Abstract
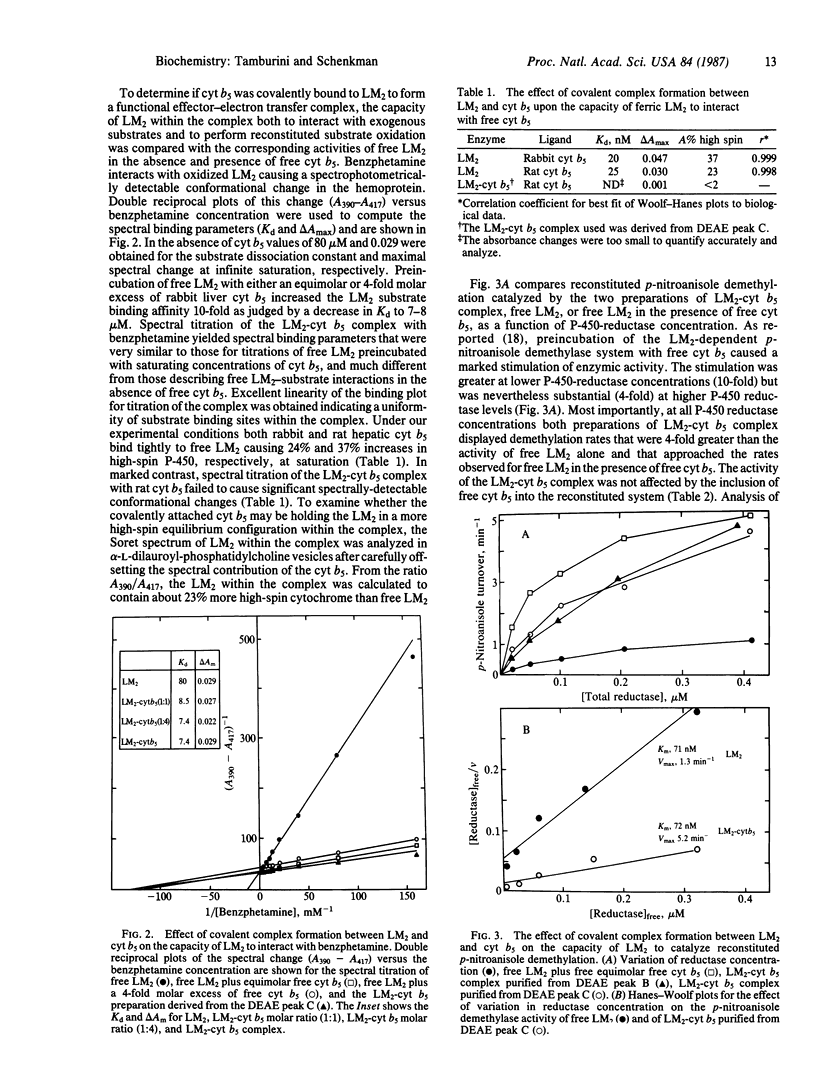
A covalent complex between rabbit hepatic microsomal cytochromes P-450 isozyme 2 (LM2) and b5 was created and purified to greater than 95% homogeneity. The purified complex was largely comprised of the two cytochromes covalently attached at the interface of the functional electron transfer-effector complex as shown by the following evidence. The spin state of the LM2 within the complex was greater than the spin state of free LM2, and the addition of free cytochrome b5 (cyt b5) did not further increase the spin state of the LM2 within the complex. The spectral binding parameters (Kd and delta Amax) for the association of benzphetamine with LM2 in the complex were identical to those observed with free LM2 in the presence of saturating concentrations of free cyt b5 and much different from those observed for LM2 in the absence of cyt b5. Reconstituted monooxygenase activity of the covalent LM2-cyt b5 complex (LM2-cyt b5) in the presence of NADPH-cytochrome P-450 reductase was much higher than the activity of free LM2 and approached the activity of free LM2 in the presence of optimal concentrations of free cyt b5. Furthermore, the Km for the flavoprotein in supporting either free LM2 or LM2-cyt b5-dependent p-nitroanisole demethylation were similar. (iv) Less than 20-25% of the cyt b5 within the complex could be reduced by free NADH-cytochrome b5 reductase (NADH-cyt b5 reductase) albeit at a slow rate. The implications of this data to the current understanding of the mechanism and stoichiometry of protein interactions in the hepatic mixed function oxidase system are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backes W. L., Sligar S. G., Schenkman J. B. Kinetics of hepatic cytochrome P-450 reduction: correlation with spin state of the ferric heme. Biochemistry. 1982 Mar 16;21(6):1324–1330. doi: 10.1021/bi00535a034. [DOI] [PubMed] [Google Scholar]
- Bechtold R., Bosshard H. R. Structure of an electron transfer complex. II. Chemical modification of carboxyl groups of cytochrome c peroxidase in presence and absence of cytochrome c. J Biol Chem. 1985 Apr 25;260(8):5191–5200. [PubMed] [Google Scholar]
- Bonfils C., Balny C., Maurel P. Direct evidence for electron transfer from ferrous cytochrome b5 to the oxyferrous intermediate of liver microsomal cytochrome P-450 LM2. J Biol Chem. 1981 Sep 25;256(18):9457–9465. [PubMed] [Google Scholar]
- Chiang J. Y. Interaction of purified microsomal cytochrome P-450 with cytochrome b5. Arch Biochem Biophys. 1981 Oct 15;211(2):662–673. doi: 10.1016/0003-9861(81)90502-6. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Strittmatter P. Characterization of the interaction of amphipathic cytochrome b5 with stearyl coenzyme A desaturase and NADPH:cytochrome P-450 reductase. J Biol Chem. 1980 Jun 10;255(11):5184–5189. [PubMed] [Google Scholar]
- Dailey H. A., Strittmatter P. Modification and identification of cytochrome b5 carboxyl groups involved in protein-protein interaction with cytochrome b5 reductase. J Biol Chem. 1979 Jun 25;254(12):5388–5396. [PubMed] [Google Scholar]
- Guengerich F. P. Oxidation-reduction properties of rat liver cytochromes P-450 and NADPH-cytochrome p-450 reductase related to catalysis in reconstituted systems. Biochemistry. 1983 Jun 7;22(12):2811–2820. doi: 10.1021/bi00281a007. [DOI] [PubMed] [Google Scholar]
