Abstract
Autism is a neurodevelopmental disorder characterized by aberrant reciprocal social interactions, impaired communication, and repetitive behaviors. While the etiology remains unclear, strong evidence exists for a genetic component, and several synaptic genes have been implicated. SHANK genes encode a family of synaptic scaffolding proteins located postsynaptically on excitatory synapses. Mutations in SHANK genes have been detected in several autistic individuals. To understand the consequences of SHANK mutations relevant to the diagnostic and associated symptoms of autism, comprehensive behavioral phenotyping on a line of Shank1 mutant mice was conducted on multiple measures of social interactions, social olfaction, repetitive behaviors, anxiety-related behaviors, motor functions, and a series of control measures for physical abilities. Results from our comprehensive behavioral phenotyping battery indicated that adult Shank1 null mutant mice were similar to their wildtype and heterozygous littermates on standardized measures of general health, neurological reflexes and sensory skills. Motor functions were reduced in the null mutants on open field activity, rotarod, and wire hang, replicating and extending previous findings (Hung et al., 2008). A partial anxiety-like phenotype was detected in the null mutants in some components of the light ↔ dark task, as previously reported (Hung et al., 2008) but not in the elevated plus-maze. Juvenile reciprocal social interactions did not differ across genotypes. Interpretation of adult social approach was confounded by a lack of normal sociability in wildtype and heterozygous littermates. All genotypes were able to discriminate social odors on an olfactory habituation/dishabituation task. All genotypes displayed relatively high levels of repetitive self-grooming. Our findings support the interpretation that Shank1 null mice do not demonstrate autism-relevant social deficits, but confirm and extend a role for Shank1 in motor functions.
1. Introduction
Shank1 is a member of the Shank family of scaffolding proteins which are localized in the postsynaptic densities of neuronal excitatory synapses, and which bind to the complex of synaptic proteins including PSD-95, Homer, GKAP and cortactin (Naisbitt et al., 1999, Sheng and Kim, 2000, Sala et al., 2001, Bockers et al., 2004, Cheng et al., 2006, Hung et al., 2010). Mice with a null mutation in Shank1 displayed smaller, thinner postsynaptic densities, an altered composition of postsynaptic density proteins, and reduced size of dendritic spines in the hippocampus (Hung et al., 2008). Electrophysiological phenotypes of Shank1 knockout mice included decreased synaptic strength with retained hippocampal synaptic plasticity. Behavioral phenotypes of male Shank1 null mutants included reduced open field activity, reduced latencies to fall from a rotarod, higher anxiety-like scores on the light ↔ dark test, and altered performance on learning and memory tasks, as compared to wildtype littermate controls. In addition, Shank1 levels were altered in the hippocampus and cortex of Fmr1 mice, a model of Fragile X syndrome (Schutt et al., 2009). These findings are consistent with a role for Shank1 in hippocampal mechanisms mediating cognitive processes (Bourgeron, 2009).
The SHANK family of genes has been implicated in the etiology of autism (Bourgeron, 2009, Buxbaum, 2009). Mutations in SHANK3 were detected in a small subset of some (Durand et al., 2007, Moessner et al., 2007, Abu-Elneel et al., 2008, Bourgeron, 2009, Gauthier et al., 2009) but not all (Qin et al., 2009, Sykes et al., 2009) cohorts of individuals with autism spectrum disorders. In addition, the human SHANK3 gene is located within a region of chromosome 22q13 in which deletions are strongly associated with Phelan-McDermid Syndrome, a developmental disorder characterized by learning disabilities, language deficits, hypotonia, and social deficits analogous to those seen in autism (Goizet et al., 2000, Prasad et al., 2000, Manning et al., 2004, Jeffries et al., 2005, Vorstman et al., 2006, Phelan, 2008). Mutations in the SHANK2 genes were reported in a small number of individuals with autism spectrum disorders and mental retardation (Berkel et al., 2010, Pinto et al., 2010). While SHANK1 mutations have not yet been detected in individuals with autism spectrum disorders, to our knowledge, the similarities in structure and localization between Shank1 and Shank3 raise the possibility that alterations in Shank1 could have consequences relevant to some of the symptoms of autism. Moreover, Shank1 is almost exclusively expressed in brain, while other Shank proteins are also expressed in peripheral organs including heart, kidney, liver, and spleen (Lim et al., 1999).
Shank1 knockout mice offer a model system to test the hypothesis that a mutation in Shank1 could result in behaviors relevant to the symptoms of autism. We developed multiple mouse behavioral assays relevant to the three diagnostic symptoms of autism to evaluate hypotheses about autism candidate genes. Social interaction deficits are evaluated in an automated three-chambered social approach task and from videotapes of reciprocal social interactions in freely moving pairs of mice. Communication deficits are evaluated as alterations in olfactory habituation/dishabituation to social and non-social odors, scent marking to social olfactory cues, and ultrasonic vocalization responses to social cues. Stereotyped, repetitive behaviors with restricted interests are scored on measures of spontaneous stereotypies, repetitive self-grooming, and resistance to change in spatial habits. Developmental milestones, general health, sensory abilities, motor functions, anxiety-related behaviors, and learning and memory tasks are assayed both as control measures and for relevance to associated symptoms of autism. The present study employed many of these behavioral assays to investigate possible autism-relevant phenotypes in Shank1 mutant mice. The first publication of phenotypes in Shank1 knockout mice reported behaviors in males only, and in wildtype versus null mutants only (Hung et al. 2008). To repeat the previous behavioral assays and extend their scope, both males and females of all three genotypes (Shank1 -/-, +/-, and +/+) were tested in the present experiments. Further behavioral analyses conducted in the present study included open field activity, rotarod coordination and balance, two anxiety-related tasks, juvenile and adult social behavior, olfactory habituation/dishabituation for non-social and social odors, sensory tasks including acoustic startle threshold, prepulse inhibition, hot plate and tail flick pain sensitivity and an overall battery of general health parameters and neurological reflexes.
2. Results
Measures of general health, empty cage behaviors, and neurological reflexes
Table 1 lists the scores for the measures of general health and neurological reflexes for all genotypes of Shank1 mice, and for a separate breeding line of the hybrid C57B6/129Jae (B6/Jae) used as the background strain in the present Shank1 line. Scores for the B6/Jae background strain are provided for illustrative purposes, but not included in the statistical comparison of genotypes unless explicitly stated. No genotype differences between the +/+, +/-, and -/- were detected on body weight, appearance of the fur, whiskers, posture, limb tone, and most measures of the neurobehavioral screen. No obvious physical abnormalities were seen in any mice. Reflexes including eye blink, ear twitch, whisker twitch, righting reflex, Preyer startle reflex as a measure of hearing, and forepaw reaching as a measure of vision, were normal for all mice. Genotype differences were observed in body temperature (F (2, 33) = 3.63, p < 0.05). A slightly higher body temperature was detected in -/- as compared to +/- only (Bonferroni-Dunn p < 0.05). Reactivity to handling did not differ across genotypes. No sex differences or sex by genotype interactions were detected on any parameter except body weight, in which males were generally larger than females. Observations of home cage behaviors in the vivarium indicated that Shank1 -/- mice were similar to littermates and in a normal range on home cage activity, nest building, huddling, and grooming, with few observations of fighting or sitting outside of the huddle.
Table 1. Measures of general health, empty cage behaviors, and neurological reflexes in Shank1 mice.
A standard battery of parameters revealed no genotype differences on most measures. Shank1 null mutants (-/-) showed slightly higher body temperature than wildtype littermates. * p < 0.05. No other significant differences were detected across genotypes on these measures. Data shown are means ± standard error of the mean (SEM) for body weight, temperature, and behaviors assessed using a 3 point ranking scale. Percentage of mice that exhibited a specific neurological reflex is expressed as percent of total mice within each genotype. The background strain, B6/Jae, scores are provided for illustrative purposes. +/+ N=14; +/- N=15; -/- N=10; B6/Jae N=15.
| Genotypes | +/+ N=15 |
+/- N=12 |
-/- N=11 |
B6/Jae N=10 |
Sig level |
|---|---|---|---|---|---|
| Body weight | 25.1±1.5 | 23.0±1.7 | 25.1±1.6 | 24.4±1.3 | NS |
| Body temperature | 36.7±.27 | 36.3±.45 | 37.6±.26 | 37.1±.20 | .03 |
| Fur condition (3 point scale) | 2 | 2 | 2 | 2 | NS |
| Bald patches (%) | 6% | 8% | 0% | 0% | NS |
| Missing whiskers (%) | 20% | 16% | 9% | 0% | NS |
| Piloerection | 0% | 0% | 0% | 0% | NS |
| Body tone (3 point scale) | 2 | 2 | 2 | 2 | NS |
| Limb tone (3 point scale) | 2 | 2 | 2 | 2 | NS |
| Skin color (3 point scale) | 2 | 2 | 2 | 2 | NS |
| Physical abnormalities | 0% | 0% | 0% | 0% | NS |
| Wild running (%) | 0% | 0% | 0% | 0% | NS |
| Stereotypies (%) | 0% | 0% | 0% | 0% | NS |
| Exploration (3 point scale) | 2 | 1.9 ± 0.8 | 2 | 1.8 ± 0.3 | NS |
| Trunk curl (%) | 86% | 83% | 63% | 100% | NS |
| Forepaw reach (%) | 100% | 100% | 100% | 100% | NS |
| Righting reflex (%) | 100% | 100% | 100% | 100% | NS |
| Corneal reflex (%) | 90% | 100% | 93% | 100% | NS |
| Whisker twitch (%) | 80% | 83% | 91% | 100% | NS |
| Ear twitch (%) | 74% | 67% | 73% | 100% | NS |
| Auditory startle (%) | 85% | 100% | 100% | 100% | NS |
| Struggle/vocalization (%) | 0% | 0% | 0% | 10% | NS |
| Dowel biting (3 point scale) | 1.67±.13 | 1.42±.15 | 1.54±0.16 | 1.6±.16 | NS |
Social behaviors in juvenile and adult Shank1 mice
Figure 1 illustrates bouts of reciprocal social interactions between juvenile Shank1 +/+, +/-, and -/- mice paired with juvenile C57BL/6J (B6) mice of the same sex. No significant differences were observed across genotypes for nose-to-nose sniff (Panel A, F (2, 24) = 0.89, NS), anogenital sniff (Panel B, F (2, 24) = 2.41, NS), body sniff (Panel C, F (2, 24) = 1.96, NS), push-crawl (Panel D, F (2, 24) = 0.43, NS), push-past (Panel E, F (2, 24) = 0.46, NS) or follow events (Panel F, F (2, 24) = 1.23, NS).
Figure 1. Shank1 mice exhibit normal juvenile reciprocal social interaction behaviors.
