American Physiologic Society. This study was approved by the Animal Ethics Committee of the University of Uberaba (# 047/2009).
Carrageenan-induced peritonitis
Mice were pretreated (30 min, s.c.) with 200 µL of saline, 15d-PGJ2 (3, 10 or 30 µg·kg−1) or 15d-PGJ2-NC (3, 10 or 30 µg·kg−1). Peritonitis was induced by i.p. administration of carrageenan (500 µg per cavity; 200 µL). In order to test whether empty nanocapsules could affect neutrophil migration, a control group of mice were pretreated with 200 µL of empty nanocapsules (corresponding to the highest dose, 30 µg·kg−1) followed by injection of saline or i.p. carrageenan (500 µg per cavity; 200 µL). Four hours after carrageenan administration, the mice were killed by CO2 inhalation, the peritoneal cavity was washed for 3 min with 3 mL phosphate-buffered saline (PBS) containing 1 mM ethylenediamine tetraacetic acid, and the solution recovered.
Neutrophil migration induced by LPS
Mice were treated s.c. with either saline (200 µL), 15d-PGJ2 (3, 10 or 30 µg·kg−1) or 15d-PGJ2-NC (3, 10 or 30 µg·kg−1); 30 min later, LPS (100 ng per cavity) was injected i.p. Four hours after LPS administration all mice were killed by CO2 inhalation, the peritoneal cavity washed for 3 min with 3 mL of PBS containing 1 mM ethylenediamine tetraacetic acid, and the solution recovered.
Immunization procedure
On day 0, the mice received a single subcutaneous injection of 500 µg of the proteic antigen methylated bovine serum albumin (mBSA) in 200 µL of an emulsion containing 100 µL of saline and 100 µL of complete Freund's adjuvant. The mice received booster injections of mBSA dissolved in incomplete Freund's adjuvant on days 7 and 14. Non-immunized (NI) mice were given similar injections, but without the antigen. Twenty-one days after the initial injection, the immunized and NI mice were treated with 15d-PGJ2 (3, 10 or 30 µg·kg−1) or 15d-PGJ2-NC (3, 10 or 30 µg·kg−1), and challenged with either mBSA (30 µg per cavity, i.p.) or saline control (immunized mice). The selection of the mBSA dose was based on a previous study showing that this amount was effective in inducing massive neutrophil migration (Vieira et al., 2009).
Leucocyte and neutrophil counts
Total counts were performed in a Newbauer chamber, and differential cell counts (100 cells total) were carried out on cytocentrifuge (Cientec, Piracicaba, SP, Brazil) slides stained with Rosenfeld. Data are reported as means ± SD of the number of neutrophils per cavity, and each count was carried out at least twice, with differences of less than 10%.
Cytokine measurements
TNF-α, IL-1β and IL-12p70 levels in the exudates from carrageenan-challenged mice were detected by enzyme-linked immunosorbent assay using protocols supplied by the manufacturer (R&D Systems, Minneapolis, MN, USA). After all standard procedures, the optical density was measured at 490 nm. Results are expressed as pg·mL−1 of each cytokine, based on the standard curves.
Determination of serum 15d-PGJ2 concentrations
Mice were injected with the highest dose (30 µg·kg−1) of either 15d-PGJ2 or 15d-PGJ2-NC, and after 1, 2, 4, 8, 12 and 24 h, five animals from each group were placed under deep anaesthesia, and blood samples collected by cardiac puncture. The blood samples were centrifuged at 1300×g, at 4°C for 10 min. The supernatant was rapidly frozen, and stored at −70°C for later measurement of 15d-PGJ2 levels using specific enzyme-linked immunosorbent assay kits, according to the recommendations of the supplier (Assay Designs, Ann Arbor, MI, USA). Results are expressed as pg·mL−1 of 15d-PGJ2, determined from the standard curves.
Cellular toxicity: haemolytic assays
Haemolytic effects induced by the PLGA nanocapsule and 15d-PGJ2-NC suspensions were determined using the percentage of haemoglobin released from human erythrocytes (0.15% haematocrit) obtained from Hemocentro/Unicamp (protocol 396/03, approved by the Ethics in Research Committee, FCM/Unicamp). Red blood cells were treated with PLGA nanocapsule and 15d-PGJ2-NC suspensions, to provide amounts ranging from 6.25 to 125 ng. The cells were kept at 37°C for 15 min, and then centrifuged (at 1500×g) for 3 min and the amount of haemoglobin present in the supernatant determined at a wavelength of 412 nm. Data are expressed as percentage of haemolytic effect (Malheiros et al., 2004).
