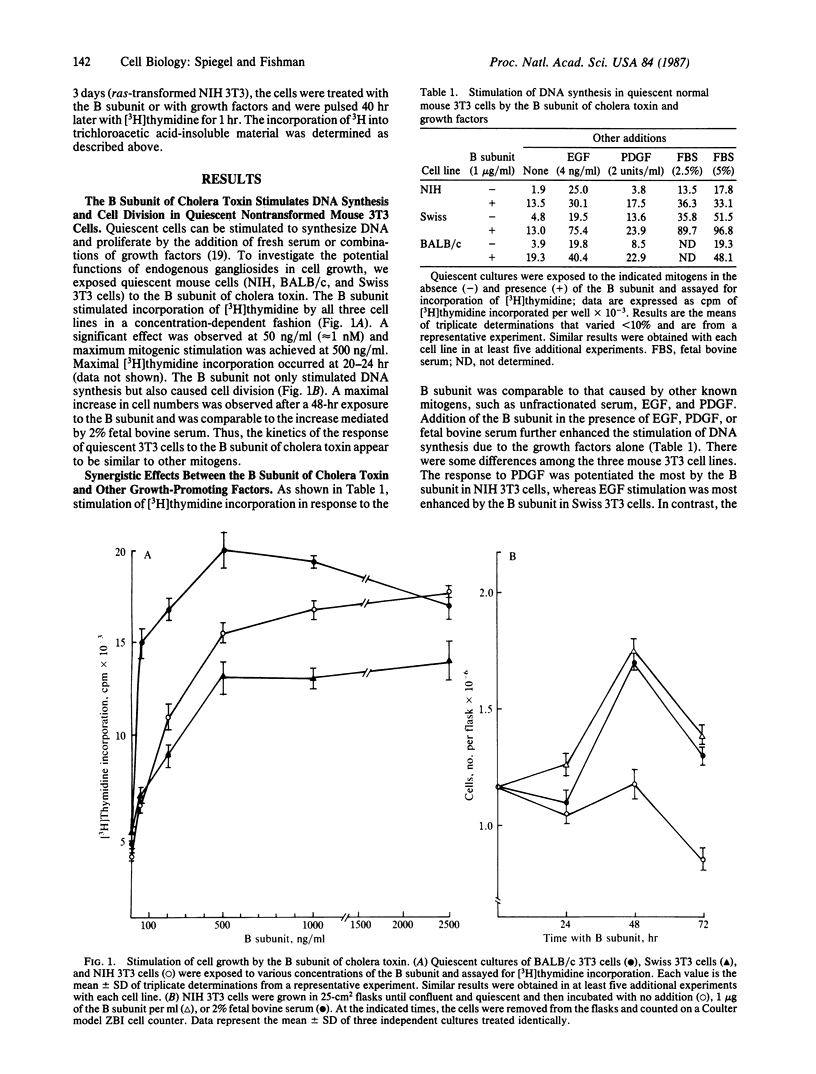
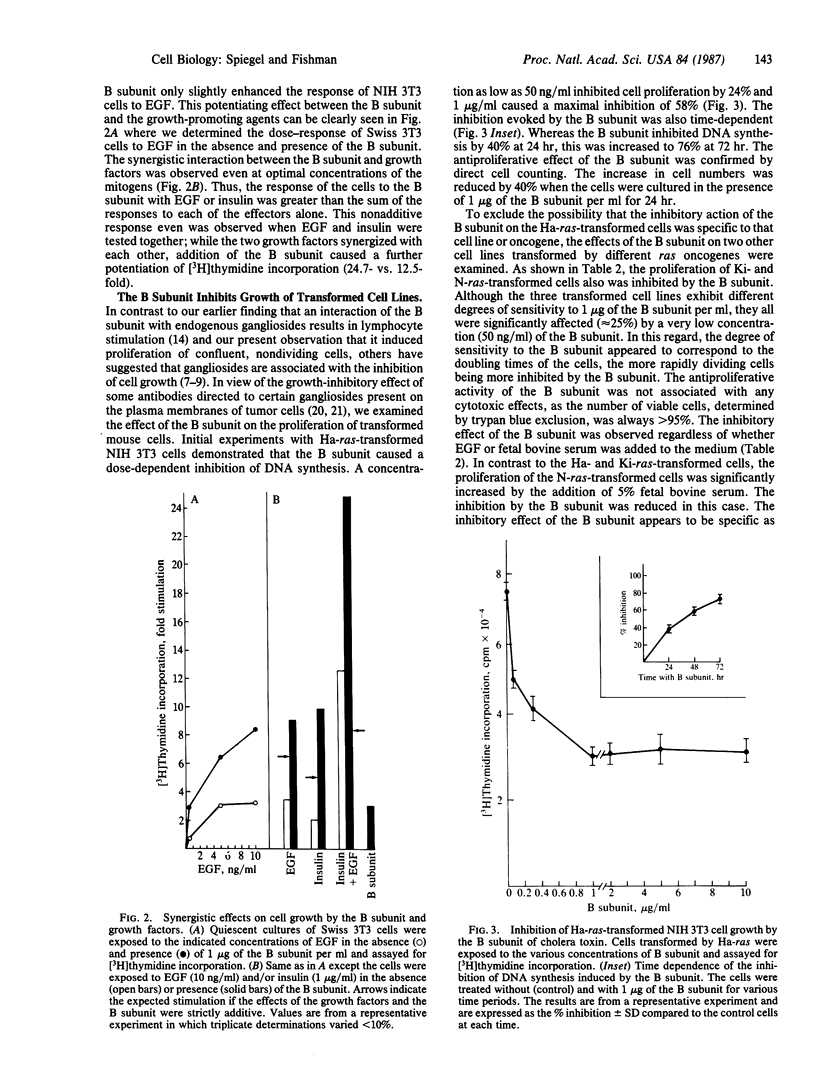
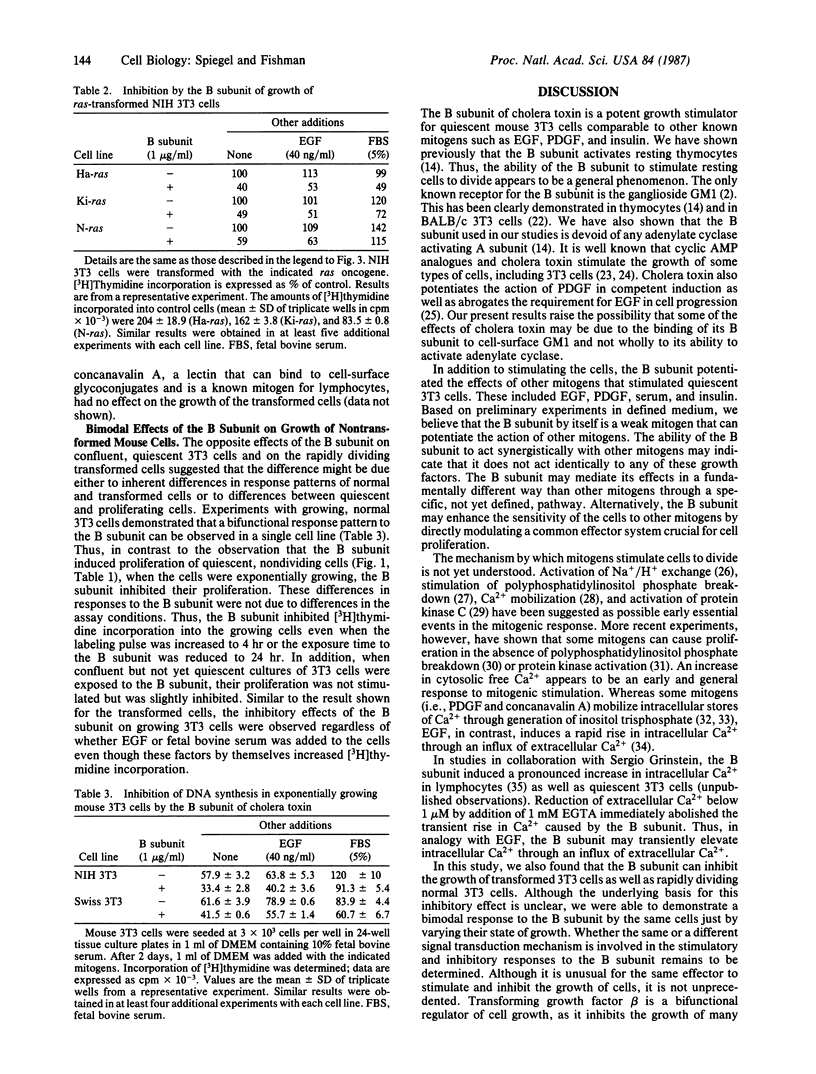
Abstract
The B subunit of cholera toxin, which binds specifically to several molecules of ganglioside galactosyl-(beta 1----3)-N-acetylgalactosyminyl(beta 1----4)-[N- acetylneuraminyl(alpha 2----3)]-galactosyl(beta 1----4)glucosyl(beta 1----1) ceramide (GM1) on the cell surface, stimulated DNA synthesis and cell division in quiescent, nontransformed mouse 3T3 cells in a dose-dependent manner. In addition, the B subunit potentiated the response of the 3T3 cells to other mitogens, such as epidermal growth factor, platelet-derived growth factor, and insulin. This synergistic effect indicates that the B subunit does not act identically to any of these growth factors but probably modulates a common effector system crucial for cell proliferation. In distinct contrast, the B subunit inhibited the growth of ras-transformed 3T3 cells as well as rapidly dividing normal 3T3 cells. Thus, the same cells, depending on their state of growth, exhibited a bimodal response to the B subunit. We conclude that endogenous gangliosides may be bimodal regulators of positive and negative signals for cell growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Watson S. P., Cuatrecasas P. Lack of association of epidermal growth factor-, insulin-, and serum-induced mitogenesis with stimulation of phosphoinositide degradation in BALB/c 3T3 fibroblasts. J Biol Chem. 1986 Jan 15;261(2):723–727. [PubMed] [Google Scholar]
- Bremer E. G., Hakomori S., Bowen-Pope D. F., Raines E., Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984 Jun 10;259(11):6818–6825. [PubMed] [Google Scholar]
- Bremer E. G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986 Feb 15;261(5):2434–2440. [PubMed] [Google Scholar]
- Critchley D. R., Streuli C. H., Kellie S., Ansell S., Patel B. Characterization of the cholera toxin receptor on Balb/c 3T3 cells as a ganglioside similar to, or identical with, ganglioside GM1. No evidence for galactoproteins with receptor activity. Biochem J. 1982 Apr 15;204(1):209–219. doi: 10.1042/bj2040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Knuth A., Meyer zum Büschenfelde K. H. Inhibition of human melanoma cell growth in vitro by monoclonal anti-GD3-ganglioside antibody. Cancer Res. 1984 Feb;44(2):806–810. [PubMed] [Google Scholar]