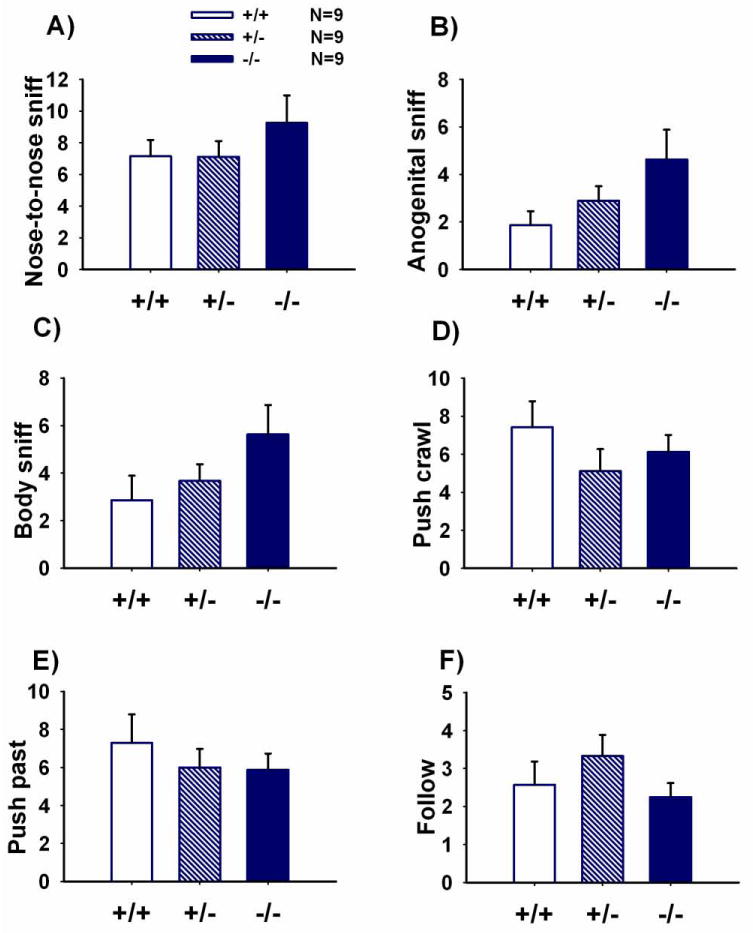
Juvenile social interaction between pairs consisting of a Shank 1 +/+, +/- or -/- mouse, age 19-22 days, with an unfamiliar sex and age-matched B6 control mouse. No significant differences were detected between the genotypes on the number of bouts of A) nose-to-nose sniff, B) anogenital sniff, C) body sniff, D) push and crawl, E) pushing past, and F) follow. N=9 per genotype. Data are shown as mean + standard error of the mean throughout Figures 1-10.
Figure 2 illustrates the sociability scores from the automated three-chambered social approach task for Shank1 mice genotypes +/+, +/-, -/- and the background B6/Jae hybrid. Sociability, defined as spending more time in the chamber with the novel mouse than in the chamber with the novel object, was not detected in any of the three genotypes, for unknown reasons. Time spent in the chamber with the novel mouse was not significantly greater than time spent in the chamber with the novel object for +/+ (F (1, 14) = 1.06, NS); +/- (F (1, 7) = 3.56, NS); -/- (F (1, 16) = 0.72, NS). B6/Jae, the genetic background on which the Shank1 mutation was bred, exhibited the expected sociability, spending significantly more time in the chamber with the novel mouse versus time in the chamber with the novel object (F (1, 11) = 12.03, p < 0.001), indicating that the background strain was not responsible for the lack of sociability in the Shank1 genotypes. The more sensitive measure of direct sniffing interaction with the novel mouse detected greater time spent sniffing the novel mouse than the novel object for -/- (F (1, 16) = 8.9, p < 0.05), and the hybrid background B6/Jae (F (1, 11) = 14.45, p < 0.005) but not +/+ (F (1, 14) = 0.33, NS) or +/- (F (1, 7) = 0.05, NS). Entries into the left and right side chambers did not differ within genotypes for +/+ (F (1, 14) = 0.87, NS); +/- (F (1, 7) = 4.77, NS); -/- (F (1, 16) = 3.74, NS). No innate side preference was observed across genotypes during the 10-minute habituation session before the start of social testing (Panel D; F (3, 48) = 2.00, NS).
Figure 2. Sociability in Shank1 mice.
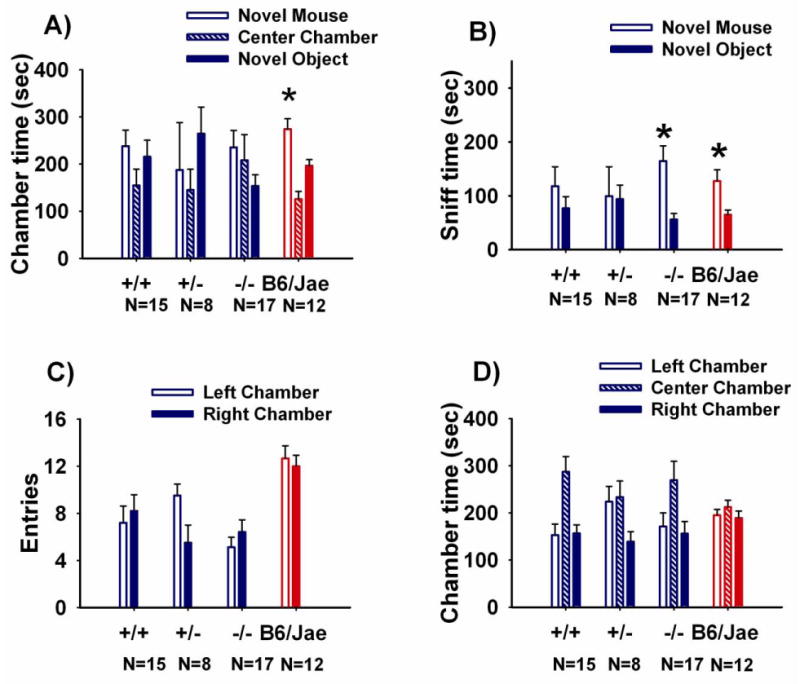
Adult sociability, assayed in an automated photocell-equipped three-chambered arena, revealed unpredicted confounds. A) None of the Shank1 genotypes displayed normal sociability, defined as more time in the side chamber with the novel mouse than in the side chamber with the novel object. In contrast, control mice from the hybrid background B6/Jae, used to breed the Shank1 mutation, displayed normal sociability for time in the chamber containing the novel mouse versus time in the chamber containing the novel object. * p < 0.05. B) Shank1 -/- and B6/Jae displayed significantly more time spent sniffing the novel mouse than time spent sniffing the novel object. C) No genotype differences were seen in the number of entries into the either the left or right side chambers. D) No innate chamber side bias was present in any group during the 10 minute habituation phase before the start of the sociability test. N=15 +/+; N =8 +/-; N=17 -/-; N=12 B6/Jae.
Olfactory abilities in Shank1 mice
Figure 3 illustrates olfactory abilities for discriminating non-social and social odors. Olfactory habituation to three identical odors and olfactory dishabituation to four novel odors detected no genotype differences on habituation to repeated exposures to water (F (2, 44) = 0.77, NS), almond (F (2, 44) = 1.08, NS), social odor from unfamiliar cage 1 (F (2, 44) = 0.19, NS), and social odor from a different, unfamiliar cage 2 (F (2, 44) = 1.14, NS). Significant habituation to each odor was observed, as seen in the decline in time spent sniffing the three swabs within the set of 3 water odors (F (2, 44) = 66.66, p < 0.001), within the set of 3 almond odors (F (2, 44) = 59.97, p < 0.001), within the set of 3 banana odors (F (2, 44) = 89.96, p < 0.001), within the set of 3 odors from unfamiliar cage 1 (F (2, 44) = 148.01, p < 0.001), and within the set of 3 odors from unfamiliar cage 2 (F (2, 44) = 77.03, p < 0.001). Genotype differences (F (2, 44) = 8.38, p < 0.001) and a genotype by trial number interaction (F (4, 44) = 3.62, p < 0.01) were observed on habituation to banana odor. Student-Newman-Keuls' posthoc analysis indicated a significant difference between Shank1 +/+ and +/- (p = 0.002) and between the Shank1 +/+ and -/- (p = 0.005) during the first trial of banana exposure only (+/+ vs. +/-, p < 0.001; +/+ vs. -/-, p < 0.001). All genotypes showed a significant dishabituation when presented with a new odor, as seen in an increase in time spent sniffing the first swab of water to almond (F (2, 44) = 50.32, p < 0.001); almond to banana (F (2, 44) = 101.65, p < 0.001); banana to unfamiliar social odor from cage 1 (F (2, 44) = 167.94, p < 0.001); and unfamiliar cage 1 to unfamiliar cage 2 (F (2, 44) = 86.92, p < 0.001).
Figure 3. No deficits in non-social and social olfactory capabilities in Shank1 mice.
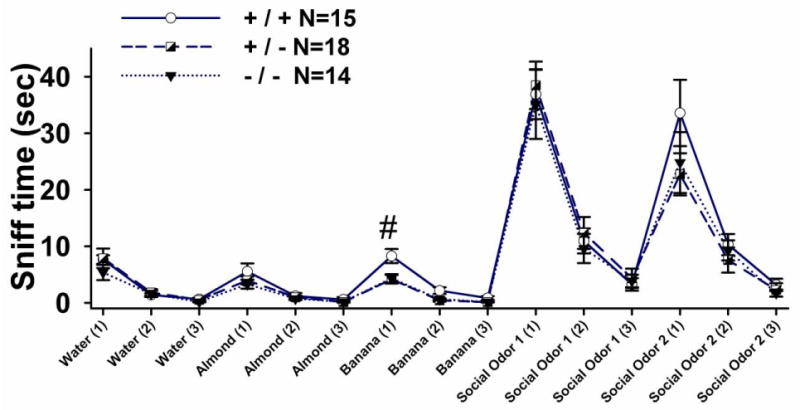
Olfactory habituation/dishabituation confirmed normal olfactory abilities in all three genotypes of Shank1 mice for non-social and social odors. All genotypes displayed significant habituation and dishabituation to non-social and social odors. The first presentation of a water-soaked swab elicited moderate sniffing that declined across the second and third exposure to water (habituation). The next presentation, a swab soaked in almond extract, elicited significantly more sniffing (dishabituation), which declined across the second and third presentation of the almond odor (habituation). Similarly, sniffing resumed at a high level to the next new odor, a swab soaked in banana flavoring (dishabituation), and declined across the three banana presentations (habituation). Shank1 +/+ mice sniffed the banana odor for a greater length of time compared to +/- and -/- littermates on the first trial. # p < 0.05. The next presentation, a swab soaked in almond extract, elicited significantly more sniffing (dishabituation), which declined across the second and third presentation of the almond odor (habituation). The next presentation, a social odor swab wiped across the bottom surface of a plastic cage that contained four unfamiliar mice of the same sex but a different strain, 129S1/SvImJ, elicited significantly more sniffing (dishabituation), which declined across the second and third presentation of this first social odor (habituation). The final presentation, a swab wiped across the bottom surface of a plastic cage that contained a second set of four unfamiliar mice of the same sex but a different strain, FVBS/Ant, elicited significantly more sniffing (dishabituation), which declined across the second and third presentation of the second social odor (habituation). N=15 +/+; N =18 +/-; N=14 -/-.
