Abstract
2-Aminothiazoles are a new class of small molecules with antiprion activity in prion-infected neuroblastoma cell lines.1 We report here structure-activity studies undertaken to improve the potency and physiochemical properties of 2-aminothiazoles, with a particular emphasis on achieving and sustaining high drug concentrations in the brain. The results of this effort include the generation of informative structure-activity relationships (SARa) and the identification of lead compounds that are orally absorbed and achieve high brain concentrations in animals. The new aminothiazole analog (5-methylpyridin-2-yl)-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (27), for example, exhibited an EC50 of 0.94 µM in prion-infected neuroblastoma cells (ScN2a-cl3) and reached a concentration of ~25 µM in the brains of mice following three days oral administration in a rodent liquid diet. The studies described herein suggest 2-aminothiazoles as promising new leads in the search for effective therapeutics for prion diseases.
Keywords: Neurodegenerative disease, prion disease, Creutzfeldt-Jakob disease, 2-aminothiazoles, structure-activity relationships
Introduction
The protein misfolding diseases are a family of debilitating neurological disorders associated with the misprocessing of cellular proteins into alternate non-native isoforms that confer cellular toxicity, often associated with oligomeric deposits derived from misfolded protein. Prominent examples of these disorders include Alzheimer's disease, Huntington's disease, Parkinson's disease, frontotemporal dementias, and the prion diseases – Creutzfeldt-Jakob disease in humans, chronic wasting disease in deer, and scrapie in sheep.2, 3 In prion disease, the endogenous prion protein (PrPC) is converted by an unknown mechanism into a protease-resistant and β-sheet–rich form denoted PrPSc. This conversion can occur spontaneously, result from inherited mutations in the PrPC gene, or be triggered by infection with exogenous PrPSc. Prion diseases are invariably fatal, and no viable treatments for these devastating disorders are currently available.
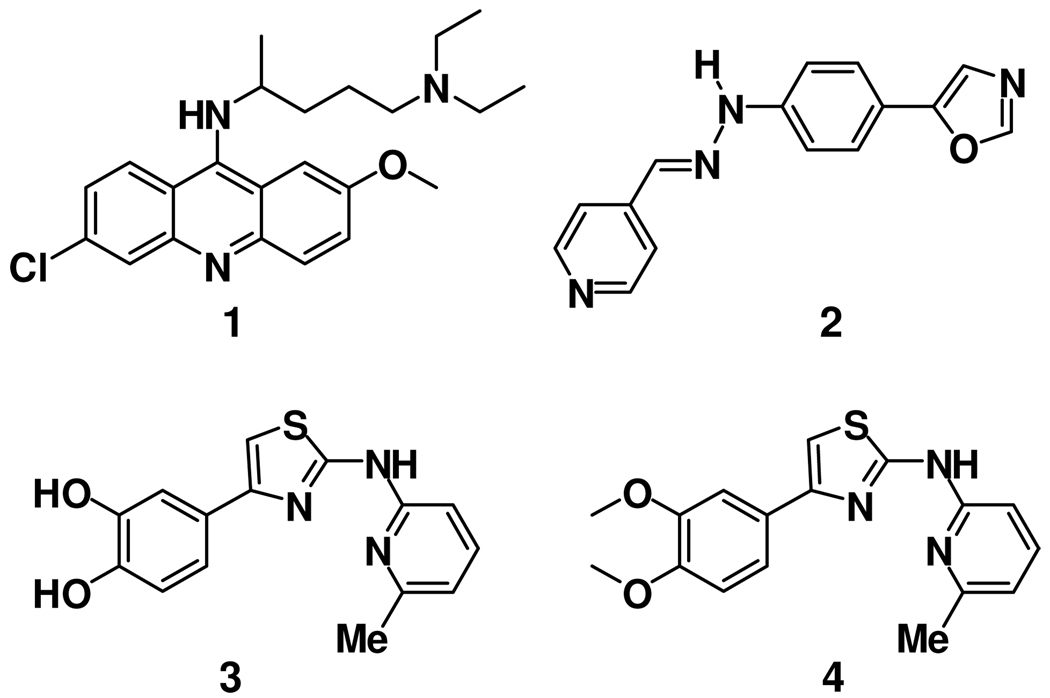
While the mechanisms of protein misfolding and subsequent disease progression remain unclear, it is well known that infectious forms of animal prions can be propagated in cell culture, notably in prion-infected, murine neuroblastoma (ScN2a) cell lines.4, 5 Various immunological methods are available to measure prion load in these cell lines and so they have provided a valuable means for evaluating the antiprion properties of large and small molecules alike. Among small molecules that have been reported to possess antiprion properties are the acridines6, 7 (e.g., quinacrine, 1) and structurally related tricyclic antidepressants; dimeric8 and chimeric9 analogs of 1; statins10; 2,4-diphenylthiazole and 2,4-diphenyloxazole amides11; pyrazolones12, 13; indole-3-glyoxylamides14; and pyridyl hydrazones15 (e.g., “compound B”, 2). In addition, larger molecules of a polyanionic chemotype (suramin, pentosan polysulfate) or polycationic chemotype (dendritic polyamines, cationic polysaccharides16) have been reported to exhibit antiprion activity in cells, although it seems unlikely such species could be used therapeutically. In fact, no small molecule has yet been shown to be broadly effective against a range of prion strains in an animal model of disease17 and only hydrazone 2 has been reported to significantly extend survival in animals (albeit strain-dependent and at high doses).15 One possible explanation for this poor track record is that the majority of small molecules investigated to date were originally designed for other purposes (e.g. malaria, hyperlipidemia), and not optimized for either antiprion effects or for the ability to cross the blood-brain barrier (BBB).