- Eva A., Tronick S. R., Gol R. A., Pierce J. H., Aaronson S. A. Transforming genes of human hematopoietic tumors: frequent detection of ras-related oncogenes whose activation appears to be independent of tumor phenotype. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4926–4930. doi: 10.1073/pnas.80.16.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R., Facci L., Skaper S. D., Varon S. Gangliosides stimulate astroglial cell proliferation in the absence of serum. J Cell Physiol. 1986 Jan;126(1):147–153. doi: 10.1002/jcp.1041260120. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Schmid E., Franke W. W., Wiegandt H. Exogenous glycosphingolipids suppress growth rate of transformed and untransformed 3T3 mouse cells. Exp Cell Res. 1975 May;92(2):259–270. doi: 10.1016/0014-4827(75)90379-1. [DOI] [PubMed] [Google Scholar]
- Kinders R. J., Rintoul D. A., Johnson T. C. Ganglioside GM1 sensitizes tumor cells to growth inhibitory glycopeptides. Biochem Biophys Res Commun. 1982 Jul 30;107(2):663–669. doi: 10.1016/0006-291x(82)91542-x. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Olashaw N. E., Pledger W. J., O'Keefe E. J. Cyclic AMP potentiates down regulation of epidermal growth factor receptors by platelet-derived growth factor. Biochem Biophys Res Commun. 1982 Nov 16;109(1):83–91. doi: 10.1016/0006-291x(82)91569-8. [DOI] [PubMed] [Google Scholar]
- Lingwood C. A., Hakomori S. Selective inhibition of cell growth and associated changes in glycolipid metabolism induced by monovalent antibodies to glycolipids. Exp Cell Res. 1977 Sep;108(2):385–391. doi: 10.1016/s0014-4827(77)80045-1. [DOI] [PubMed] [Google Scholar]