Repetitive self-grooming in Shank1 mice
Figure 4 illustrates self-grooming scores for all three genotypes of Shank mice genotypes +/+, +/-, -/- and the inbred strain, B6/Jae hybrid during a 10-minute testing session. No significant differences in the cumulative time spent self-grooming were detected across genotypes (F (2, 29) =0.80, NS). No sex differences were detected in the self-grooming assay. Comparison to a previous experiment with standard B6 mice indicates that Shank wildtypes and B6/Jae engaged in comparatively high levels of self-grooming.
Figure 4. Shank1 mice did not exhibit genotype differences in repetitive self-grooming.
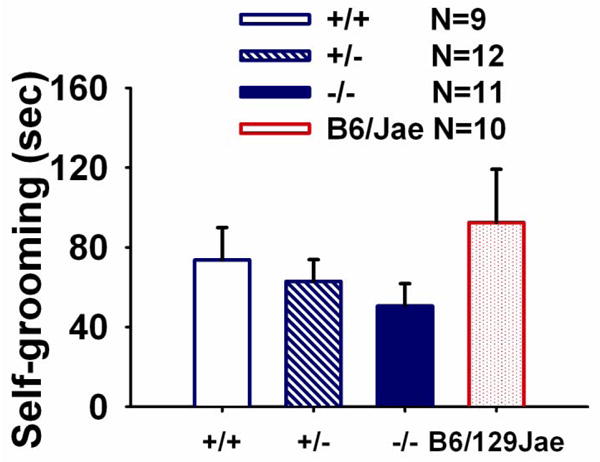
Cumulative time spent self-grooming was scored over a 10 minute session in a clean, empty mouse cage. Shank1 +/+, +/- and -/- mice did not differ on the amount of time spent self-grooming during a ten minute testing session. N=9 +/+; N =12 +/-; N=11 -/-; N=10 B6/Jae. The background strain, B6/Jae, scores are provided for illustrative purposes.
Anxiety-like traits assessed in Shank1 mice
Figure 5 illustrates a mild anxiety-like phenotype in Shank1 -/- mice using the light ↔ dark test for anxiety-like behavior. Total number of transitions between the light and dark compartments was significantly different (Panel A, F (2, 40) = 9.18, p < 0.01). Bonferroni-Dunn posthoc analysis confirmed fewer transitions in the Shank1 null mutant -/- mice as compared to wildtype +/+ littermate controls (p < 0.05) and heterozygotes +/- (p < 0.05). No differences between genotypes were observed on cumulative time spent in the dark chamber (Panel B, F (2, 40) = 0.38, NS) or the latency to first entry into the dark chamber (Panel C, F (2, 40) = 2.38, NS).
Figure 5. Mild anxiety-related behavior in Shank1 null mutant mice in the light ↔ dark test.
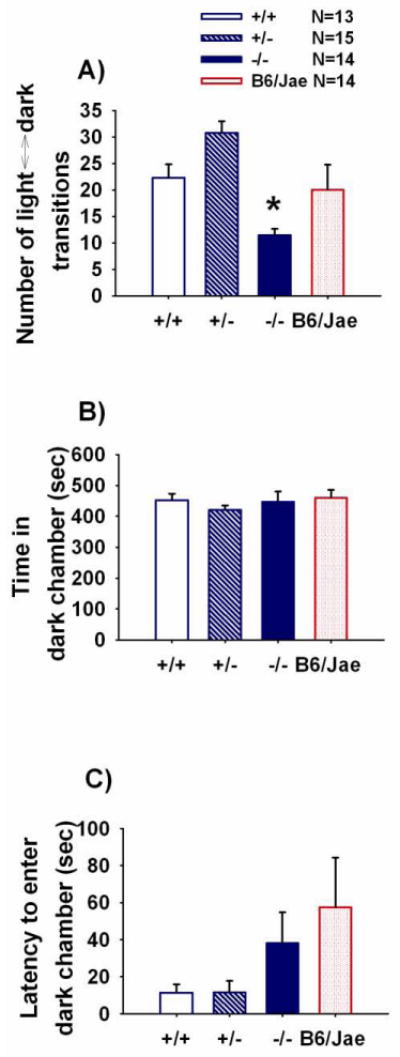
Basal performance on an anxiety-related task, light ↔ dark exploration A) Shank1 null -/- mutants displayed reduced transitions between the light and dark compartments of the test apparatus compared to +/+ and +/- littermates. * p < 0.05. B) No significant differences were detected across Shank1 genotypes on time spent in the dark chamber. C) No significant differences were detected across Shank1 genotypes on latency to enter the dark portion of the apparatus. N=13 +/+; N =15 +/-; N=14 -/-; N=14 B6/Jae. Scores for the background strain, B6/Jae, are provided for illustrative purposes but were not included in the statistical analysis.
Figure 6 illustrates anxiety-like behavior assessed by the elevated plus-maze task in Shank1 mice and inbred B6/Jae. Percentage of time spent on the open arms did not differ significantly across genotypes (Panel A, F (2, 39) = 0.42, NS). Entries into the open arm segments did not differ across genotypes (Panel B, F (2, 39) = 0.14, NS). As an internal control for locomotion, total entries into both the open and closed arms were summed. No significant difference in total entries was detected across genotypes were detected (Panel C, F (2, 39) = 0.43, NS). B6/Jae scores during the light ↔ dark and elevated plus-maze tasks are presented for qualitative comparisons.
Figure 6. No genotype differences in anxiety-like behaviors on the elevated plus-maze.
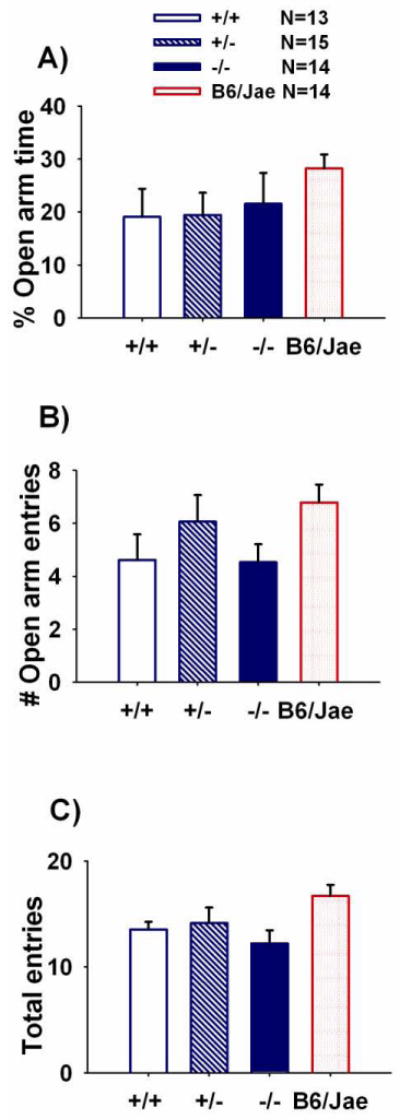
Elevated plus-maze data revealed no significant genotype differences in A) percentage of time spent on the open segments, B) entries into the open arm segments, C) total entries. N=13 +/+; N=15 +/-; N=14 -/-; N=14 B6/Jae. Score for the background strain, B6/Jae, are provided for illustrative purposes.
Locomotor abilities and motor learning in Shank1 mice
Figure 7 illustrates the four parameters assessed on open field exploratory locomotion for Shank1 +/+, +/-, -/- and B6/Jae during a 30-minute test session. Activity levels in the inbred hybrid B6/Jae group are presented on each locomotor parameter for qualitative comparisons. The time course for total distance traversed in the novel open field over a 30 minute time period was highly significant, as expected, representing habituation to the novel open field (Panel A, F (5, 39) = 56.0, p < 0.0001). A significant effect of genotype on total distance traversed was detected (F (2, 39) = 4.66, p < 0.01). Student-Newman-Keuls' posthoc analysis indicated a significant difference between -/- and +/+ (p = 0.050) and -/- and +/- (p = 0.004). Time spent in the center of the test arena differed across time bins (Panel B, F (5, 39) = 4.19, p < 0.01). A significant effect of genotype on time in the center of the arena was detected (Panel B, F (2, 39) = 3.63, p < 0.05). Center time was lower in -/- compared to +/+ (p = 0.050). Horizontal activity over the 30 minute test period was reduced, as expected, representing habituation to the novel open field (Panel C, F (5, 39) = 103.8, p < 0.001). Horizontal activity did not differ across genotypes (F (3, 39) = 1.11, NS). Vertical activity in the novel open field over a 30 minute time period was reduced (Panel D, F (5, 39) = 12.11, p < 0.001). Vertical activity did not differ across genotypes (F (3, 39) = 2.57, NS).
Figure 7. Shank1 null mutants display reduced exploratory locomotion in a novel open field.
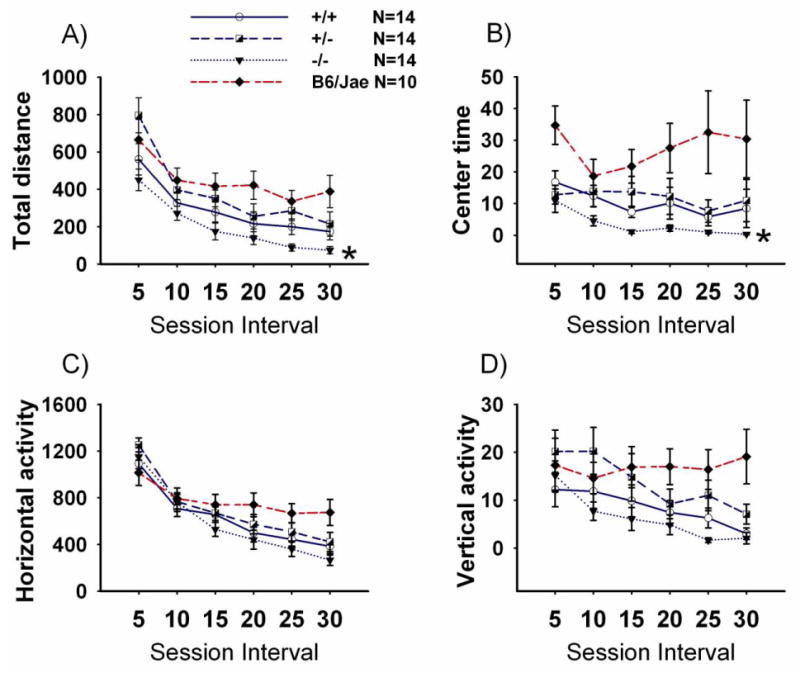
Total distance, center time, horizontal activity and vertical activity and were assayed in 5-minute time bins across a 30 minute session in a novel open field arena, for the three Shank1 genotypes and hybrid background mice, B6/Jae. A) Shank1 null mutants -/- traversed less total distance in a novel open field as compared to +/+ and +/- littermates. * p < 0.05. B) Shank1 null mutants -/- spent less time in the center of the open field as compared to +/+ littermates. * p < 0.05. C) Horizontal activity did not differ across genotypes and D) vertical activity did not differ across genotypes. N=14 +/+; N=14 +/-; N=14 -/-; N=10 B6/Jae. Scores for the hybrid background mice, B6/Jae, are provided for illustrative purposes.