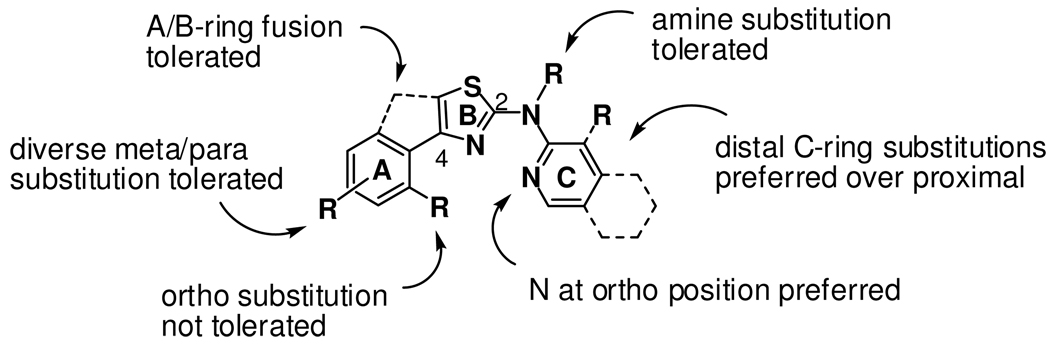
We recently described the discovery of antiprion small molecules containing the 2-aminothiazole ring system (e.g., 3, Figure 1).1 Preliminary mechanistic profiling of aminothiazole analogs indicated that they neither diminish the expression of PrPC nor denature PrPSc, thus suggesting that a mechanism influencing PrPSc formation or clearance is more likely.1 For example, the compounds might inhibit as-yet unidentified auxiliary macromolecules18 that promote prion replication or enhance the activity of proteins that facilitate the clearance of PrPSc. Here we report structure-activity studies aimed at improving the potency of 2-aminothiazoles in vitro and altering the physiochemical properties of these molecules so as to increase their likelihood of accessing the brain in vivo. The SAR studies reveal those positions in 2-aminothiazole structure that are sensitive or insensitive to modification (Figure 2). Similarly, structural modifications that improve metabolic stability and permeability are reported, as are the brain concentrations of a representative analog in mice. Together, these data suggest that 2-aminothiazoles represent a promising new class of drug leads for prion diseases.
Figure 1.
Structures of small molecules with antiprion properties.
Figure 2.
Summary of structure-antiprion activity relationships for 2-aminothiazole analogs. The three rings are arbitrarily denoted A, B, and C for convenience.
Results and Discussion
The antiprion action of new aminothiazole analogs was evaluated using a new ‘clone-3’ cell line (denoted ScN2a-cl319) that expresses a higher level of PrPSc as compared to the ScN2a cell line used in the original screen. In general we have found that EC50 values for antiprion compounds tend to be ~10-fold higher in the high-expressing cells, and thus the ScN2a-cl3 cell line represents a more stringent test of antiprion action. The EC50 values presented in the discussion below represent mean values from three separate determinations using ScN2a-cl3 cells. The precision of the assay is high; the coefficient of variance in mean pEC50 values is generally less than 5% (see Supporting Information). The high quality of the assay data allowed even subtle SAR trends to be assigned with some confidence. An evaluation of compound toxicity toward ScN2a-cl3 cells was carried out using the fluorescent probe calcein-AM as we have reported previously.20 Almost without exception we found that 2-aminothiazole analogs are not toxic to ScN2a-cl3 cells (Supporting Information), indicating that 2-aminothiazoles reduce PrPSc load in ScN2a-cl3 cells by a drug-like mechanism (i.e., not simply by killing cells). Notably, 2-aminothiazoles do not reduce PrPSc load in non-dividing ScN2a-cl3 cells that have been arrested in cell division by treatment with sodium butyrate. It should be noted however that lack of activity in non-dividing cells does not necessarily preclude antiprion efficacy in animals, as we found that hydrazone 2, like 2-aminothiazoles, has no effect on non-dividing ScN2a-cl3 cells (data not shown).
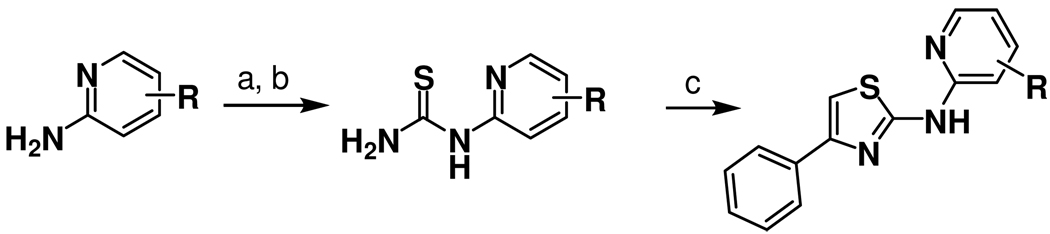
The SAR studies described herein were undertaken with the dual objectives of expanding upon nascent SAR from the primary screen1 and identifying improved aminothiazole analogs with a higher likelihood of penetrating the brain in animals. To help achieve the latter objective, we applied recently advanced21 guidelines for assessing the potential CNS activity of small molecules. These “rules of thumb” advise special attention to properties such as molecular weight (< 500 Da. preferred), polar surface area (< 90 Å2), clogP (2–5), and the number of hydrogen bond donors (< 3). The synthesis of new 2-aminothiazole analogs was carried out in both serial and parallel formats using the Hantzsch-type condensation of bromomethyl ketones with thioureas (Scheme 1 and Supporting Information). An early objective of the SAR study was to modify the catechol ring present in early screening hits like 3, since such functionality would likely limit brain exposure in vivo, and might also present metabolic and/or toxicological liabilities. Evaluation of the corresponding dimethoxyphenyl analog 4 (Figure 1) showed it to be equipotent to 3, thereby alleviating concerns that a catechol A-ring might be required for antiprion activity. With this potential liability eliminated, a systematic exploration of aminothiazole SAR was initiated.
Scheme 1.a.
aHantzsch-type synthesis of 2-aminothiazole analogs from amines via thiourea intermediates. Reagents and conditions: (a) PhSCN, acetone, reflux; (b) NaOH, MeOH, reflux; (c) bromoacetophenone, EtOH, reflux.