Statistical analysis
Data are reported as means ± SD, and the number of animals per group was five or six (as indicated in the Figure legends). The means from different treatments were compared using anova. When statistically significant differences were identified, individual comparisons were subsequently made using Bonferroni's t-test for unpaired values. Statistical significance was set at P < 0.05. The in vitro experiment was repeated twice, with similar results.
Results
15d-PGJ2-NC characterization
Both the 15d-PGJ2-NC and the unloaded nanocapsules presented a homogeneous macroscopic appearance, as opalescent bluish-white liquids. All results of the characterization of the 15d-PGJ2-NC suspensions are presented in Table 1. The hydrodynamic diameters and polydispersions of the suspensions were compatible with those of colloidal materials. The polydispersion values were lower than 0.2, which is considered ideal as this indicates that the particle size distribution falls within a narrow range. The zeta potential of the formulation indicated good solution stability, with values near −30 mV reflecting the presence of strong forces of repulsion, which tend to prevent aggregation (Schaffazick et al., 2003). A high encapsulation efficiency (77%) of 15d-PGJ2 with the PLGA nanocapsules can be explained by the fact that 15d-PGJ2 is a lipophilic compound (Figure 1).
Table 1.
Hydrodynamic diameters, polydispersions and zeta potentials of suspensions of 15d-PGJ2 loaded in PLGA nanocapsules, as a function of time
| Parameter | Time zero | 60 days |
|---|---|---|
| Diameter (nm) | 158.0 ± 0.8 | 164.5 ± 1.8 |
| Polydispersion | 0.082 ± 0.006 | 0.116 ± 0.012 |
| Zeta potential (mV) | −24.6 ± 4.3 | −30.3 ± 3.2 |
Data shown are means ± SD.
Figure 1.
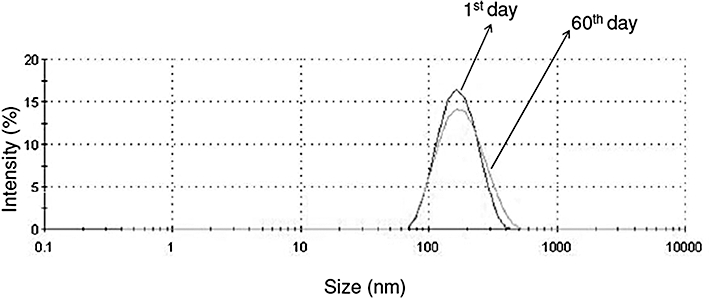
Particle size distribution (nm) by intensity (%) of the 15d-PGJ2 loaded in the PLGA NC formulation, at 0 and 60 days.
The physico-chemical stability of NC suspensions containing 15d-PGJ2 was evaluated based on measurements of size, polydispersion and zeta potential, as a function of time (time zero and after 60 days). All samples were stored in amber glass flasks at room temperature. Figure 1 shows the particle size distribution of the PLGA NC containing 15d-PGJ2, obtained from the dynamic light scattering intensity measurements. The size distribution of the formulation remained constant and unimodal throughout the 60 days. The polydispersion index, size and zeta potential did not show any statistically significant differences as a function of time (Table 1).
Effect of 15d-PGJ2 encapsulation on neutrophil migration induced by carrageenan or LPS
Compared with PBS administration, the injection of carrageenan (Cg, 500 µg per cavity, i.p.) significantly increased neutrophil migration in the mice (Figure 2A,B). Pretreatment with 15d-PGJ2-NC (1, 3 or 30 µg·kg−1; s.c.) strongly decreased the neutrophil migration induced by i.p. injection of Cg, measured 4 h later (Figure 2A) (P < 0.05). However, pretreatment with unloaded 15d-PGJ2 (1, 3 or 30 µg·kg−1; s.c.) did not reduce neutrophil migration (Figure 2B). Compared with PBS administration, the injection of LPS (100 µg per cavity, i.p.) significantly increased neutrophil migration (Figure 2C,D). LPS-induced neutrophil migration was also significantly inhibited by treatment of the mice with 15d-PGJ2-NC (1, 3 or 30 µg·kg−1; s.c.) (Figure 2C). On the other hand, pretreatment with unloaded 15d-PGJ2 (1, 3 or 30 µg·kg−1; s.c.) did not reduce LPS-induced neutrophil migration (Figure 2D).