Figure 8 illustrates performance on two tasks of motor coordination, balance and neuromuscular strength, the accelerating rotarod and the inverted wire hang test. Latency to fall over the 6 trials increased, as expected, representing a significant effect of training (Panel A, F (5, 57) = 54.85, p < 0.001). Shank1 -/- displayed shorter latencies to fall as compared to both +/+ (p < 0.05) and +/- (p < 0.05). Since no significant interaction was detected between genotype and training (F (10, 57) = 1.27, NS), individual trials by genotype were not analyzed. Neuromuscular strength, as measured by the latency to fall from the inverted wire hang task, differed by genotype (Panel B, F (2, 33) = 5.46, p < 0.01). Shank1 -/- fell off the wire faster than +/+ (Bonferroni-Dunn p = 0.008) or +/- (Bonferroni-Dunn p = 0.005).
Figure 8. Reduced motor coordination, balance and neuromuscular strength in Shank1 null mutant mice.
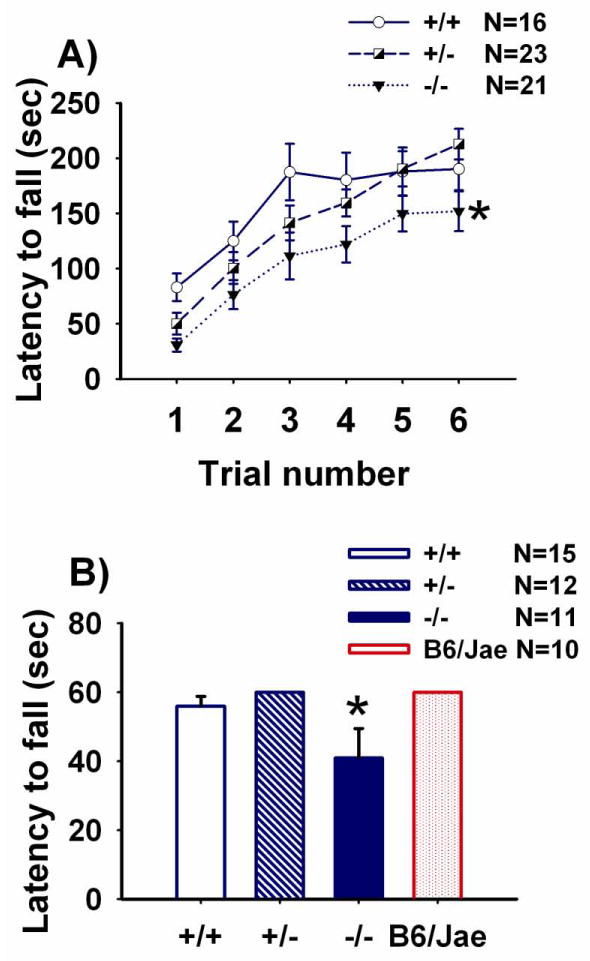
Motor coordination, balance, and neuromuscular strength were assayed. Latency to fall from an accelerating rotarod was recorded with a 300 second maximum latency. Each subject was given 6 total trials over two days, 3 trials per day, with a 30–60 minute intertrial interval. Latency to fall from an inverted wire mouse cage lid was recorded with a 60 second maximum hang time. A) Shank1 null mutants -/- fell from the accelerating rotarod faster than +/+ and +/- littermates. * p < 0.05. N=16 +/+; N=23 +/-; N=21 -/-. B) Shank1 null mutants -/- fell from the inverted wire mouse cage lid faster than +/+ and +/- littermates. * p < 0.05. N=15 +/+; N=12 +/-; N=11 -/-; N=10 B6/Jae. Scores for the hybrid background mice, B6/Jae, are provided for illustrative purposes.
Acoustic startle threshold and prepulse inhibition in Shank1 mice
Figure 9 illustrates acoustic startle at 5 decibel (dB) levels, and prepulse inhibition of acoustic startle reactivity using a 110 dB startle stimulus and 5 prepulse levels, in Shank1 mice. Startle responses to sudden loud acoustic stimuli differed by trial using various dB intensities (Panel A, F (2, 28) = 15.52, p < 0.001) but were similar across genotypes (F (2, 28) = 1.20, NS) and no trial by genotype interaction was detected (F (10, 28) = 0.81, NS). Reduced startle reactivity as prepulse intensity increased was observed, as expected (Panel B, F (2, 28) = 2.61, p < 0.05). Prepulse inhibition of acoustic startle did not differ across genotypes (F (2, 28) = 1.69, NS).
Figure 9. No genotype differences in acoustic startle or prepulse inhibition sensorimotor gating.
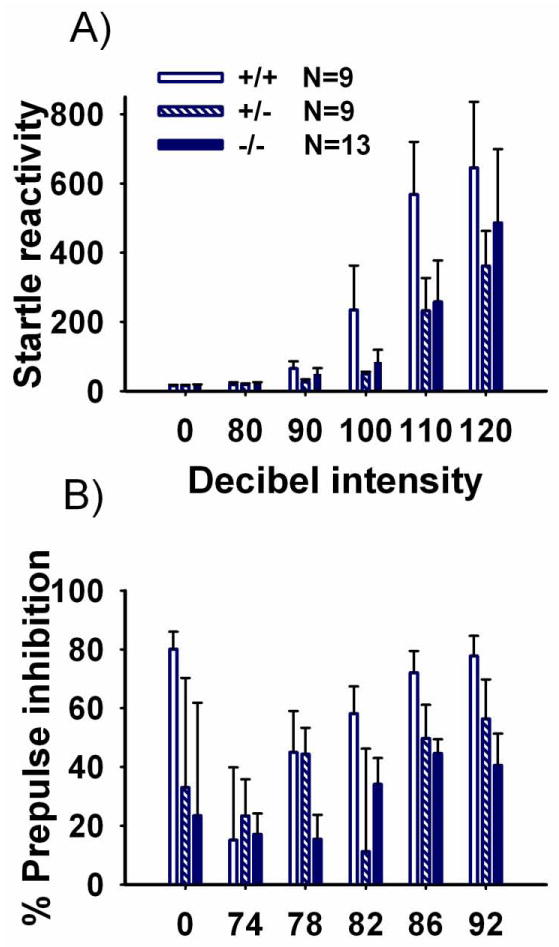
A) No genotype differences were observed in the acoustic startle response at 6 dB levels. B) No genotype differences were observed in prepulse inhibition of acoustic startle at any prepulse level. N=9 +/+; N =9 +/-; N=13 -/-.
Pain sensitivity in Shank1 mice
Figure 10 illustrates responses to both centrally mediated hot plate and spinally mediated tail flick thermal pain. No genotype differences were detected on latencies to first response on the hot plate test (Panel A, F (2, 26) = 1.19, NS) or on the tail flick test (Panel B, F (2, 27) = 0.19, NS). Responses in B6/Jae are presented for illustrative purposes.
Figure 10. Normal pain sensitivity in Shank1 mice.
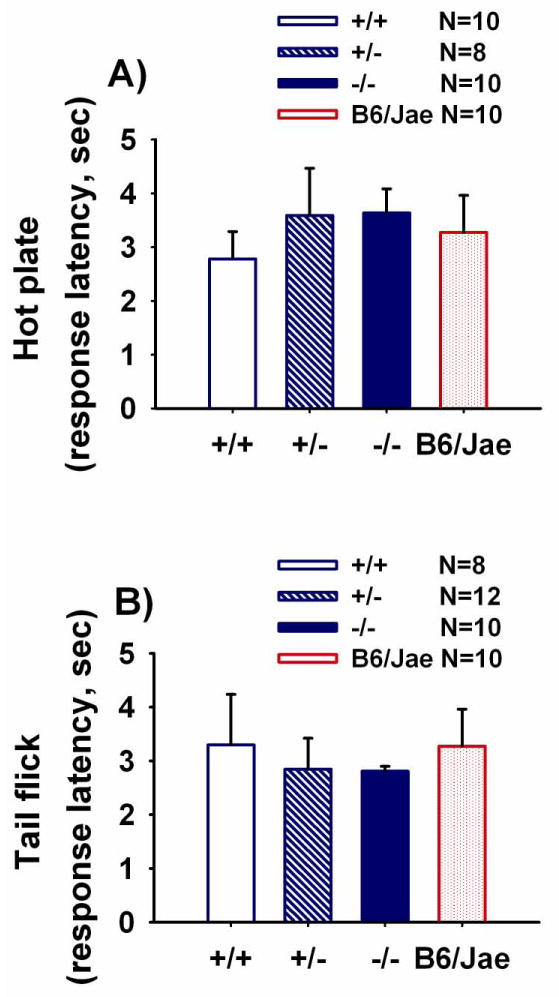
Responsiveness to painful stimuli was measured using the hot plate and tail flick tasks. No genotype differences were observed for A) latency to jump, lick or vocalize in the hot plate task. N=10 +/+; N=8 +/-; N=10 -/-; N=10 B6/Jae. No genotype differences were observed for B) latency to flick the tail out of the path of an intense light beam. N=8 +/+; N=12 +/-; N=10 -/-; N=10 B6/Jae. Scores for the genetic background mice, B6/Jae, are provided for illustrative purposes.
3. Discussion
The Shank family of proteins plays a role in the development of the postsynaptic scaffolding matrix and the maturation of dendrites in neuronal synapses in mouse brain (Boeckers et al., 1999, Naisbitt et al., 1999, Sala et al., 2001, Roussignol et al., 2005). The three Shank genes identified to date contain high homology, e.g. approximately 87% homology between Shank1 and Shank3 (Lim et al., 1999, Sheng and Kim, 2000). Expression analyses indicate that Shank1 is the only Shank isoform expressed almost exclusively in brain (Lim et al., 1999, Yao et al., 1999, Sheng and Kim, 2000). Mutations in human SHANK2 and SHANK3 genes, and in a SHANK3 binding partner, are implicated in neurodevelopmental disorders including autism (Durand et al., 2007, Abu-Elneel et al., 2008, Buxbaum, 2009, Gauthier et al., 2009, Berkel et al., 2010, Blundell et al., 2010, Pinto et al., 2010). The current study was designed to test the hypothesis that lifelong absence of Shank1 results in deficits in behavioral tasks relevant to the core symptom domains of autism.