- Hackett C. S., Strittmatter P. Covalent cross-linking of the active sites of vesicle-bound cytochrome b5 and NADH-cytochrome b5 reductase. J Biol Chem. 1984 Mar 10;259(5):3275–3282. [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Properties of electrophoretically homogeneous phenobarbital-inducible and beta-naphthoflavone-inducible forms of liver microsomal cytochrome P-450. J Biol Chem. 1976 Dec 25;251(24):7929–7939. [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Johansson I. Cytochrome b5 as electron donor to rabbit liver cytochrome P-450LM2 in reconstituted phospholipid vesicles. Biochem Biophys Res Commun. 1980 Nov 28;97(2):582–586. doi: 10.1016/0006-291x(80)90303-4. [DOI] [PubMed] [Google Scholar]
- Jansson I., Tamburini P. P., Favreau L. V., Schenkman J. B. The interaction of cytochrome b5 with four cytochrome P-450 enzymes from the untreated rat. Drug Metab Dispos. 1985 Jul-Aug;13(4):453–458. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D., Geren L. M., Millett F. Adrenodoxin interaction with adrenodoxin reductase and cytochrome P-450scc. Cross-linking of protein complexes and effects of adrenodoxin modification by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide. J Biol Chem. 1984 Aug 25;259(16):10025–10029. [PubMed] [Google Scholar]
- Mathews F. S. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45(1):1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Miwa G. T., West S. B., Huang M. T., Lu A. Y. Studies on the association of cytochrome P-450 and NADPH-cytochrome c reductase during catalysis in a reconstituted hydroxylating system. J Biol Chem. 1979 Jul 10;254(13):5695–5700. [PubMed] [Google Scholar]
- Morgan E. T., Coon M. J. Effects of cytochrome b5 on cytochrome P-450-catalyzed reactions. Studies with manganese-substituted cytochrome b5. Drug Metab Dispos. 1984 May-Jun;12(3):358–364. [PubMed] [Google Scholar]
- Ng S., Smith M. B., Smith H. T., Millett F. Effect of modification of individual cytochrome c lysines on the reaction with cytochrome b5. Biochemistry. 1977 Nov 15;16(23):4975–4978. doi: 10.1021/bi00642a006. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y., Lambeth J. D. NADPH-cytochrome P-450 reductase-cytochrome b5 interactions: crosslinking of the phospholipid vesicle-associated proteins by a water-soluble carbodiimide. Arch Biochem Biophys. 1985 Sep;241(2):386–396. doi: 10.1016/0003-9861(85)90561-2. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Pompon D., Coon M. J. On the mechanism of action of cytochrome P-450. Oxidation and reduction of the ferrous dioxygen complex of liver microsomal cytochrome P-450 by cytochrome b5. J Biol Chem. 1984 Dec 25;259(24):15377–15385. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Poulos T. L., Mauk A. G. Models for the complexes formed between cytochrome b5 and the subunits of methemoglobin. J Biol Chem. 1983 Jun 25;258(12):7369–7373. [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. Comparison of the binding sites on cytochrome c for cytochrome c oxidase, cytochrome bc1, and cytochrome c1. Differential acetylation of lysyl residues in free and complexed cytochrome c. J Biol Chem. 1980 May 25;255(10):4732–4739. [PubMed] [Google Scholar]