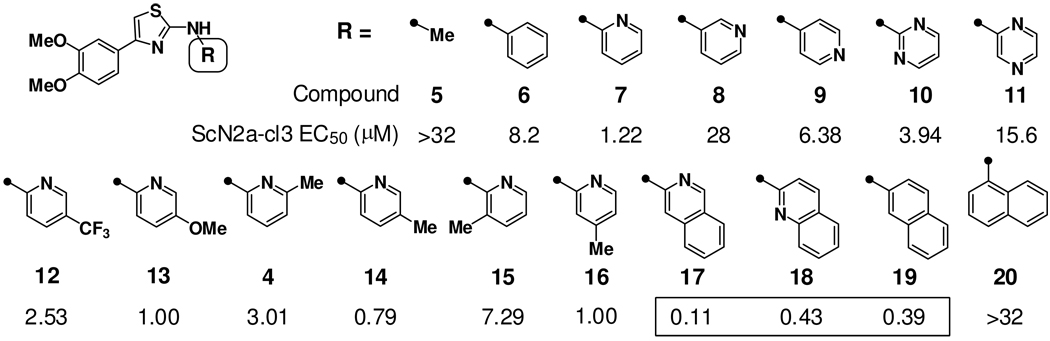
Preliminary SAR gleaned from the initial screen1 suggested a preference for 2-pyridyl type C-rings over simple aryl congeners. To evaluate more fully the C-ring SAR, a series of analogs were synthesized with alkyl, aryl, or heteroaryl groups at this position (Chart 1). The N-alkyl analog (5) was without significant activity while among regioisomeric pyridyl analogs, 2-pyridyl analog 7 was indeed more potent than the 3-pyridyl or 4-pyridyl congeners (8 and 9). Hence, analog 7 had an EC50 value (defined as the effective concentration for reducing PrPSc load in ScN2a-cl3 cells by 50%) of 1.22 µM, roughly ten-fold lower than the original1 screening hits. Replacement of the 2-pyridyl ring in 7 with 2-pyrimidyl or 2-pyrazinyl rings produced analogs of comparable (10) and reduced (11) potency, respectively. Next, we examined ring-substitution effects in the favored 2-pyridyl C-ring type. In general, ring substitution was most favorable in positions distal from the B–C ring connection, as in methyl substituted analogs 14 and 16, and especially in the more extended bicyclic C-ring analogs 17–19 (EC50 = 0.11 µM for 17). In contrast, analogs substituted proximally to the B–C ring connection (e.g., 15, 20) were notably less potent than their unsubstituted comparators. Electronic effects in the C-ring appear to be of comparably smaller importance; analogs with electron rich (13) or electron deficient (12) 2-pyridyl rings showed similar activities. The most potent analogs identified from this series were those with more extended bicyclic C-ring systems. Hence, quinoline, isoquinoline, and naphthalene analogs (17–19) were approximately ten-fold more potent than comparable monocyclic analogs, and at least 100-fold more potent than the original screening hits.
Chart 1.
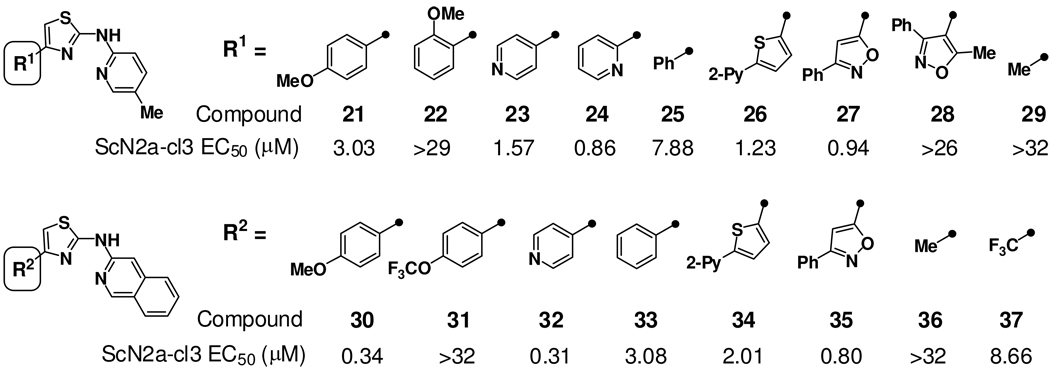
Having identified several viable new C-ring subtypes, we next explored SAR of the ‘A-ring’ positioned at C-4 of the aminothiazole ring (Figure 2). As noted above, bis-methylation of the catechol function in 3 to afford 4 was a tolerated modification. A more systematic exploration of A-ring preferences was carried out in the context of the favored 2-pyridyl and isoquinoline C-ring types. Various aromatic and heteroaromatic ring systems could be tolerated (Chart 2), but analogs bearing small alkyl groups at this position were either inactive (29, 36) or less potent (37). Analogs 25 and 33 bearing unsubstituted phenyl A-rings were between two and ten-fold less potent than pyridyl (23, 24, 32) or para-methoxyphenyl A-ring analogs (21, 30). Whereas para-methoxyphenyl analog 30 was among the most potent analogs examined (EC50 = 0.34 µM), the analogous trifluoromethoxyphenyl analog 31 was surprisingly inactive. Other favorable A-rings conferring low or sub-micromolar potencies included phenyl-substituted isoxazoles as found in analogs 27 and 35, and pyridyl-substituted thiophenes as in analogs 26 and 34.
Chart 2.