Reciprocal interactions were examined using six of the most frequent, representative parameters to characterize active social interactions in male and female juvenile Shank1 mice (Terranova et al., 1993, Terranova and Laviola, 2005, Panksepp et al., 2007, Yang et al., 2007a, Yang et al., 2007b, McFarlane et al., 2008, Yang et al., 2009). During interactions with a naive B6 partner, Shank1 null mutants displayed social interaction scores that did not differ from wildtype littermates on any parameter, including nose-to-nose sniffing, anogenital sniffing, sniffing of other body regions, following each other, pushing past, pushing under, and crawling over the other mouse. Scores for all genotypes were similar to those reported in standard social strains of mice and in wildtype mice in other knockout studies (Terranova et al., 1993, Terranova et al., 1998, File and Seth, 2003, Bolivar et al., 2007, Moy et al., 2007, Yang et al., 2007a, Yang et al., 2007b, McFarlane et al., 2008, Moy et al., 2008, Yang et al., 2009). This negative finding in Shank1 mice is in contrast to significant reciprocal social interaction deficits reported in mice with null mutations in other synaptic genes implicated in autism, including neuroligin1, neuroligin4, and homer1a (Jaubert et al., 2007, Jamain et al., 2008, Blundell et al., 2010), but similar to normal reciprocal interactions reported for neuroligin2 and neuroligin3 mutant mice (Tabuchi et al., 2007, Chadman et al., 2008, Blundell et al., 2009, Radyushkin et al., 2009).
Adult sociability was assayed using a three-chambered task for sociability, in which mice were given a choice between spending time in the side with an unfamiliar mouse or in the side with a novel object. Impaired sociability in this task was reported for mouse lines with mutations in autism candidate genes and gene regions including Slc6a4, Nlgn4, Gabrb3, Pten, Fgf17and 15q11-13 (DeLorey et al., 2008, Jamain et al., 2008, Kwon et al., 2008, Scearce-Levie et al., 2008, Moy et al., 2009a, Nakatani et al., 2009), and for the inbred strains BTBR T+tf/J and BALB/c (Sankoorikal et al., 2006, Bolivar et al., 2007, Brodkin, 2007, Moy et al., 2007, Panksepp and Lahvis, 2007, Yang et al., 2007a, Yang et al., 2007b, McFarlane et al., 2008, Yang et al., 2009). All of the Shank1 genotypes (+/+, +/-, -/-) failed to demonstrate significant sociability, measured by the amount of time spent in the chamber with the novel mouse versus time spent in the chamber containing the novel object. Further, the +/+ and +/- failed to spend more time sniffing the novel mouse than sniffing the novel object, although the -/- did display the expected social sniff preference.
We postulated that the mixed background strain, B6/Jae, was responsible for the aberrant social phenotypes in the Shank1 +/+ and other genotypes. To investigate this possibility, a separate cohort of B6/Jae mice, generated from breeding pairs of 129SvJae (Massachusetts Institute of Technology, Cambridge, MA) and B6, was tested for sociability. The hybrid background B6/Jae mice showed normal sociability. Therefore, the observed lack of sociability in the Shank1 genotypes cannot be attributed directly to their background strain. Lack of sociability in wildtype control mice is unusual but has been observed in a few other mutant lines, including Nrcam and Avpr1b knockout mice (Yang et al., 2007a, Moy et al., 2009b). One potential explanation for the absence of sociability in the Shank1 line may reside in parental care. It is possible that altered behavior of the Shank1 +/- mother could affect performance on behavioral tasks such as social approach via epigenetic modulation (Champagne, 2010). Analyses of maternal behaviors, including quantitative scoring of licking and grooming, pup retrieval latencies and qualitative nest building by the dams (Francis et al., 1999, Lonstein and Fleming, 2002), could reveal maternal influences that impacted negatively on subsequent social behaviors in the offspring. Another potential explanation for the absence of sociability in the Shank1 line may be home cage interactions among littermate pups, which may also affect later social performance (D'Andrea et al., 2007, Branchi, 2009). Analyses of home cage interactions, including huddling, nesting, allogrooming, fighting and sleeping patterns, could determine whether littermate interactions may have impacted negatively on sociability scores in the Shank1 +/+ but not in the B6/Jae hybrid. Because the background strain comes from a separate breeding line than the original Shank1 +/- breeding pairs, potential differences in maternal care, sibling interactions, and other home cage differences may explain the observed difference in sociability scores between the +/+ Shank1 and the B6/Jae hybrid, despite the fact that the genetic make-up of the two groups should be identical. In addition, genetic drift across generations could have introduced variations in the mixture of genes from the two original background strains across individual subject mice. 129SvJae is known to exhibit relatively low levels of maternal care, low object exploration and low exploratory activity (Kim et al., 2005, Champagne et al., 2007, Silverman and Crawley, unpublished observations). It is possible that the Shank1 +/- breeding pairs and their offspring incorporated a higher penetrance of 129SvJae phenotypes than the B6/Jae hybrid line.
A third measure of social interest conducted in the present study was a component of the olfactory habituation/dishabituation task. The olfactory cues were designed to measure familiar and unfamiliar odors, with and without social valence. In the olfactory habituation/dishabituation test, Shank1 wildtypes, heterozygotes and null mutants all showed normal levels of sniffing, habituation, and dishabituation, to the non-social odors, i.e. water, almond, and banana, and to two social odors taken from two different cage bottoms containing soiled litter from unfamiliar mice strains. These results indicate normal sensory abilities in both the main and accessory olfactory systems in all genotypes. In addition, the amount of time spent sniffing social odors, as measured by the height of the peaks, did not differ between genotypes, and were similar to the peak heights obtained in previous experiments with B6 mice and wildtypes from oxytocin, galanin and vasoactive intestinal peptide mutant mouse lines (Crawley et al., 2007; Stack et al., 2008; Wrenn et al., 2003). These results indicate normal interest in social odors. Thus, the Shank1 genotypes appear to have both the normal olfactory abilities and the normal interest in social pheromones that are considered essential for mouse social behavior (Cheal and Sprott, 1971; Dantzer and Bluthe, 1993; Pfeiffer and Johnston, 1994; Ryan et al., 2008; Wrenn et al., 2003). Related findings from our laboratory suggest impairments in olfactory scent marking and ultrasonic vocalizations, independent of olfactory ability, in Shank1 null mutants in two tasks relevant to the second diagnostic symptom of autism, impaired communication skills (personal communication, Wohr, Roullet, Laboratory of Behavioral Neuroscience, National Institute of Mental Health).
The third diagnostic symptom of autism includes high levels of repetitive behaviors (Richler et al., 2007, Happe and Ronald, 2008, Richler et al., 2010). All three Shank1 genotypes exhibited high self-grooming scores in a 10-minute self-grooming assay. Scores were approximately 2 fold higher than usually observed for B6 control mice in past experiments in our laboratory. High levels of self-grooming have been reported in other genetic mouse models of autism (Yang et al., 2007a, McFarlane et al., 2008, McNaughton et al., 2008, Etherton et al., 2009, Yang et al., 2009, Blundell et al., 2010, Mines et al., 2010, Ryan et al., 2010, Silverman et al., 2010). However, self-grooming scores in Shank1 wildtype littermates and also in the B6/Jae hybrid background control group indicate that the present scores are not related to genotype. Conditions present in the animal facility or during the behavioral testing may have contributed to the higher grooming scores in the present experiment, as compared to previous grooming scores from other batches of mice.
Anxiety is considered an associated symptom of autism, i.e. present in a subset of individuals with autism spectrum disorders (American Psychiatric Association, 1994, de Bruin et al., 2007, Simonoff et al., 2008, Hallett et al., 2009). Shank1 null mutant mice displayed an anxiety-like phenotype, reduced transitions in the light ↔ dark task, replicating earlier findings (Hung et al., 2008). Number of transitions is often considered to be the more robust, sensitive component of this light ↔ dark conflict paradigm (Crawley and Goodwin, 1980, Blumstein and Crawley, 1983, Mathis et al., 1995, Holmes et al., 2003d). However, other parameters of the light ↔ dark task, and scores on the elevated plus-maze task for anxiety-like behaviors, did not differ across genotypes. The present results indicate a mild anxiety-like phenotype attributable to the Shank1 mutation, as previously reported (Hung et al., 2008). However, the possibility exists that the lower transitions in the light ↔ dark conflict task were the result of impaired motor abilities.
Motor functions were evaluated on multiple measures of activity, neuromuscular strength and coordination and balance, as previously described (Chadman et al., 2008; Holmes et al., 2001; Holmes et al., 2002; Holmes et al., 2003a; Holmes et al., 2003d; Karlsson et al., 2008; McDonald et al., 2001; Silverman et al., 2010). On the novel open field test, Shank1 null mutants had lower scores on the parameters of total distance traversed and center time, replicating Hung et al. (2008). Additional lower total distance scores in Shank1 null mutants were observed during the olfactory scent marking task developed by members of our laboratory (personal communication, Wohr, Roullet, Laboratory of Behavioral Neuroscience, National Institute of Mental Health). However, null mutants did not differ from wildtype littermates on open field parameters of horizontal and vertical activity. Shank1 null mutants displayed faster latencies to fall from the accelerating rotarod, as compared to wildtype and heterozygote littermates, replicating previous findings (Hung et al., 2008). The rotarod deficit in Shank1 null mutants was significant both during the initial testing day and the second testing day. Null mutants fell off the rotarod in approximately 30 seconds on the first trial and improved to approximately 150 seconds latency by the sixth training trial. In comparison, wildtype littermates fell off the rotarod in approximately 85 seconds on the first trial and improved to approximately 190 seconds by the sixth training trial. Shank1 null mutants displayed motor deficits on a third task, inverted wire hang. In the inverted wire hang task, neuromuscular strength was evaluated by the ability of the subject mouse to hang upside down from a wire screen over a 60 second trial. An approximately 30% reduction was seen in the amount of time that Shank1 null mutants were able to hang upside down as compared to their wildtype littermates.