Figure 2.
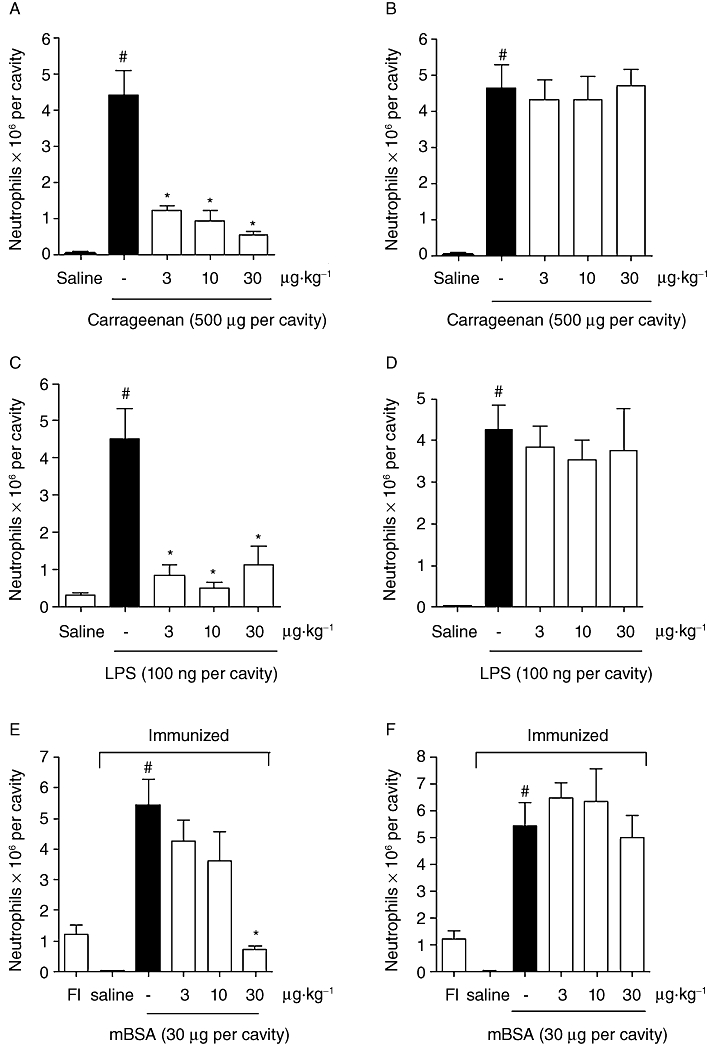
Anti-inflammatory effect of 15d-PGJ2-NC or unloaded 15d-PGJ2 under different inflammatory stimuli. The mice were treated with saline (0.2 mL, s.c.) or 15d-PGJ2-NC (3, 10 or 30 µg·kg−1, s.c.), and then injected i.p. with Cg at a dose of 500 µg per cavity (A), LPS at a dose of 100 ng per cavity (C), or mBSA challenge at a dose of 30 µg per cavity (E); sham immunized mice (FI) without the mBSA antigen were used as control. Another set of mice was treated with saline (0.2 mL, s.c.) or unloaded 15d-PGJ2 (3, 10 or 30 µg·kg−1, s.c.), and then injected i.p. with Cg at a dose of 500 µg per cavity (B), LPS at a dose of 100 ng per cavity (D), or mBSA challenge at a dose of 30 µg per cavity (F). Neutrophil migration was evaluated after 4 h. The values are means (±SD) of 6 animals per group. *P < 0.05 compared with carrageenan, LPS or mBSA group; #P < 0.05 compared with saline group (anova followed by Bonferroni's t-test).
Inhibition of the accumulation of neutrophils induced by immune peritonitis by low doses of 15d-PGJ2-NC
Intraperitoneal challenge of immunized mice with antigen (mBSA) induced a significant accumulation of neutrophils in the peritoneum compared with that induced by saline in immunized mice or by antigen in NI mice (Figure 2E,F). This accumulation of neutrophils was inhibited by treatment with 15d-PGJ2-NC (1, 3 or 30 µg·kg−1; s.c.) (Figure 2E). However, unloaded 15d-PGJ2 (1, 3 or 30 µg·kg−1; s.c.) did not have an inhibitory effect on this recruitment of neutrophils (Figure 2E,F).