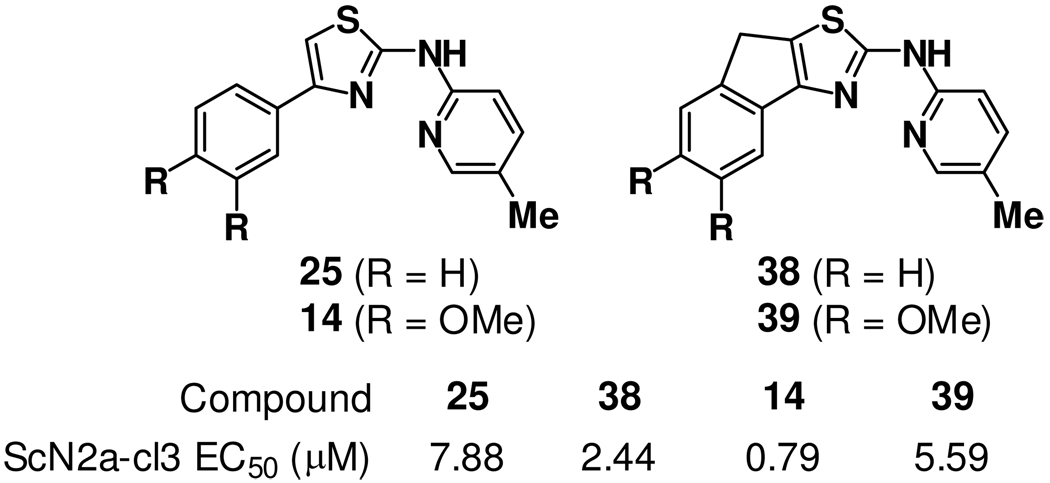
The general tolerance of para or meta substitution on the A-ring can be contrasted with an apparent intolerance for substitution at the ortho position. This effect was evident in both six-membered (compare 21 and 22) and five-membered (compare 27 and 28) A-rings and was true regardless of C-ring chemotype. These findings suggest perhaps that a co-planar arrangement of the A- and B-ring is important for activity, the presence of an ortho substituent as in 22 and 28 disfavoring such a conformation. To test this supposition, we prepared fused tricyclic analogs 38 and 39 (Figure 3) in which a co-planar conformation is enforced by A–B ring fusion. In both cases the A–B ring fusion was tolerated, being somewhat favored in the case of 38 (as compared to 25) and somewhat disfavored in the case of 39 (as compared to 14). This observation that ortho substitution is tolerated only in the context of A–B ring fusion supports the notion that a co-planar conformation of these ring systems is important for activity.
Figure 3.
Chemical structures and antiprion activity of analogs 38 and 39, in which ring fusion enforces a co-planar A/B-ring conformation. Activities of the corresponding unconstrained analogs 25 and 14 are shown for comparison.
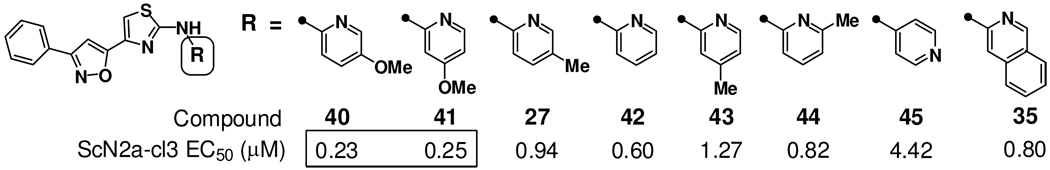
Among the new A-ring variants examined (Chart 2), phenylisoxazole 27 was notable for its sub-micromolar potency and improved stability to rat liver microsomes as compared to phenyl (25) and pyridyl (23) congeners (Table 1). This finding led to a reinvestigation of favored C-ring types in the context of the phenylisoxazole A-ring (Chart 3). Perhaps not surprisingly, the SAR of phenylisoxazole A-ring analogs was not completely reconcilable with SAR in the original dimethoxyphenyl A-ring series. Thus, whereas extended bicyclic C-rings (isoquinoline, quinoline) were optimal in combination with the dimethoxyphenyl A-ring (Chart 1), the most potent phenylisoxazole analogs were those bearing methoxypyridine C-rings, as in analogs 40 (EC50 = 0.23 µM) and 41 (EC50 = 0.25 µM). By comparison, other pyridine (27, 42–45) and quinoline (35) C-ring analogs were between four- and twenty-fold less potent.
Table 1.
In vitro microsome stability, permeability data, and calculated physiochemical properties for select 2-aminothiazole analogs.
| Antiprion activity |
Microsome stabilitya |
Permeabilityb (10−6 cm/sec) |
Calculated propertiesc | |||||
|---|---|---|---|---|---|---|---|---|
| Compound | ScN2a-cl3 EC50 (µM) |
T1/2 (min) | PA→B | PB→A | MW | PSA (Å2) |
ClogP | HBD |
| 25 | 7.88 | 10 | n.d | n.d | 267.4 | 66.0 | 4.5 | 1 |
| 23 | 1.57 | 20 | 32.6 | 42.3 | 268.3 | 78.9 | 3.2 | 1 |
| 27 | 0.94 | 151 | 6.8 | 8.3 | 334.4 | 92.1 | 4.9 | 1 |
| 28 | >26 | 19 | 11.2 | 8.6 | 348.4 | 92.1 | 5.1 | 1 |
| 12 | 2.53 | 150 | 9.4 | 8.7 | 381.4 | 84.5 | 4.4 | 1 |
| 13 | 1.00 | 45 | 20.1 | 24.4 | 343.4 | 93.7 | 3.3 | 1 |
| 17 | 0.11 | 83 | 13.2 | 10.5 | 363.4 | 84.5 | 4.5 | 1 |
| 18 | 0.43 | 69 | 9.2 | 8.9 | 363.4 | 84.5 | 4.9 | 1 |
rat liver microsomes.
Permeability across MDR1-MDCK cell monolayers in the apical to basal (absorptive) and basal to apical (secretory) directions.
Molecular Weight (MW), Polar Surface Area (PSA) and calculated n-octanol water partition coefficient (ClogP) values calculated using MarvinSketch 4.1.8. HBD = number of hydrogen bond donors. n.d. = not determined.
Chart 3.
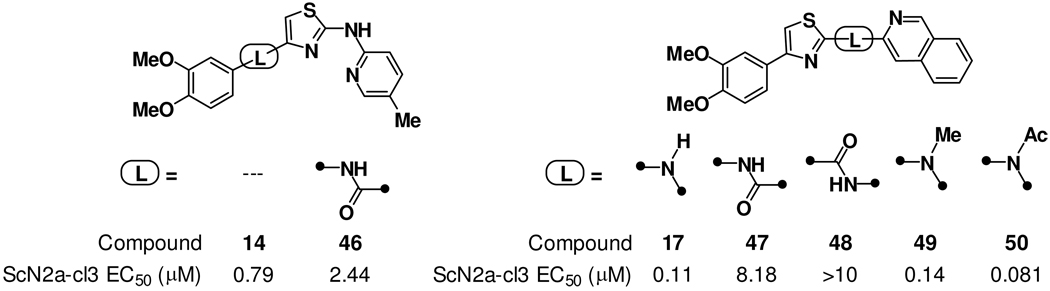
We also examined SAR relating to the nature of connection between the A-, B-, and C-rings. For example, insertion of an amide function between the A- and B-ring in analog 14 produced analog 46 of comparable potency (Figure 4). However, the consequent introduction of an additional hydrogen bond donor in 46 was judged undesirable with respect to CNS properties and so amide-linked analogs like 46 were not pursued further. With respect to the B–C ring connection, methylation (as in 49) or acylation (as in 50) of the amine linkage in analog 17 was well tolerated. In contrast, replacement of the amine linkage in 17 with amide linkages (as in 47 and 48) led to a ~100-fold loss of potency (Figure 4). Overall, activity data derived from analogs 47–50 suggest that proper spacing of the B- and C-rings is important for antiprion activity, whereas the presence of a hydrogen bond donor in the B–C ring linkage is not. This latter finding is significant since the complete elimination of hydrogen bond donors in analogs like 49 and 50 would predict for better permeability across the BBB in animals.