Hyperreactivity and hyporeactivity to sensory stimuli, and reduced sensorimotor gating, have been reported in children with autism (Rogers et al., 2003, Rogers and Ozonoff, 2005, Kern et al., 2006, Perry et al., 2007, Orekhova et al., 2008, Jones et al., 2009). Shank1 null mutant mice displayed scores that did not differ from wildtype littermates on acoustic startle, including very loud tones, up to 120db. Sensorimotor gating, measured by prepulse inhibition of acoustic startle, did not differ across Shank1 genotypes. Responses to painful stimuli did not differ across Shank1 genotypes, as measured on two standard nociception tasks, the centrally mediated hot plate test and the spinal reflex tail flick test. Thus, sensory abilities appear to be unaffected by the Shank1 mutation.
The major phenotypic outcomes of the Shank1 deletion were impairments on three motor tasks, open field, rotarod, and wire hang, along with indications of anxiety-like behavior on the light ↔ dark task. These findings fully corroborate results reported in the original description of this Shank1 mutant line (Hung et al., 2008). Individual motor task parameters that replicated exactly were reduced distance traversed and less time spent in the center of a novel open field, reduced latency to fall from an accelerating rotarod, and fewer transitions between chambers in the light ↔ dark test of anxiety-related behaviors, as compared to wildtype littermates. Shorter latencies for the Shank1 null mutants to fall on the hanging wire test, as conducted in the present experiments only, provides a further corroboration of motor deficits. It is important to note that Shank1 knockouts did not demonstrate an anxiety-like phenotype on the elevated plus-maze, a task conducted in the present experiments but not in the previous report. Hung et al. (2008) reported impairments in contextual fear conditioning and improved retention of spatial memory in the radial arm maze in Shank1 knockouts, while learning and memory tasks were not conducted in the current phenotyping battery. Conversely, the current study analyzed social behaviors, which were not tested previously. Three social tasks were employed herein: juvenile reciprocal social interactions, adult social approach in an automated three-chambered apparatus, and olfactory habituation/dishabituation to social odors. Results indicate no social deficits in Shank1 null mutants. The adult social approach task yielded inconclusive data due to low sociability in the wildtype and heterozygote littermate comparison groups. Other new findings reported herein were normal general health and neurological reflexes, and no genotype differences on repetitive behaviors, sensory abilities including acoustic startle, prepulse inhibition, hot plate, tail flick, and olfactory discrimination of non-social and social odors. The present studies therefore replicate the previously reported motor phenotypes, and provide a full characterization of normal physical, sensory, and social phenotypes in Shank1 heterozygote and null mutant mice, as compared to wildtype littermates.
Taken together, our comprehensive phenotyping results yield a profile of motor deficits and a mild-anxiety-like phenotype in mice with null mutations in the Shank1 gene, as compared to wildtype littermates. Lack of robust social deficits in Shank1 null mice support the interpretation that the absence of Shank1 in mice does not directly affect sociability, but may play a larger role in cognitive abilities, including motor learning on the rotarod, and on fear conditioning and radial maze learning and memory as previously reported (Hung, et al., 2008), consistent with the actions of Shank1 on the maturation of postsynaptic densities. Our findings from Shank1 mutant mice on social tasks and measures of repetitive behavior indicate the absence of phenotypic outcomes relevant to the first and third diagnostic symptoms of autism.
4. Experimental Procedures
Mice
Shank1 mutant mice were generated as previously described (Hung et al., 2008). Briefly, a 2 kb BstXIHindIII fragment containing exons 14 and 15 encoding almost the entire PDZ domain was replaced by the PGK-neo cassette in the same transcriptional orientation as Shank1. Chimeric mice were produced by injecting targeted ES cell clones into C57BL/6 blastocysts. Heterozygous offspring were backcrossed into both C57BL/6 (B6) and 129SvJae (129Jae) strains. These two lines of mice, generated on two independent background strains, were imported to the National Institute of Mental Health (NIMH) in Bethesda, MD for subsequent breeding. High mortality of Shank1 mice was obtained in the B6 background strain. Very low locomotion was obtained for Shank1 mice of all genotypes in the 129Jae background strain. Therefore, the two lines were crossed, to produce a mixed C57BL/6/129SvJae (B6/Jae) background for the Shank1 mutation, consistent with the original studies of Shank1 mutants (Hung et al., 2008). The cross of heterozygous offspring was inbred for at least three generations. Therefore, the Shank1 mice used for the present experiments were on a 50-50% B6/Jae hybrid genetic background. To understand the baseline behavioral phenotypes of the hybrid B6/Jae genetic background, breeding pairs of the 129SvJae (129Jae) background strain were imported (gift from R. Jaenisch, Massachusetts Institute of Technology, Cambridge, MA) and bred with B6 to generate a B6/Jae hybrid background control line of mice.
Heterozygous Shank1 males and females were bred in a conventional mouse vivarium at the NIMH in Bethesda, Maryland, using harem breeding trios. After two weeks with a male, females were separated into individual cages. Pups were kept with the dam until weaning at 21 days of age. After weaning, juveniles were housed by sex in standard plastic cages in groups not exceeding four per cage. All experiments were conducted with littermates of each genotype. Mice were housed in a conventional animal facility on a 12h-12h light-dark cycle (lights on from 0700 hr to 1900 hr). Cages were housed in ventilated racks in colony rooms maintained at ∼20°C temperature and ∼55% humidity. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. All experiments were conducted and analyzed by investigators who were uninformed of the genotype during the behavioral tasks. All procedures were conducted in strict compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and approved by the NIMH Animal Care and Use Committee.
Genotyping
Genotyping of mouse tail DNA was conducted using standard PCR methods. Briefly, 0.5 cm tail snips were digested and DNA isolated using the REDExtract-N-Amp Tissue PCR kit (Sigma Aldrich, St. Louis, MO). The PCR reaction utilized primers that recognize sequences from the deleted segment in forward (WT-F; CAA ACC CCC ATC GAG GAA TTC) and reverse (WT-R; CCA GGA CTG ACT GGG CTA GC), and Neo primers that were generated against the Neo cassette introduced in the targeting vector reading forward (Neo-F; GCT TGG GTG GAG AGG CTA TTC) and reverse (Neo-R; CAA GGT GAG ATG ACA GGA GAT C). The PCR reaction was run on a 1.5% agarose gel.
Behavioral tests
Behavioral experiments were conducted in dedicated behavioral testing rooms during the standard light phase, usually between 0900 and 1700 hr. Mice were brought to a holding room in the hallway of the testing area at least one hour prior to the start of the behavioral test. Order of testing was as follows (1) juvenile play at age 20-22 days, (2) elevated plus-maze at age 5-6 weeks, (3) light ↔ dark exploration at age 6-7 weeks, (4) open field locomotion and rotarod at age 7–9 weeks, (5) adult social approach at 8–10 weeks, (6) general health, neurological reflexes and pain sensitivity at age 9–11 weeks, (7) self-grooming at age 10-12 weeks, and (8) olfactory habituation/dishabituation at age 12–14 weeks. For each experiment, male and female mice were used in approximately equal proportions. Data from males and females were subsequently compared for sex differences in each behavioral task.
Juvenile reciprocal social interaction
Juvenile reciprocal social interactions were tested in mice between postnatal days 20-22 in the Noldus PhenoTyper Observer 3000 chamber (Noldus Information Technology, Leesburg, VA), as previously described (Yang et al., 2007b, Chadman et al., 2008, McFarlane et al., 2008, Yang et al., 2009). The floor of the arena was covered with a 0.5-cm layer of clean bedding. Subjects were individually housed in standard mouse cages for 1 hour prior to the play session. An individual Shank1 mouse was then placed in the arena, with an age and sex matched juvenile B6 partner. Interactions were recorded for 10 min, the period during which the majority of social interactions occur, using a digital videocamera (Noldus Information Technology, Leesburg, VA). B6 mice were chosen as the partners because this strain exhibits high sociability and is neither unusually high nor unusually low on most behavioral traits (Moy et al., 2007, Moy et al., 2008). Behaviors were subsequently scored from digital videotapes by a highly trained observer, using Noldus Observer 8.0XT software (Noldus Information Technology, Leesburg, VA). The observer was uninformed of the genotype during scoring. Parameters of juvenile mouse social behaviors were chosen from the established literature and from our previous studies (Laviola and Terranova, 1998, Bolivar et al., 2007, Yang et al., 2007a, Yang et al., 2007b, Chadman et al., 2008, McFarlane et al., 2008, Yang et al., 2009). Investigative behaviors included anogenital sniffing, nose-to-nose sniffing and body sniffing. Affiliative behaviors include following a partner or being followed around the cage without any fast, sudden, or running movements, pushing underneath the partner's anterior body area or crawling over the back of a partner, and pushing past between the play partner and the cage wall.
Sociability
Social approach was tested in an automated three-chambered apparatus using methods previously described (Crawley, 2007, Yang et al., 2007a, Chadman et al., 2008, Yang et al., 2009, Silverman et al., 2010). The apparatus was a rectangular, three-chambered box made from clear polycarbonate. Retractable doorways within the two dividing walls allowed access to the side chambers. Number of entries and time spent in the chambers were automatically recorded from photocells embedded in the doorways (equipment and software built by George Dold and co-workers, Research Services Branch, NIH, Bethesda, MD). A top mounted CCTV camera (Security Cameras Direct, Luling, TX) was placed over the boxes to record the session, for subsequent scoring of the videos for time spent sniffing the novel mouse and novel object. The apparatus was cleaned with 70% ethanol and water between subjects. At least five minutes elapsed between cleaning and the start of the next test session to allow for ethanol evaporation and clearance of ethanol vapor odors. Mice used as the novel stimulus target were 129S1/SvImJ, aged 12–20 weeks old, bred and maintained in the NIMH vivarium from breeding pairs originally obtained from The Jackson Laboratory, and matched to the subject mice by sex and age. Stimulus mice were habituated to the apparatus and to the wire cup enclosure, several days before the start of experiments, for 15 minutes during 3 habituation sessions per day for 2 days. The location (left or right) of the novel object and novel mouse alternated across subjects. The subject mouse was allowed to acclimate to the apparatus before the sociability test, with 10 minutes in the central chamber with the doors closed, followed by 10 minutes in the entire empty arena with the doors open. The subject was then briefly confined to the center chamber while a novel object (inverted wire pencil cup, Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com) was placed in one of the side chambers and a novel mouse was placed inside an identical inverted wire cup in the other side chamber. Novel mice were enclosed in a wire cup to ensure that all social approach was initiated by the subject, and to avoid complications of fighting and sexual activity, while allowing visual, olfactory, auditory, and partial tactile contact through the widely spaced wire bars. A plastic drinking cup (Solo Cup Company, Highland Park, IL) containing a lead weight was placed on the top of the inverted wire cups, to prevent climbing and sitting on top of the inverted wire cup during the sociability phase test. Time spent in each chamber and number of entries into each chamber were calculated by the automated software, based on the movements of the subject mouse in sequentially breaking and unbreaking a series of photocell beams embedded in the openings between chambers. Number of entries served as a within-task control for levels of general exploratory locomotion. Lack of innate side preference was confirmed during the initial 10 minutes of habituation to the entire arena. After both stimuli were positioned, the doors were simultaneously re-opened and the subject was allowed access to all three chambers for 10 minutes. An observer uninformed of the genotypes scored the videos with a stopwatch for cumulative time in which the subject mouse sniffed the target mouse. At the end of each testing day, test chambers were thoroughly cleaned with Alconox (Alconox, White Plains, NY) detergent diluted with warm water, followed by extensive rinsing with hot water and air drying.