In order to determine whether the empty nanocapsules induced any alteration in the immune responses of the mice, neutrophil migration was investigated in saline-injected and Cg-injected mice using empty nanocapsules. In neither case was there any significant change (P > 0.05) in neutrophil migration (Figure 3).
Figure 3.
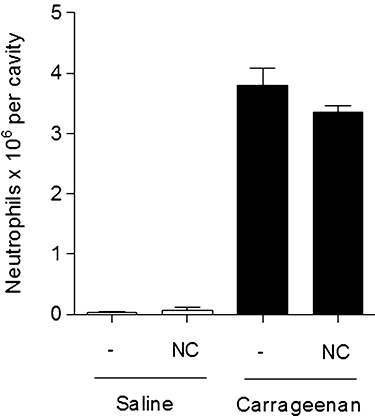
Effect of empty nanocapsules (NC) on the migration of neutrophils. Possible interference in homeostasis was evaluated using a quantity of NC corresponding to a dosage of 30 µg·kg−1. The mice were treated with saline (0.2 mL, s.c.) or with NC followed by injection with saline. A separate set of mice was treated with saline (0.2 mL, s.c.), or NC and then injected i.p. with carrageenan at a dose of 500 µg per cavity. Neutrophil migration was determined after 4 h. The values are means (±SD) of six animals per group.
Effect of low doses of 15d-PGJ2-NC on Cg-induced release of neutrophil chemotactic cytokines
The possible interference of 15d-PGJ2-NC in the release of TNF-α, IL-1β and IL-12p70 cytokines was investigated in order to examine the possibility that the regulation of neutrophil migration by 15d-PGJ2-NC could be related to a reduction in the release of neutrophil chemotactic mediators. There was a dose-dependent decrease in the levels of inflammatory cytokines in the peritoneal exudate of mice pretreated with 15d-PGJ2-NC and challenged with Cg, in contrast to mice pretreated with PBS and injected with Cg (Figure 4A,C and E). On the other hand, there was no reduction in the cytokine levels in the peritoneal exudate of animals treated with the same concentration (3, 10 or 30 µg·kg−1) of non-encapsulated 15d-PGJ2 (Figure 4B,D and F).
Figure 4.
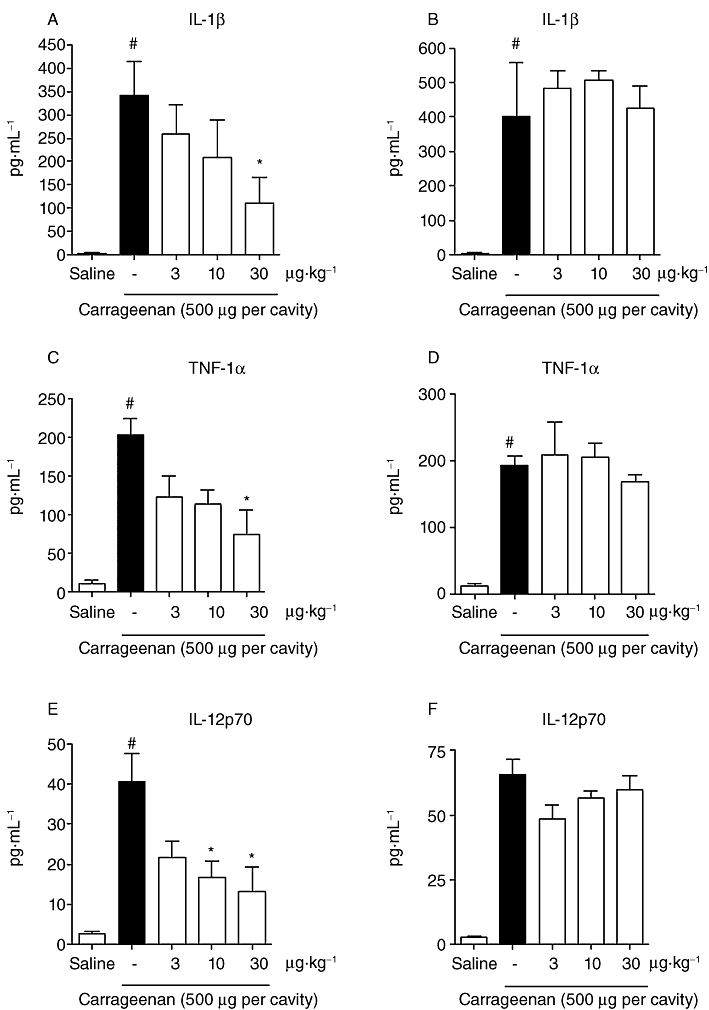
Effect of 15d-PGJ2-NC (3, 10 or 30 µg·kg−1) on (A) IL-1β (C) TNF-α and (E) IL-12p70, and effect of unloaded 15d-PGJ2 (3, 10 or 30 µg·kg−1) on (B) IL-1β (D) TNF-α and (F) IL-12p70, in serum of animals after i.p. injection of Cg (500 µg per cavity). The values are means (±SD) of six animals per group. *P < 0.05 compared with carrageenan group; #P < 0.05 compared with saline group (anova followed by Bonferroni's t-test).