Figure 4.
Chemical structures and antiprion activity of analogs with modified A–B ring linkages (46) or B–C ring linkages (47–50). The introduction of an amide linkage was better tolerated at the A–B ring connection (46) than at the B–C ring connection (47 and 48). Alkylation or acylation of the amino B–C ring linkage was tolerated (17 vs 49 and 50) demonstrating that a hydrogen bond donor is not required in this position.
The SAR studies described above have revealed a number of structural determinants in the antiprion activities of 2-aminothiazoles. Just as importantly, many of the new analogs possess physiochemical properties that predict permeability across the BBB. In fact, most small molecules do not readily traverse the BBB and/or are subject to active efflux mediated by drug resistance transporters (e.g., P-glycoprotein transporter; P-gp) expressed in the endothelial cells that constitute the BBB.22 Efflux by P-gp is much more difficult to predict than is passive permeation of the BBB. To address the potential for efflux, a subset of aminothiazole analogs were evaluated for permeability in P-gp-expressing Multidrug resistance-1 Madin-Darby canine kidney (MDR1-MDCK) cell mono-layers, an assay that has been utilized as an in-vitro predictor of in-vivo BBB permeability.23 All eight analogs evaluated showed good permeability in this assay, and more importantly, none appeared likely to be P-gp substrates based on absorptive (apical to basalateral) and secretory (basalateral to apical) permeability values (Table 1). In fact, analogs 28, 12, 17, and 18 showed greater permeability in the absorptive direction, indicating net active transport across the BBB. Furthermore, analogs 17 and 18, as well as 12 and 27, displayed excellent stability to rat liver microsomes in vitro. On the basis of antiprion potency, metabolic stability, and permeability, optimized 2-aminothiazole analogs like 27, 13, 17, and 18 were considered as candidates for further study in animals.
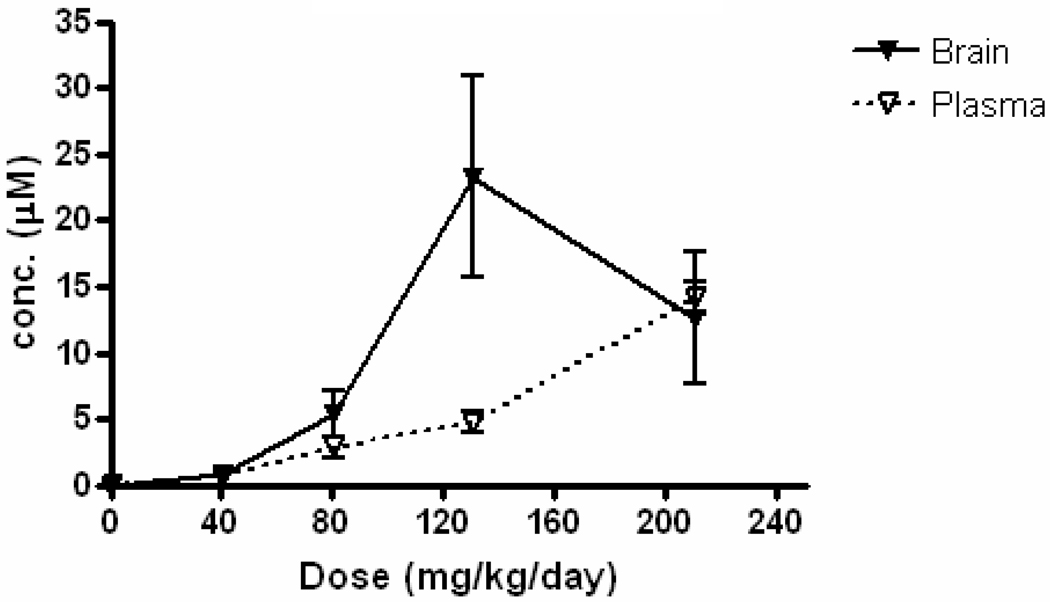
While a full account of the pharmacokinetic optimization and pharmacological evaluation of 2-aminothiazoles will appear in a subsequent communication, we present here the results of a representative feeding experiment in which compound 27 was administered at escalating doses (0, 40, 80, 130, or 210 mg/kg/day) to wild-type FVB mice for three days as part of a rodent liquid diet (Figure 5). This protocol is suitable for subsequent animal efficacy trials, where daily dosing for well over 100 days is required (administration by oral gavage is not practical for such long-term experiments). Brain and plasma concentrations of compound 27 were measured after the 3-day administration period. Increasing doses of 27 resulted in a linear increase in plasma concentrations (Figure 5). Doses up to 130 mg/kg/day also resulted in linear increases in brain concentrations. While significant variability between animals was seen at the two highest doses, concentrations of 27 in brain generally exceeded those in plasma. Mean brain concentrations of 27 were in excess of the compound’s in vitro activity (EC50 = 0.94 µM), surpassing it by as much as 25-fold at the higher doses. Since the reported concentrations were determined at an arbitrary time point following three days of feeding, they represent pseudo steady-state rather than peak concentrations. Full pharmacokinetic parameters were not determined as part of this study, as the intent was to evaluate drug concentrations in brain and plasma at pseudo steady-state. Differences in feeding behavior among individual animals may partially explain the observed variability within certain animal cohorts. Overall, the excellent brain concentrations achieved in these studies confirm that 2-aminothiazole analogs such as 27 are absorbed following oral administration and achieve and maintain high concentrations in the brains of animals. These results prompted us to select several 2-aminothiazole analogs as candidates for further investigation in mouse models of prion disease.