Olfactory habituation/dishabituation
The ability to discriminate non-social and social odors was measured using modifications of the olfactory habituation/dishabituation task, as previously described (Wrenn et al., 2003, Crawley et al., 2007, Stack et al., 2008, Yang and Crawley, 2009). Subjects were individually tested for time spent sniffing cotton tipped swabs (6 in. length, Solon Manufacturing Company, Solon, Maine) suspended from the cage lid. The olfactory cues were designed to measure familiar and unfamiliar odors, with and without social valence. Sequences of three identical swabs assayed habituation to the same odor. Switching to a different odor on the swab assayed dishabituation, i.e. recognition that an odor is new. Swabs were dipped in (1) distilled water, (2) almond extract (McCormick, Hunt Valley, MD; 1:100 dilution), (3) banana flavoring (McCormick, Hunt Valley, MD; 1:100 dilution), (4) wiped in a zig-zag pattern across the bottom surface of a plastic cage that contained four unfamiliar mice of a different strain, 129S1/SvImJ, but the same sex (social odor 1) (5) wiped in a zig-zag pattern across the bottom surface of a second plastic cage of four unfamiliar mice of another different strain, FVBS/Ant, but the same sex (social odor 2). The order of swab presentation was: water, water, water, almond, almond, almond, banana, banana, banana, social odor 1, social odor 1, social odor 1, social odor 2, social odor 2, and social odor 2. Time spent sniffing the swab was quantitated with a stopwatch by an observer uninformed of genotypes of the subject mouse. Sniffing was scored when the nose was within 2 cm of the cotton swab. Each swab was presented for a 2 min period, immediately following the last swab presentation, for a total session length of approximately 30 min per mouse. Each test session was conducted in a clean mouse cage containing fresh litter.
Self-grooming
Mice were scored for spontaneous grooming behaviors as previously described (Yang et al., 2007b, McFarlane et al., 2008). Each mouse was placed individually into a standard mouse cage, (46 cm length × 23.5 cm wide × 20 cm high), illuminated at ∼ 40 lux. A thin (1 cm) layer of bedding reduced neophobia, while preventing digging, a potentially competing behavior. A front mounted CCTV camera (Security Cameras Direct, Luling, TX) was placed approximately 1 meter from the cages to record the sessions. After a 5-minute habituation period in the test cage, each mouse was scored with a stopwatch for 10 minutes for cumulative time spent grooming all body regions. A trained observer uninformed of the genotypes scored the videos.
Open field locomotion
General exploratory locomotion in a novel open field environment was assessed as previously described (Holmes et al., 2001, Holmes et al., 2002, Holmes et al., 2003a, Holmes et al., 2003d, Bailey et al., 2007, Chadman et al., 2008, Karlsson et al., 2008, Silverman et al., 2010). Individual mice were placed in a VersaMax Animal Activity Monitoring System (AccuScan Instruments, Columbus, OH, USA) for a 30-minute test session. The testing room was illuminated with overhead lighting at ∼ 200 lux. The chambers consisted of clear Plexiglas sides and floor, approximately 40 × 40 × 30.5 cm. Mice were placed in the center of the open field at the initiation of the testing session. Photocells at standard heights for recording activity were aligned 8 to a side, dividing the chamber into 64 equal squares. Horizontal activity, total distance, vertical activity and center time were automatically collected using the Versamax activity monitor and analyzer software system. Test chambers were cleaned with 70% ethanol between test subjects. At least five minutes between cleaning and the start of the next session was allowed for ethanol evaporation and odor dissipation.
Accelerating rotarod
Motor coordination, balance, and motor learning were assessed using an accelerating rotarod (Ugo Basile, Schwenksville, PA) as previously described (Holmes et al., 2001, Paylor et al., 2006). Mice were placed on a cylinder which slowly accelerated from 4 to 40 revolutions per minute over a 5 minute (300 second) test session. The task requires the mice to walk forward in order to remain on top of the rotating cylinder rod. Mice were given 3 trials per day with a 30–60 minute intertrial rest interval. Mice were tested over two consecutive days for a total of 6 trials. Latency to fall was recorded with a 300 second maximum latency.
Inverted wire hang
The wire hang test, used to evaluate limb strength, was performed as described previously (McDonald et al., 2001). The task was performed by placing the mouse on the wire bars of a standard mouse cage lid, allowing the mouse to obtain its grip and then swiftly inverting the lid over an empty mouse cage to avoid injury. Latency to fall into the empty cage was measured with a stopwatch over a 60 second maximum test session. Mice that fell in less than 10 seconds were given a second trial. A clean wire lid was used for each mouse. The trained experimenter was uninformed of genotypes.
Elevated plus-maze
The elevated plus-maze test was performed as previously described (Holmes et al., 2001, Holmes et al., 2002, Holmes et al., 2003d, Bailey et al., 2007, Karlsson et al., 2008). The apparatus (San Diego Instruments, San Diego, CA) was comprised of two open arms (30 × 5 cm2) and two closed arms (30 × 5 × 15 cm3) that extended from a common central platform (5 × 5 cm2). A small raised lip (0.25 cm) around the edges of the open arms helped prevent mice from slipping off. The apparatus was constructed from polypropylene and Plexiglas, with a white floor and clear walls, and elevated to a height of 38 cm above floor level. One hour after bringing the mice to the testing facility, each mouse was placed on the center square facing an open arm and allowed to freely explore the apparatus under a light intensity of ∼ 30 lux for 5 minutes. The 5 min session was recorded by a top mounted CCTV camera (Security Cameras Direct, Luling, TX), placed approximately 1 meter from the maze. The maze was cleaned with 70% ethanol and water between subjects, with at least five minutes between cleaning and the start of the next test session, to allow for ethanol evaporation and clearance of ethanol vapor odors. Each 5 minute session was scored by a trained observer using Noldus Observer 8.0 XT software (Noldus Information Technology, Leesburg, VA). Behaviors scored were time spent in the open arms, number of open arm entries, and number of open and closed arm entries combined to give a total entries measure of general exploratory activity. An open or closed arm entry was defined as all four paws into an arm. A center entry was defined as both forepaws being placed into the center.
Light ↔ dark exploration test
The light ↔ dark exploration test was conducted as previously described (Crawley and Goodwin, 1980, Mathis et al., 1995, Holmes et al., 2001, Holmes et al., 2002, Holmes et al., 2003b, Holmes et al., 2003c, Holmes et al., 2003d, Karlsson et al., 2008). The apparatus consisted of a polypropylene cage (44 × 21 × 21 cm3) separated into two compartments by a partition, with a rectangular opening (12 × 5 cm2) at floor level. The larger compartment (28 cm long) was open topped, transparent, and lit using overhead fluorescent ceiling lights (∼400 lux). The smaller compartment (14 cm long) had black painted sides and was covered at the top with black Plexiglas, creating a closed dark space (∼ 5 lux). The subject mouse was individually placed in the light compartment, facing away from the partition, and allowed to freely explore the apparatus for 10 min. The apparatus was cleaned with 70% ethanol after each subject. The number of transitions, i.e. entries between the two compartments, and the total time spent in the dark compartment were detected by photocells located within the partition, across the opening between the two chambers. Data from the beam breaks were automatically analyzed by dedicated software (fabricated by Bruce Smith, George Dold, and co-workers, Research Services Branch, NIH, Bethesda, MD). The latency to enter the dark side was scored by a trained observer using a stopwatch.
Acoustic startle threshold
Acoustic startle was measured in separate experiments using the SR-Lab System (San Diego Instruments, San Diego, CA) as previously described (Paylor and Crawley, 1997, Chadman et al., 2008). Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5-minute acclimation period. Over the next 8 minutes, mice were presented with each of six trial types across five discrete blocks of trials for a total of 30 trials. The intertrial interval was 10-20 s. One trial type measured the response to no stimulus (baseline movement). The other five trial types measured the response to a startle stimulus alone, consisting of a 40 millisecond (ms) sound burst of 80, 90, 100, 110 or 120 dB. Startle amplitude was measured every 1 ms over a 65 ms period beginning at the onset of the startle stimulus. The maximum startle amplitude over this sampling period was taken as the dependent variable. A background noise level of 70 dB was maintained over the duration of the test session.
Prepulse inhibition of acoustic startle
Prepulse inhibition of acoustic startle was conducted as previously described (Paylor and Crawley, 1997, Dulawa and Geyer, 2000, Holmes et al., 2001, Chadman et al., 2008). Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5-minute acclimation period. Over the next 10.5 minutes, mice were presented with each of seven trial types across six discrete blocks of trials for a total of 42 trials. The intertrial interval was 10-20 s. One trial type measured the response to no stimulus (baseline movement) and another measured the response to the startle stimulus alone (acoustic startle response) which was a 40 ms 110 dB sound burst. The other five trial types were acoustic prepulse plus acoustic startle stimulus trials. Prepulse tones were 20 ms at 74, 78, 82, 86, and 90 dB, presented 100 ms prior to the 110 dB startle stimulus. Startle amplitude was measured every 1 ms over a 65 ms period beginning at the onset of the startle stimulus. The maximum startle amplitude over this sampling period was taken as the dependent variable. A background noise level of 70 dB was maintained over the duration of the test session.
Hot plate pain sensitivity
Response to an acute thermal stimulus was measured using the hot plate test as described previously (Blakeman et al., 2003, Wiesenfeld-Hallin et al., 2005, Bailey et al., 2007, Chadman et al., 2008). The mouse was placed on a flat, black metal surface (IITC Life Science, Inc., Woodland Hills, CA) maintained at 55 °C and surrounded by a square transparent plexiglass barrier to prevent jumping off. The latency to the first paw lick, jump or vocalization was measured by an observer using a foot pedal-controlled timer. A maximum cut-off time of 30 s was used to prevent the risk of tissue damage to the paws.
Tail flick pain assessment
Response to thermal stimulation of the tail was conducted as previously described (Blakeman et al., 2003, Wiesenfeld-Hallin et al., 2005, Bailey et al., 2007, Chadman et al., 2008). Mice were gently held in place with the tail lying along the groove of the tail-flick monitor (Columbus Instruments, Columbus, OH). An intense photobeam was directed at the tail. The latency for the mouse to move its tail out of the path of the beam was timed automatically by the apparatus. To prevent any tissue damage there was a maximum cutoff latency of 10 seconds.
