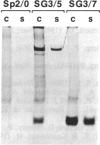
Abstract
We have cloned the genomic DNA fragments encoding the heavy and light chain variable regions of monoclonal antibody 17-1A, and we have inserted them into mammalian expression vectors containing genomic DNA segments encoding human gamma 3 and kappa constant regions. The transfer of these expression vectors containing mouse-human chimeric immunoglobulin genes into Sp2/0 mouse myeloma cells resulted in the production of functional IgG that retained the specific binding to the surface antigen 17-1A expressed on colorectal carcinoma cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulianne G. L., Hozumi N., Shulman M. J. Production of functional chimaeric mouse/human antibody. Nature. 1984 Dec 13;312(5995):643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- Curtis P. J. Cloning of mouse carbonic anhydrase mRNA and its induction in mouse erythroleukemic cells. J Biol Chem. 1983 Apr 10;258(7):4459–4463. [PubMed] [Google Scholar]
- Girardet C., Vacca A., Schmidt-Kessen A., Schreyer M., Carrel S., Mach J. P. Immunochemical characterization of two antigens recognized by new monoclonal antibodies against human colon carcinoma. J Immunol. 1986 Feb 15;136(4):1497–1503. [PubMed] [Google Scholar]
- Gough N. M., Bernard O. Sequences of the joining region genes for immunoglobulin heavy chains and their role in generation of antibody diversity. Proc Natl Acad Sci U S A. 1981 Jan;78(1):509–513. doi: 10.1073/pnas.78.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn D. M., Steplewski Z., Herlyn M. F., Koprowski H. Inhibition of growth of colorectal carcinoma in nude mice by monoclonal antibody. Cancer Res. 1980 Mar;40(3):717–721. [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley M. E., Wang C. H., Ekenberg S. J., Zuelke M. S., Kelso D. M. Particle concentration fluorescence immunoassay (PCFIA): a new, rapid immunoassay technique with high sensitivity. J Immunol Methods. 1984 Feb 24;67(1):21–35. doi: 10.1016/0022-1759(84)90082-6. [DOI] [PubMed] [Google Scholar]
- Levy R., Miller R. A. Biological and clinical implications of lymphocyte hybridomas: tumor therapy with monoclonal antibodies. Annu Rev Med. 1983;34:107–116. doi: 10.1146/annurev.me.34.020183.000543. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Wims L. A., Morrison S. L. A variant of the dextran-binding mouse plasmacytoma J558 with altered glycosylation of its heavy chain and decreased reactivity with polymeric dextran. Biochemistry. 1981 Aug 18;20(17):4827–4835. doi: 10.1021/bi00520a004. [DOI] [PubMed] [Google Scholar]
- Max E. E., Maizel J. V., Jr, Leder P. The nucleotide sequence of a 5.5-kilobase DNA segment containing the mouse kappa immunoglobulin J and C region genes. J Biol Chem. 1981 May 25;256(10):5116–5120. [PubMed] [Google Scholar]
- Morrison S. L., Johnson M. J., Herzenberg L. A., Oi V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L. Sequentially derived mutants of the constant region of the heavy chain of murine immunoglobulins. J Immunol. 1979 Aug;123(2):793–800. [PubMed] [Google Scholar]
- Morrison S. L. Transfectomas provide novel chimeric antibodies. Science. 1985 Sep 20;229(4719):1202–1207. doi: 10.1126/science.3929380. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Mitchell E. B., Jouhal S. S., Flanagan J. G., Rabbitts T. H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985 Mar 21;314(6008):268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Hawley T., Shulman M. J., Traunecker A., Köhler G., Hozumi N. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears H. F., Atkinson B., Mattis J., Ernst C., Herlyn D., Steplewski Z., Häyry P., Koprowski H. Phase-I clinical trial of monoclonal antibody in treatment of gastrointestinal tumours. Lancet. 1982 Apr 3;1(8275):762–765. doi: 10.1016/s0140-6736(82)91811-6. [DOI] [PubMed] [Google Scholar]
- Sears H. F., Herlyn D., Steplewski Z., Koprowski H. Effects of monoclonal antibody immunotherapy on patients with gastrointestinal adenocarcinoma. J Biol Response Mod. 1984;3(2):138–150. [PubMed] [Google Scholar]
- Sears H. F., Herlyn D., Steplewski Z., Koprowski H. Phase II clinical trial of a murine monoclonal antibody cytotoxic for gastrointestinal adenocarcinoma. Cancer Res. 1985 Nov;45(11 Pt 2):5910–5913. [PubMed] [Google Scholar]
- Shawler D. L., Bartholomew R. M., Smith L. M., Dillman R. O. Human immune response to multiple injections of murine monoclonal IgG. J Immunol. 1985 Aug;135(2):1530–1535. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Naito T., Hama K., Noma T., Honjo T. Construction of chimaeric processed immunoglobulin genes containing mouse variable and human constant region sequences. Nature. 1985 Apr 4;314(6010):452–454. doi: 10.1038/314452a0. [DOI] [PubMed] [Google Scholar]