Figure 5.
Brain and plasma concentrations (µM) of aminothiazole 27 in mice after three days of feeding. Compound 27 was administered at the indicated doses as part of a rodent liquid diet (n = 3 per dosing group).
In conclusion, we have identified improved 2-aminothiazole analogs that possess EC50 values as low as 81 nM in ScN2a-cl3 cells. The SAR revealed in this study suggests action at one or more defined molecular targets, the identification of which remains to be established. The physiochemical properties of many 2-aminothiazole analogs are favorable for possible therapeutic use in prion diseases. Preliminary animal studies demonstrate that members of the 2-aminothiazole class are orally absorbed when formulated appropriately in liquid rodent diet and can achieve steady-state brain concentrations well in excess of their in vitro potencies. Whether any of the 2-aminothiazoles described herein can extend the lives of humans or experimental animals with prion disease is unknown but will be of considerable interest.
Experimental Section
General
Reagents and solvents were purchased from Aldrich Chemical, Acros Organics, Alfa Aesar, AK Scientific, or TCI America and used as received unless otherwise indicated. Air and/or moisture sensitive reactions were carried out under an argon atmosphere in oven-dried glassware using anhydrous solvents from commercial suppliers. Air and/or moisture sensitive reagents were transferred via syringe or cannula and were introduced into reaction vessels through rubber septa. Solvent removal was accomplished with a rotary evaporator at ca. 10–50 Torr. Automated column chromatography was carried out using a Biotage SP1 system and silica gel cartridges from Biotage. Analytical TLC plates from EM Science (Silica Gel 60 F254) were employed for TLC analyses. Melting points were determined with an electrothermal capillary melting point apparatus and are uncorrected. 1H NMR spectra were recorded on a Varian INOVA-400 400MHz spectrometer. Chemical shifts are reported in δ units (ppm) relative to TMS as an internal standard. Coupling constants (J) are reported in hertz (Hz). Characterization data are reported as follows: chemical shift, multiplicity (s=singlet, d=doublet, t=triplet, q=quartet, br=broad, m=multiplet), coupling constants, number of protons, mass to charge ratio.
All analogs submitted for testing (3–50) were judged to be of 95% or higher purity based on analytical LC/MS analysis. LC/MS analyses were performed on a Waters Micromass ZQ/Waters 2795 Separation Module/Waters 2996 Photodiode Array Detector system controlled by MassLynx 4.0 software. Separations were carried out on an XTerra® MS C18 5µm 4.6×50mm column at ambient temperature using a mobile phase of water-acetonitrile containing 0.05% trifluoroacetic acid. Gradient elution was employed wherein the acetonitrile-water ratio was increased linearly from 5 to 95% acetonitrile over 2.5 minutes, then maintained at 95% acetonitrile for 1.5 min., and then decreased to 5% acetonitrile over 0.5 min, and maintained at 5% acetonitrile for 0.5 min. Compound purity was determined by integrating peak areas of the liquid chromatogram, monitored at 254 nm.
General procedure for preparing thiourea intermediates from amines
Neat phenyl isothiocyanate (1.1 mmol, 1.1 equiv) is added dropwise to a stirred solution of the aniline, aminopyridine, or other amine building block (1 mmol) in acetone (10 mL) at room temperature. The reaction mixture is heated to reflux for 1–3 hours until judged complete (LC/MS), and then cooled, poured into water-ice, and stirred for an additional 30 min. The benzoyl thiourea precipitate is collected by filtration and washed with more water. This crude material is dissolved in methanol (20mL) and treated with 5 mL of aqueous 1N NaOH. The reaction mixture is heated to 80°C until hydrolysis is judged complete (LC/MS). After cooling, the reaction mixture is poured into water-ice and sufficient aqueous 1N HCl is added to produce a neutral (pH~7) solution. The thiourea intermediate typically precipitates from the neutral solution and is collected by filtration and dried. This two-step procedure provides thiourea intermediates in 50–95% overall yield, with purities generally >90% as determined by 1H NMR. These intermediates are used in the next step without further purification.
General procedure for preparing 2-aminothiazole analogs
An ethanolic solution (~10 mL) of the desired thiourea (1 mmol) and the requisite bromoacetophenone (1 mmol) is heated at reflux for 3–5 hours, or until the reaction is judged complete (LC/MS). The reaction mixture is then poured into water-ice (20mL) and stirred for another 30 minutes. A solution of aqueous 1N Na2CO3 is then added to produce a solution of pH~ 8. The aminothiazole product typically precipitates from this solution and is collected by filtration and washed with water. Crude aminothiazoles are purified by column chromatography on silica gel (~40–80% ethyl acetate-hexanes). Relevant fractions are collected and concentrated to afford the desired product in 60–95% yields, with purity of >95% as determined by 1H NMR.