General health and neurological reflexes
General health was evaluated using measures described previously (Crawley and Paylor, 1997, Bailey et al., 2007, Crawley, 2007). General health assessment included assessing physical condition of fur and whiskers as well as limb and body tone. Empty cage behaviors were scored by placing the mouse into a clean, empty cage and noting incidents of wild running, stereotypies, and excessive exploration levels. Neurological reflex tests included forepaw reaching, righting reflex, trunk curl, whisker twitch, pinnae response, eyeblink response and auditory startle. The reactivity level of the mice was assessed with tests measuring responsiveness to petting, intensity of a dowel biting response and level of vocalization during handling. Home cage observations involved scoring the activity of all mice in a home cage for approximately 15-minute at three different daily time points (9:00 am, 3:00 pm, and 8:00 pm). The experimenter scored incidence of excessive fighting, grooming, stereotypies, isolated mice, lack of huddling and quality of nest building.
Statistical analysis
Genotype differences were analyzed with a one-way analysis of variance (ANOVA). Significant ANOVA results were followed by Bonferroni-Dunn posthoc analysis for the light ↔ dark exploration task, elevated plus-maze, juvenile play, hot plate and tail flick pain assessments, inverted wire hang and repetitive self-grooming. Acoustic startle threshold, prepulse inhibition, accelerating rotarod, open field locomotion and olfactory habituation/dishabituation were analyzed using a Repeated Measures ANOVA. Significant Repeated Measures ANOVA results were followed by a Student-Newman-Keuls' analysis, where applicable, via StatView statistical software (Citewise.com, Acton, MA) and SigmaPlot version 11.0 (Systat Inc., San Jose, CA). Measures of general health and neurological reflexes that utilized continuous variables, such as temperature and weight, were analyzed with a one-way ANOVA followed by a Bonferroni-Dunn post-hoc, where applicable. Measures of general health and neurological reflexes that utilized a rating of present or absent were analyzed for genotype differences using a Chi-squared statistic. Reflexes or physical parameters that were rated on a 3-point ranking scale were analyzed using a non-parametric Kruskal- Wallis for ranks ANOVA. Social approach was analyzed using a within groups Repeated Measures ANOVA, to compare time spent in the side chambers in the sociability test. Since the times spent in each of the three chambers added to 10 minutes, and therefore were not independent, the test condition factor compared time spent only in the right versus left chambers. Center chamber times are shown in the graphs for illustrative purposes. Time spent sniffing the novel object versus the novel mouse and entries into the side chambers were similarly analyzed using within groups Repeated Measures ANOVA. Since the inbred hybrid B6/Jae mice were not littermates of the Shank1 mice, data collected from this group are shown for illustrative purposes but were not included in statistical analyses, except in the case of social approach, as part of the within genotype comparisons. All data were graphed using SigmaPlot version 11.0 (Systat Inc., San Jose, CA).
Acknowledgments
Supported by the National Institute of Mental Health Intramural Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao K, Kosik KS. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9:153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington D.C: American Psychiatric Association; 1994. [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- Blakeman KH, Hao JX, Xu XJ, Jacoby AS, Shine J, Crawley JN, Iismaa T, Wiesenfeld-Hallin Z. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117:221–227. doi: 10.1016/s0306-4522(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Blumstein LK, Crawley JN. Further characterization of a simple, automated exploratory model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1983;18:37–40. doi: 10.1016/0091-3057(83)90247-2. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockers TM, Segger-Junius M, Iglauer P, Bockmann J, Gundelfinger ED, Kreutz MR, Richter D, Kindler S, Kreienkamp HJ. Differential expression and dendritic transcript localization of Shank family members: identification of a dendritic targeting element in the 3′ untranslated region of Shank1 mRNA. Mol Cell Neurosci. 2004;26:182–190. doi: 10.1016/j.mcn.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, Wex H, Langnaese K, Bockmann J, Garner CC, Gundelfinger ED. Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci. 1999;19:6506–6518. doi: 10.1523/JNEUROSCI.19-15-06506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin Neurosci. 2009;11:35–43. doi: 10.31887/DCNS.2009.11.1/jdbuxbaum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic perspectives on development: Evolving insights on the origins of variation. Dev Psychobiol. 2010;52:e1–e3. [Google Scholar]
- Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav. 2007;91:325–334. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- D'Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res. 2007;183:60–66. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- de Bruin EI, de Nijs PF, Verheij F, Hartman CA, Ferdinand RF. Multiple complex developmental disorder delineated from PDD-NOS. J Autism Dev Disord. 2007;37:1181–1191. doi: 10.1007/s10803-006-0261-4. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Spiegelman D, Piton A, Lafreniere RG, Laurent S, St-Onge J, Lapointe L, Hamdan FF, Cossette P, Mottron L, Fombonne E, Joober R, Marineau C, Drapeau P, Rouleau GA. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Goizet C, Excoffier E, Taine L, Taupiac E, El Moneim AA, Arveiler B, Bouvard M, Lacombe D. Case with autistic syndrome and chromosome 22q13.3 deletion detected by FISH. Am J Med Genet. 2000;96:839–844. [PubMed] [Google Scholar]
- Hallett V, Ronald A, Happe F. Investigating the association between autistic-like and internalizing traits in a community-based twin sample. J Am Acad Child Adolesc Psychiatry. 2009;48:618–627. doi: 10.1097/CHI.0b013e31819f7116. [DOI] [PubMed] [Google Scholar]
- Happe F, Ronald A. The ‘fractionable autism triad’: a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003a;28:1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003b;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003c;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003d;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Sung CC, Brito IL, Sheng M. Degradation of postsynaptic scaffold GKAP and regulation of dendritic spine morphology by the TRIM3 ubiquitin ligase in rat hippocampal neurons. PLoS One. 2010;5:e9842. doi: 10.1371/journal.pone.0009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF, Kang SH, Schwarz MK, Seeburg PH, Berman RF. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–154. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Jeffries AR, Curran S, Elmslie F, Sharma A, Wenger S, Hummel M, Powell J. Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A. 2005;137:139–147. doi: 10.1002/ajmg.a.30780. [DOI] [PubMed] [Google Scholar]
- Jones CR, Happe F, Baird G, Simonoff E, Marsden AJ, Tregay J, Phillips RJ, Goswami U, Thomson JM, Charman T. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47:2850–2858. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2008;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, Johnson DG, Mehta JA, Schroeder JL. The pattern of sensory processing abnormalities in autism. Autism. 2006;10:480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- Kim D, Chae S, Lee J, Yang H, Shin HS. Variations in the behaviors to novel objects among five inbred strains of mice. Genes Brain Behav. 2005;4:302–306. doi: 10.1111/j.1601-183X.2005.00133.x. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, Mason RP, Lee EY, Wu H, Parada LF. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68:3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Terranova ML. The developmental psychobiology of behavioural plasticity in mice: the role of social experiences in the family unit. Neurosci Biobehav Rev. 1998;23:197–213. doi: 10.1016/s0149-7634(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem. 1999;274:29510–29518. doi: 10.1074/jbc.274.41.29510. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Fleming AS. Parental behaviors in rats and mice. Curr Protoc Neurosci. 2002;Chapter 8(Unit 8):15. doi: 10.1002/0471142301.ns0815s17. [DOI] [PubMed] [Google Scholar]
- Manning MA, Cassidy SB, Clericuzio C, Cherry AM, Schwartz S, Hudgins L, Enns GM, Hoyme HE. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. 2004;114:451–457. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- Mathis C, Neumann PE, Gershenfeld H, Paul SM, Crawley JN. Genetic analysis of anxiety-related behaviors and responses to benzodiazepine-related drugs in AXB and BXA recombinant inbred mouse strains. Behav Genet. 1995;25:557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Miller KM, Li C, Deng C, Crawley JN. Motor deficits in fibroblast growth factor receptor-3 null mutant mice. Behav Pharmacol. 2001;12:477–486. doi: 10.1097/00008877-200111000-00009. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One. 2010;5:e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D'Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009a;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nonneman RJ, Young NB, Demyanenko GP, Maness PF. Impaired sociability and cognitive function in Nrcam-null mice. Behav Brain Res. 2009b;205:123–131. doi: 10.1016/j.bbr.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neurosci Lett. 2008;434:218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelan MC. Deletion 22q13.3 syndrome. Orphanet J Rare Dis. 2008;3:14. doi: 10.1186/1750-1172-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI, Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010 doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C, Prasad AN, Chodirker BN, Lee C, Dawson AK, Jocelyn LJ, Chudley AE. Genetic evaluation of pervasive developmental disorders: the terminal 22q13 deletion syndrome may represent a recognizable phenotype. Clin Genet. 2000;57:103–109. doi: 10.1034/j.1399-0004.2000.570203.x. [DOI] [PubMed] [Google Scholar]
- Qin J, Jia M, Wang L, Lu T, Ruan Y, Liu J, Guo Y, Zhang J, Yang X, Yue W, Zhang D. Association study of SHANK3 gene polymorphisms with autism in Chinese Han population. BMC Med Genet. 2009;10:61. doi: 10.1186/1471-2350-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Richler J, Bishop SL, Kleinke JR, Lord C. Restricted and repetitive behaviors in young children with autism spectrum disorders. J Autism Dev Disord. 2007;37:73–85. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Dev Psychopathol. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF, Bockaert J, Fagni L. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25:3560–3570. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–354. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Schutt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284:25479–25487. doi: 10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113(Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Crawley JN (unpublished observations).
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes NH, Toma C, Wilson N, Volpi EV, Sousa I, Pagnamenta AT, Tancredi R, Battaglia A, Maestrini E, Bailey AJ, Monaco AP. Copy number variation and association analysis of SHANK3 as a candidate gene for autism in the IMGSAC collection. Eur J Hum Genet. 2009;17:1347–1353. doi: 10.1038/ejhg.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova, Laviola . Scoring of social interactions and play in mice during adolescence. Hoboken, NJ: John Wiley & Sons., Inc.; 2005. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol. 1993;26:467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. J Comp Psychol. 1998;112:3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Crawley JN, Hokfelt T. Galanin and spinal nociceptive mechanisms: recent results from transgenic and knock-out models. Neuropeptides. 2005;39:207–210. doi: 10.1016/j.npep.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social Approach Behaviors are Similar on Conventional Versus Reverse Lighting Cycles, and in Replications Across Cohorts, in BTBR T+ tf/J, C57BL/6J, and Vasopressin Receptor 1B Mutant Mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Hata Y, Hirao K, Deguchi M, Ide N, Takeuchi M, Takai Y. Synamon, a novel neuronal protein interacting with synapse-associated protein 90/postsynaptic density-95-associated protein. J Biol Chem. 1999;274:27463–27466. doi: 10.1074/jbc.274.39.27463. [DOI] [PubMed] [Google Scholar]