Kinetics of 15d-PGJ2 in the serum
The release kinetics of encapsulated-15d-PGJ2 in mouse serum was determined at different points in time, and compared with results obtained for unloaded 15d-PGJ2 (Figure 5). High amounts of 15d-PGJ2 were released using 15d-PGJ2-NC (dose of 30 µg·kg−1), with a peak achieved between 2 and 8 h following its administration. Furthermore, high amounts of 15d-PGJ2 were still detected in the serum after 24 h, indicating a sustained release of 15d-PGJ2 from the carrier and explaining the more pronounced anti-inflammatory activity observed after treatment with encapsulated 15d-PGJ2. When the same concentration of non-encapsulated 15d-PGJ2 was administered, only low amounts of 15d-PGJ2 were detected in the serum, even after only a few hours. The area under the curve (AUC0–24) obtained for 15d-PGJ2-NC (590 519 ± 23 113) was significantly different (P < 0.001) from that obtained after treatment with non-encapsulated 15d-PGJ2 (178 020 ± 46 909).
Figure 5.
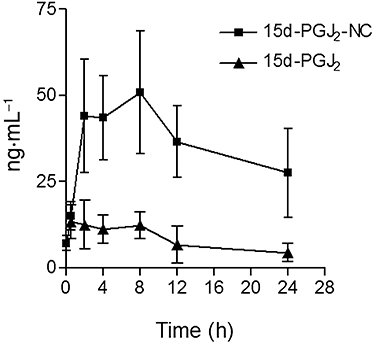
Kinetics of 15d-PGJ2 in the serum of the mice. Mice received subcutaneous injections of 15d-PGJ2-NC or unloaded 15d-PGJ2. After 1, 2, 4, 8, 12 and 24 h, a set of five animals from each group were killed and the concentration of 15d-PGJ2 in the serum measured by enzyme-linked immunosorbent assay. The values are expressed as ng·mL−1.
Haemolytic effects of the PLGA nanocapsule and 15d-PGJ2-NC suspensions
The concentration required for the onset of haemolysis (Conset) provoked by the PLGA nanocapsule and 15d-PGJ2-NC suspensions was 15.6 ng·mL−1 (corresponding to haemolysis rates of 1.79% and 1.38%, respectively). The low concentration (or light scattering by the volume of PLGA nanocapsules) did not permit determination of the total haemolytic concentration (Ctotal). However, the PLGA nanocapsule and 15d-PGJ2-NC suspensions showed low cytotoxicities, reflected in the haemolytic percentages (40% and 35%, respectively) obtained at the highest concentration tested (125 ng·mL−1) (Figure 6).
Figure 6.
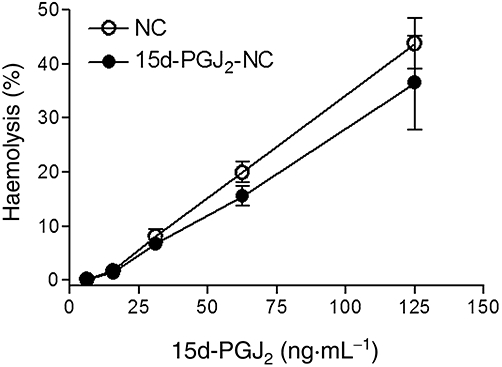
Haemolytic effects of the PLGA nanocapsule and of the PLGA nanocapsule containing 15d-PGJ2 suspensions (0.15% haematocrit, pH 7.4, 37°C, n = 6 per group).