- Marcus D. M. A review of the immunogenic and immuno-modulatory properties of glycosphingolipids. Mol Immunol. 1984 Nov;21(11):1083–1091. doi: 10.1016/0161-5890(84)90118-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Svennerholm L., Paulson J. C. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Morris J. D., Metcalfe J. C., Smith G. A., Hesketh T. R., Taylor M. V. Some mitogens cause rapid increases in free calcium in fibroblasts. FEBS Lett. 1984 Apr 24;169(2):189–193. doi: 10.1016/0014-5793(84)80316-6. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. B. Cholera toxin stimulates division of 3T3 cells. J Cell Physiol. 1979 Mar;98(3):469–474. doi: 10.1002/jcp.1040980305. [DOI] [PubMed] [Google Scholar]
- Robb R. J. The suppressive effect of gangliosides upon IL 2-dependent proliferation as a function of inhibition of IL 2-receptor association. J Immunol. 1986 Feb 1;136(3):971–976. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of DNA synthesis in quiescent cultured cells: exogenous agents, internal signals, and early events. Curr Top Cell Regul. 1980;17:59–88. doi: 10.1016/b978-0-12-152817-1.50007-9. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Fishman P. H., Weber R. J. Direct evidence that endogenous GM1 ganglioside can mediate thymocyte proliferation. Science. 1985 Dec 13;230(4731):1285–1287. doi: 10.1126/science.2999979. [DOI] [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kaibuchi K., Kawahara Y., Fukuzaki H., Takai Y. Induction of protein kinase C activation and Ca2+ mobilization by fibroblast growth factor in Swiss 3T3 cells. FEBS Lett. 1985 Oct 28;191(2):205–210. doi: 10.1016/0014-5793(85)80009-0. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Arita M., Nagai Y. GQ1b, a bioactive ganglioside that exhibits novel nerve growth factor (NGF)-like activities in the two neuroblastoma cell lines. J Biochem. 1983 Jul;94(1):303–306. doi: 10.1093/oxfordjournals.jbchem.a134344. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Gol R. A., Chang A., Chiu I. M., Reddy E. P., Tronick S. R., Aaronson S. A. Mechanism of activation of an N-ras oncogene of SW-1271 human lung carcinoma cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3670–3674. doi: 10.1073/pnas.81.12.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]