- Salemme F. R. An hypothetical structure for an intermolecular electron transfer complex of cytochromes c and b5. J Mol Biol. 1976 Apr 15;102(3):563–568. doi: 10.1016/0022-2836(76)90334-x. [DOI] [PubMed] [Google Scholar]
- Sligar S. G., Cinti D. L., Gibson G. G., Schenkman J. B. Spin state control of the hepatic cytochrome P450 redox potential. Biochem Biophys Res Commun. 1979 Oct 12;90(3):925–932. doi: 10.1016/0006-291x(79)91916-8. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Miki N., Miyake Y., Yamano T. Interaction and electron transfer between cytochrome b5 and cytochrome P-450 in the reconstituted p-nitroanisole O-demethylase system. J Biochem. 1982 Dec;92(6):1793–1803. doi: 10.1093/oxfordjournals.jbchem.a134109. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Miki N., Yamano T. NADH- and NADPH-dependent reconstituted p-nitroanisole O-demethylation system containing cytochrome P-450 with high affinity for cytochrome b5. J Biochem. 1980 May;87(5):1457–1467. doi: 10.1093/oxfordjournals.jbchem.a132887. [DOI] [PubMed] [Google Scholar]
- Tamburini P. P., Gibson G. G. Thermodynamic studies of the protein-protein interactions between cytochrome P-450 and cytochrome b5. Evidence for a central role of the cytochrome P-450 spin state in the coupling of substrate and cytochrome b5 binding to the terminal hemoprotein. J Biol Chem. 1983 Nov 25;258(22):13444–13452. [PubMed] [Google Scholar]
- Tamburini P. P., MacFarquhar S., Schenkman J. B. Evidence of binary complex formations between cytochrome P-450, cytochrome b5, and NADPH-cytochrome P-450 reductase of hepatic microsomes. Biochem Biophys Res Commun. 1986 Jan 29;134(2):519–526. doi: 10.1016/s0006-291x(86)80451-x. [DOI] [PubMed] [Google Scholar]
- Tamburini P. P., Schenkman J. B. Mechanism of interaction between cytochromes P-450 RLM5 and b5: evidence for an electrostatic mechanism involving cytochrome b5 heme propionate groups. Arch Biochem Biophys. 1986 Mar;245(2):512–522. doi: 10.1016/0003-9861(86)90244-4. [DOI] [PubMed] [Google Scholar]
- Tamburini P. P., White R. E., Schenkman J. B. Chemical characterization of protein-protein interactions between cytochrome P-450 and cytochrome b5. J Biol Chem. 1985 Apr 10;260(7):4007–4015. [PubMed] [Google Scholar]
- Vermilion J. L., Ballou D. P., Massey V., Coon M. J. Separate roles for FMN and FAD in catalysis by liver microsomal NADPH-cytochrome P-450 reductase. J Biol Chem. 1981 Jan 10;256(1):266–277. [PubMed] [Google Scholar]
- Waldmeyer B., Bechtold R., Bosshard H. R., Poulos T. L. The cytochrome c peroxidase.cytochrome c electron transfer complex. Experimental support of a hypothetical model. J Biol Chem. 1982 Jun 10;257(11):6073–6076. [PubMed] [Google Scholar]
- Waldmeyer B., Bosshard H. R. Structure of an electron transfer complex. I. Covalent cross-linking of cytochrome c peroxidase and cytochrome c. J Biol Chem. 1985 Apr 25;260(8):5184–5190. [PubMed] [Google Scholar]
- Weber H., Weis W., Staudinger H. Bestimmung des Standardredoxpotentials (pH 7.0) von Cytochrom b5 (Fe+2)-Cytochrom b5 (Fe+3) in mikrosomalen Membranen verschiedener Zustände. Hoppe Seylers Z Physiol Chem. 1971 Jan;352(1):109–110. [PubMed] [Google Scholar]