Discussion
Several studies have demonstrated that PPAR-γ agonists possess anti-inflammatory properties (Ricote et al., 1998; Kawahito et al., 2000; Napimoga et al., 2008a; Hassumi et al., 2009). PPAR-γ agonists therefore represent a novel class of immunomodulatory drugs that could be useful for the management of inflammatory diseases such as arthritis, sepsis, inflammatory pain and colitis, among others (Cuzzocrea et al., 2003; Shan et al., 2004; Kaplan et al., 2005; Chima et al., 2008; Napimoga et al., 2008b; Pena-dos-Santos et al., 2009). However, it has been shown that 15d-PGJ2 is extremely efficient, compared with some of the commercial PPAR-γ agonists, partly due to its mechanism of intracellular action. Thiazolidinediones, or 15d-PGJ2, bind to the PPAR-γ/RXR heterodimer that competes for recruitment of the coactivators CREB binding protein (CBP) and p-300 (Varga and Nagy, 2008). The limited availability of these coactivators inhibits the activation of the transcription factors activator protein-1 (AP-1), NF-kB, signal transducer and activation of transcription, and nuclear factor of activated T cells, and consequently inhibits pro-inflammatory gene expression. However, 15d-PGJ2 may also exert PPAR-γ-independent anti-inflammatory effects through direct modification of cysteine residues of the inhibitor-kB kinase and NF-kB subunits, and direct inhibition of the extracellular regulated kinase (ERK1/2) (Zingarelli and Cook, 2005). Thus, the use of 15d-PGJ2 as a pharmacological tool may be more effective than the use of other PPAR-γ agonists.
It has been estimated that >50% of the 15d-PGJ2 added exogenously to a biological system binds to albumin, while in cardiomyocyte cell cultures >80% were found to bind to culture medium supplemented with 10% FCS, with ∼16% binding to (or being metabolized by) cardiomyocytes, leaving <4% detectable by liquid chromatography/tandem mass spectrometry (Rajakariar et al., 2007). For this reason it has been necessary to use quantitatively larger amounts of pure 15d-PGJ2 (1 mg·kg−1) in inflammatory models, in order to observe an anti-inflammatory effect (Kawahito et al., 2000; Kaplan et al., 2005; Napimoga et al., 2008a). These findings justified our investigation of the anti-inflammatory effects of exogenously administered 15d-PGJ2, loaded into PLGA nanocapsules to minimize losses and improve efficiency.
As with any new drug carrier system, a key question is whether the drug encapsulated in the carrier has retained its biological activity. The present results demonstrate that 30 µg·kg−1 of 15d-PGJ2-NC has anti-inflammatory activity; this dose is 33 times smaller than the dose required to achieve the same action using unloaded 15d-PGJ2 (1 mg·kg−1) (Napimoga et al., 2008a). A further advantage of this method of drug delivery is the prolonged release of 15d-PGJ2, with high amounts of 15d-PGJ2 detectable in the serum even 24 h after its administration (Figure 4). Controversy exists over whether 15d-PGJ2 is synthesized in vivo, and whether it possesses relevant pathophysiological functions. However, in a previous study using the liquid chromatography/tandem mass spectrometry assay with samples obtained from a resolving inflammation, up to 5 ng·mL−1 of 15d-PGJ2 was observed in vivo, confirming that 15d-PGJ2 is an endogenously generated protective PGD2 metabolite formed during resolving inflammatory reactions (Rajakariar et al., 2007). Because PPAR-γ agonists, and especially 15d-PGJ2, possess anti-inflammatory effects, we have enhanced the biological effects of endogenously produced 15d-PGJ2. The peak concentration obtained during 15d-PGJ2 release from the nanocapsules was around 50 ng·mL−1, which is 10 times greater than that found by Rajakariar et al., (2007) during demonstration of the importance of 15d-PGJ2 in the resolution of inflammation. This may explain the observed decrease in the recruitment of neutrophils as well as pro-inflammatory cytokines. Conversely, the same amount of unloaded 15d-PGJ2 did not show anti-inflammatory activity.