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-(6-methylpyridin-2-yl)-amine (4)
Intermediate 51 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 92% yield; mp 264–266 °C. 1H NMR (DMSO-d6) δ 11.41 (br. s., 1H, NH), 7.63 (t, J = 7.78 Hz, 1H), 7.42 – 7.50 (m, 2H), 7.32 (s, 1H), 7.00 (d, J = 8.42 Hz, 1H), 6.91 (d, J = 8.24 Hz, 1H), 6.82 (d, J = 7.33 Hz, 1H), 3.82 (s, 3H), 3.79 (s, 3H), 2.49 (s, 3H); LCMS (ESI) m/z 328 (MH+)
4-(3,4-dimethoxyphenyl)-N-methyl-1,3-thiazol-2-amine (5)
Methyl thiourea (Aldrich Chemical) was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 60% yield; mp 120–123 °C. 1H NMR (DMSO-d6) δ 7.53 (d, J = 4.76 Hz, 1H), 7.35 – 7.41 (m, 2H), 6.91 – 6.97 (m, 2H), 3.79 (s, 3H), 3.76 (s, 2H), 2.86 (d, J = 4.76 Hz, 3H); LCMS (ESI) m/z 251 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-pyridin-2-yl-amine (6)
Intermediate 62 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 62% yield; mp 211–214 °C. 1H NMR (DMSO-d6) δ 11.37 (s, 1H, NH), 8.27 – 8.33 (m, 1H), 7.66 – 7.74 (m, 1H), 7.43 – 7.50 (m, 2H), 7.31 (s, 1H), 7.09 (d, J = 8.24 Hz, 1H), 6.99 (d, J = 8.42 Hz, 1H), 6.92 (ddd, J = 0.92, 5.08, 7.19 Hz, 1H), 3.82 (s, 3H), 3.78 (s, 3H); LCMS (ESI) m/z 314 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-pyridin-3-yl-amine (7)
Intermediate 57 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 80% yield; mp 251–254 °C. 1H NMR (DMSO-d6) δ 11.25 (s, 1H, NH), 9.47 (s, 1H), 8.39 – 8.55 (m, 2H), 7.95 (s, 1H), 7.48 – 7.55 (m, 2H), 7.47 (s, 1H), 6.95 – 7.06 (m, 1H), 3.87 (s, 3H), 3.81 (s, 3H); LCMS (ESI) m/z 314 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-pyridin-4-yl-amine (8)
Pyridin-4-yl-thiourea (Alfa Aesar) was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 56% yield; mp 205–207 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.71 (s, 1H, NH), 8.41 (d, J = 6.23 Hz, 2H), 7.62 – 7.69 (m, 2H), 7.52 (dd, J = 2.01, 8.24 Hz, 1H), 7.48 (d, J = 2.01 Hz, 1H), 7.39 (s, 1H), 7.03 (d, J = 8.42 Hz, 1H), 3.85 (s, 3H), 3.79 (s, 3H); LCMS (ESI) m/z 314 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-phenyl-amine (9)
N-Phenylthiourea (AK Scientific) was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 67% yield; mp 170–174 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.23 (s, 1H, NH), 7.71 (dd, J = 0.92, 8.61 Hz, 2H), 7.49 – 7.53 (m, 1H), 7.47 (t, J = 1.92 Hz, 1H), 7.30 – 7.39 (m, 2H), 7.22 (s, 1H), 6.99 – 7.05 (m, 1H), 6.93 – 6.99 (m, 1H), 3.82 (s, 3H), 3.78 (s, 3H); LCMS (ESI) m/z 313 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-pyrimidin-2-yl-amine (10)
Intermediate 61 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 62% yield; mp 225–228 °C. 1H NMR (DMSO-d6) δ 11.80 (br. s., 1H, NH), 8.65 (s, 1H), 8.64 (s, 1H), 7.49 (s, 1H), 7.46 (d, J = 1.28 Hz, 1H), 7.43 (s, 1H), 7.04 (td, J = 0.73, 4.85 Hz, 1H), 7.00 (d, J = 8.06 Hz, 1H), 3.82 (s, 3H), 3.78 (s, 3H); LCMS (ESI) m/z 315 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-pyrazin-2-yl-amine (11)
Intermediate 58 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 66% yield; mp 205–207 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.83 (s, 1H, NH), 8.50 (d, J = 1.46 Hz, 1H), 8.32 (dd, J = 1.46, 2.75 Hz, 1H), 8.13 (d, J = 2.93 Hz, 1H), 7.47 – 7.51 (m, 1H), 7.46 (d, J = 2.01 Hz, 1H), 7.43 (s, 1H), 7.01 (d, J = 8.24 Hz, 1H), 3.83 (s, 3H), 3.79 (s, 3H); LCMS (ESI) m/z 315 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-(5-trifluoromethylpyridin-2-yl)-amine (12)
Intermediate 55 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 68% yield; mp 244–247 °C. 1H NMR (DMSO-d6) δ 11.90 (br. s., 1H, NH), 8.68 (s, 1H), 8.05 (dd, J = 2.56, 8.79 Hz, 1H), 7.47 – 7.51 (m, 1H), 7.46 (d, J = 2.01 Hz, 1H), 7.44 (s, 1H), 7.24 (d, J = 8.79 Hz, 1H), 7.00 (d, J = 8.24 Hz, 1H), 3.82 (s, 3H), 3.79 (s, 3H); LCMS (ESI) m/z 382 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-(5-methoxypyridin-2-yl)-amine (13)
Intermediate 56 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 56% yield; mp 222–225 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.32 (br. s., 1H, NH), 8.05 (d, J = 2.93 Hz, 1H), 7.42 – 7.49 (m, 3H), 7.27 (s, 1H), 7.11 (d, J = 8.97 Hz, 1H), 7.00 (d, J = 8.42 Hz, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 3.78 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 160.5, 150.8, 149.4, 149.2, 149.0, 146.9, 131.9, 128.7, 126.5, 118.7, 112.5, 112.1, 110.0, 103.8, 56.6, 56.2, 56.1; LCMS (ESI) m/z 344 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-(5-methylpyridin-2-yl)-amine (14)
Intermediate 52 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 89% yield; mp 190–192 °C. 1H NMR (DMSO-d6) δ 11.38 (br. s., 1H, NH), 8.15 (s, 1H), 7.60 (d, J = 8.24 Hz, 1H), 7.43 – 7.49 (m, 2H), 7.30 (s, 1H), 7.05 (d, J = 8.24 Hz, 1H), 6.99 (d, J = 8.24 Hz, 1H), 3.82 (s, 3H), 3.78 (s, 3H), 2.24 (s, 3H); LCMS (ESI) m/z 328 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-(3-methylpyridin-2-yl)-amine (15)