It is difficult to maintain the stability of nanocapsules in circulation, while at the same time providing adequate local drug bioavailability in the target tissue (Goppert and Muller, 2005; Aggarwal et al., 2009). These properties are determined by the characteristics of nanocapsule systems, including particle size, shape, surface charge (zeta potential), solubility, surface modifications (including targeting), as well as by the route of administration. Interaction with proteins in the blood, which is influenced by particle size, polymer type and surface features, might cause steric stabilization of the nanocapsules, thus inhibiting protein binding and interaction with red blood cells, while increasing the blood circulation time, all of which influence the therapeutic effect of the drug (Cedervall et al., 2007; Aggarwal et al., 2009). The cytotoxicity of PLGA nanoparticles has been studied both in terms of the time required for blood clotting to occur and in relation to haemolytic effects. Haemolysis caused by PLGA nanocapsules was found to depend on the concentration (Kim et al., 2005) and the type of nanoparticles, with surfactant-modified PLGA showing more pronounced haemolytic effects compared with PEGylated PLGA nanocapsules (Cenni et al., 2008). However, PLGA nanocapsules normally presented low cytotoxicity, and blood compatibility, as was also observed in the present study.
Although more research is needed, the current findings clearly indicate the anti-inflammatory potential of the novel 15d-PGJ2 carrier formulation, applied by systemic administration. The 15d-PGJ2-NC suspensions showed good physicochemical stability, measured in terms of size, polydispersion and zeta potential, as these parameters did not change with time. Importantly, the formulation was significantly more effective than pure 15d-PGJ2, even using a much smaller drug dose, and was less cytotoxic. The maintenance of high levels of prostaglandin over longer periods, as obtained using this novel approach, could be an important step towards the control of inflammatory reactions.
Acknowledgments
The authors gratefully acknowledge financial support from the Brazilian funding agencies FAPESP, CNPq, FUNDUNESP and FAPEMIG (PPM 097/09). NFSM was supported by a research fellowship provided by CAPES.
Glossary
Abbreviations
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- 15d-PGJ2-NC
15d-PGJ2-loaded nanocapsules
- Cg
carrageenan
- IL-1β
interleukin 1β
- IL-12p70
interleukin 12p70
- LPS
endotoxin
- mBSA
methylated bovine serum albumin
- NF-κB
nuclear factor κ-light-chain enhancer of activated B cells
- PLGA
poly(D,L-lactide-co-glycolide)
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- TNF-α
tumour necrosis factor α
Conflict of interest
There is no conflict of interest to declare.
Supporting Information
Teaching Materials; Figs 1–6 as PowerPoint slide.
References
- Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A, Zilberstein AC, Jäger E, Campos MM, Morrone FB, Calixto JB, et al. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br J Pharmacol. 2009;158:1104–1111. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun RP, Spiegelman BM. PPAR-γ and the molecular control of adipogenesis. J Endocrinol. 1997;155:217–218. doi: 10.1677/joe.0.1550217. [DOI] [PubMed] [Google Scholar]
- Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni E, Granchi D, Avnet S, Fotia C, Salerno M, Micieli D, et al. Biocompatibility of poly(D,L-lactide-co-glycolide) nanoparticles conjugated with alendronate. Biomaterials. 2008;29:1400–1411. doi: 10.1016/j.biomaterials.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008;36:2849–2857. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Ianaro A, Wayman NS, Mazzon E, Pisano B, Dugo L, et al. The cyclopentenone prostaglandin 15-deoxy-delta(12,14)-PGJ2 attenuates the development of colon injury caused by dinitrobenzene sulphonic acid in the rat. Br J Pharmacol. 2003;138:678–688. doi: 10.1038/sj.bjp.0705077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elron-Gross I, Glucksam Y, Biton IE, Margalit R. A novel Diclofenac-carrier for local treatment of osteoarthritis applying live-animal MRI. J Control Release. 2009;135:65–70. doi: 10.1016/j.jconrel.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Fessi H, Puiseiux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–R4. [Google Scholar]
- Fukui N, Honda K, Ito E, Ishikawa K. Peroxisome proliferator-activated receptor gamma negatively regulates allergic rhinitis in mice. Allergol Int. 2009;58:247–253. doi: 10.2332/allergolint.08-OA-0047. [DOI] [PubMed] [Google Scholar]
- Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13:179–187. doi: 10.1080/10611860500071292. [DOI] [PubMed] [Google Scholar]
- Hassumi MY, Silva-Filho VJ, Campos-Júnior JC, Vieira SM, Cunha FQ, Alves PM, et al. PPAR-gamma agonist rosiglitazone prevents inflammatory periodontal bone loss by inhibiting osteoclastogenesis. Int Immunopharmacol. 2009;9:1150–1158. doi: 10.1016/j.intimp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Housley WJ, O'Conor CA, Nichols F, Puddington L, Lingenheld EG, Zhu L, et al. PPAR-γ regulates retinoic acid-mediated DC induction of Tregs. J Leukoc Biol. 2009;86:293–301. doi: 10.1189/jlb.1208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie P, Venkatraman SS, Min F, Freddy BYC, Huat GL. Micelle-like nanoparticles of star-branched PEO-PLA copolymers as chemotherapeutic carrier. J Control Release. 2005;110:20–33. doi: 10.1016/j.jconrel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Jones AK, al-Janabi MA, Solanki K, Sobnack R, Greenwood A, Doyle DV, et al. In vivo leucocyte migration in arthritis. Arthritis Rheum. 1991;34:270–275. doi: 10.1002/art.1780340304. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Cook JA, Hake PW, O'Connor M, Burroughs TJ, Zingarelli B. 15-Deoxy-delta(12,14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock. 2005;24:59–65. doi: 10.1097/01.shk.0000167108.88376.f2. [DOI] [PubMed] [Google Scholar]
- Kawahito Y, Kondo M, Tsubouchi Y, Hashiramoto A, Bishop-Bailey D, Inoue K, et al. 15-deoxy-delta(12,14)-PGJ(2) induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J Clin Invest. 2000;106:189–197. doi: 10.1172/JCI9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, El-Shall H, Dennis D, Morey T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf B Biointerfaces. 2005;40:83–91. doi: 10.1016/j.colsurfb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- Malheiros SVP, Pinto LMA, Gottardo L, Yockaichiya DK, Fraceto LF, Meirelles N, et al. A new look at the hemolytic effect of local anesthetics considering their real membrane/water partitioning at pH 7.4. Biophys Chem. 2004;110:213–221. doi: 10.1016/j.bpc.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Vieira SM, Dal-Secco D, Freitas A, Souto FO, Mestriner FL, et al. Peroxisome proliferator-activated receptor-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J2, reduces neutrophil migration via a nitric oxide pathway. J Immunol. 2008a;180:609–617. doi: 10.4049/jimmunol.180.1.609. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, et al. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J Pharmacol Exp Ther. 2008b;324:313–321. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- Padilla J, Kaur K, Cao HJ, Smith TJ, Phipps RP. Peroxisome proliferator activator receptor-γ agonists and 15-deoxy-Δ12, 14/12,14-PGJ2 induce apoptosis in normal and malignant B-lineage cells. J Immunol. 2000;165:6941–6948. doi: 10.4049/jimmunol.165.12.6941. [DOI] [PubMed] [Google Scholar]
- Pena-dos-Santos DR, Severino FP, Pereira SA, Rodrigues DB, Cunha FQ, Vieira SM, et al. Activation of peripheral kappa/delta opioid receptors mediates 15-deoxy-(Delta12,14)-prostaglandin J2 induced-antinociception in rat temporomandibular joint. Neuroscience. 2009;163:1211–1219. doi: 10.1016/j.neuroscience.2009.07.052. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-delta12,14-prostaglandin J2. Proc Natl Acad Sci USA. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Riley T, Govender T, Stolnik S, Xiong CD, Garnett MC, Illum L, et al. Colloidal stability and drug incorporation aspects of micellar-like PLA-PEG nanoparticles. Colloids Surf B Biointerfaces. 1999;16:147–159. [Google Scholar]
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Schaffazick SR, Guterres SS, Freitas LL, Pohlmann AR. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Quim Nova. 2003;26:726–737. [Google Scholar]
- Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A potential role of 15-deoxy-delta(12,14)-prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J Biol Chem. 2004;279:37939–37950. doi: 10.1074/jbc.M402424200. [DOI] [PubMed] [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-Deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Nagy L. Nuclear receptors, transcription factors linking lipid metabolism and immunity: the case of peroxisome proliferator-activated receptor gamma. Eur J Clin Invest. 2008;38:695–707. doi: 10.1111/j.1365-2362.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Vieira SM, Lemos HP, Grespan R, Napimoga MH, Dal-Secco D, Freitas A, et al. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br J Pharmacol. 2009;158:779–789. doi: 10.1111/j.1476-5381.2009.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