Intermediate 54 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 92% yield; mp 264–266 °C. 1H NMR (DMSO-d6) δ 10.65 (bs, 1H, NH), 8.21 (d, J = 5.0 Hz, 1H), 7.67 (d, J = 6.8 Hz, 1H), 7.51 (s, 1H), 7.50 (dd, J = 10.4, 1.5, 1H), 7.39 (s, 1H), 7.00 (s, 1H), 6.98 (s, 1H), 3.82 (s, 3H), 3.77 (s, 3H), 2.37 (s, 3H); LCMS (ESI) m/z 328 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-(4-methylpyridin-2-yl)-amine (16)
Intermediate 53 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 68% yield; mp 211–213 °C. 1H NMR (DMSO-d6) δ 11.46 (br. s., 1H, NH), 8.19 (d, J = 5.49 Hz, 1H), 7.48 (s, 1H), 7.46 (d, J = 2.01 Hz, 1H), 7.33 (s, 1H), 6.99 (d, J = 8.24 Hz, 1H), 6.93 (s, 1H), 6.83 (d, J = 4.94 Hz, 1H), 3.83 (s, 3H), 3.79 (s, 3H), 2.31 (s, 3H); LCMS (ESI) m/z 328 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-isoquinolin-3-yl-amine (17)
Intermediate 59 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 78% yield; mp 200–203 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.40 (s, 1H, NH), 9.19 (s, 1H), 8.03 (d, J = 8.24 Hz, 1H), 7.80 (d, J = 8.06 Hz, 1H), 7.65 (td, J = 1.19, 7.55 Hz, 1H), 7.47 – 7.55 (m, 3H), 7.39 – 7.45 (m, 1H), 7.29 (s, 1H), 7.01 (d, J = 8.24 Hz, 1H), 3.84 (s, 3H), 3.79 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 161.4, 150.1, 149.6, 149.0, 148.7, 148.4, 138.3, 130.6, 128.6, 127.8, 125.7, 124.7, 124.3, 118.6, 111.4, 109.6, 103.1, 103.0, 56.0, 56.0; LCMS (ESI) m/z 364 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-quinolin-2-yl-amine (18)
Intermediate 60 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 80% yield; mp 257–259 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.82 (br. s., 1H, NH), 8.23 (d, J = 8.97 Hz, 1H), 7.83 – 7.88 (m, 2H), 7.69 (td, J = 1.37, 7.65 Hz, 1H), 7.47 – 7.53 (m, 2H), 7.44 (s, 1H), 7.39 – 7.44 (m, 1H), 7.28 (d, J = 8.79 Hz, 1H), 7.02 (d, J = 8.24 Hz, 1H), 3.84 (s, 3H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 159.7, 151.2, 149.5, 149.4, 149.1, 146.6, 138.5, 130.6, 128.6, 128.5, 126.6, 124.7, 124.4, 118.7, 113.5, 112.6, 110.0, 105.8, 56.2, 56.2; LCMS (ESI) m/z 364 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-naphthalen-2-yl-amine (19)
Intermediate 64 was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 68% yield; mp 221–224 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H, NH), 8.54 (s, 1H), 7.87 (d, J = 8.97 Hz, 1H), 7.81 (t, J = 9.16 Hz, 2H), 7.53 – 7.61 (m, 3H), 7.47 (t, J = 7.42 Hz, 1H), 7.31 – 7.38 (m, 1H), 7.29 (s, 1H), 7.05 (d, J = 8.79 Hz, 1H), 3.89 (s, 3H), 3.81 (s, 3H); LCMS (ESI) m/z 363 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-naphthalen-1-yl-amine (20)
Naphthalen-1-yl-thiourea (TCI America) was reacted with commercially available 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 59% yield; mp 244–246 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.24 (br. s., 1H, NH), 8.27 – 8.33 (m, 1H), 8.23 (dd, J = 2.93, 7.51 Hz, 1H), 7.96 (dt, J = 2.38, 4.76 Hz, 1H), 7.70 (d, J = 8.06 Hz, 1H), 7.51 – 7.61 (m, 3H), 7.41 – 7.48 (m, 2H), 7.21 (s, 1H), 7.01 (d, J = 8.97 Hz, 1H), 3.83 (s, 3H), 3.79 (s, 3H); LCMS (ESI) m/z 363 (MH+)
[4-(4-Methoxyphenyl)-thiazol-2-yl]-(5-methylpyridin-2-yl)-amine (21)
Intermediate 52 was reacted with commercially available 2-bromo-1-(4-methoxyphenyl)-ethanone according to the general procedure to afford the title compound in 73% yield; mp 246–248 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.60 (br. s., 1H, NH), 8.21 (s, 1H), 7.79 – 7.94 (m, J = 8.61 Hz, 2H), 7.69 (br. s., 1H), 7.32 (br. s., 1H), 7.13 (br. s., 1H), 6.97 – 7.04 (m, J = 7.87 Hz, 2H), 3.81 (s, 3H), 2.28 (s, 3H); LCMS (ESI) m/z 298 (MH+)
[4-(2-Methoxyphenyl)-thiazol-2-yl]-(5-methylpyridin-2-yl)-amine (22)
Intermediate 52 was reacted with commercially available 2-bromo-1-(2-methoxyphenyl)-ethanone according to the general procedure to afford the title compound in 78% yield; mp 228–230 °C. 1H NMR (400 MHz, methanol-d4) δ 8.34 (s, 1H), 8.00 (dd, J = 1.92, 8.70 Hz, 1H), 7.94 (d, J = 7.87 Hz, 1H), 7.62 (s, 1H), 7.40 – 7.49 (m, 1H), 7.25 (d, J = 8.79 Hz, 1H), 7.20 (d, J = 8.42 Hz, 1H), 7.08 – 7.16 (m, 1H), 4.02 (s, 3H), 2.41 (s, 3H); LCMS (ESI) m/z 298 (MH+)
(5-Methylpyridin-2-yl)-[4-pyridin-4-yl)-thiazol-2-yl]-amine (23)
Intermediate 52 was reacted with commercially available 2-bromo-1-pyridin-4-yl-ethanone according to the general procedure to afford the title compound in 72% yield; mp decomposition at 291 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.58 (br. s., 1H, NH), 8.90 – 8.95 (m, 2H), 8.42 – 8.46 (m, 2H), 8.40 (s, 1H), 8.16 – 8.20 (m, 1H), 7.62 (dd, J = 2.38, 8.42 Hz, 1H), 7.07 (d, J = 8.24 Hz, 1H), 2.25 (s, 3H); LCMS (ESI) m/z 269 (MH+)
(5-Methylpyridin-2-yl)-(4-pyridin-2-yl-thiazol-2-yl)-amine (24)
Intermediate 52 was reacted with commercially available 2-bromo-1-pyridin-2-yl-ethanone according to the general procedure to afford the title compound in 62% yield; mp 184–190 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.31 (s, 1H, NH), 8.59 (dt, J = 0.92, 4.76 Hz, 1H), 8.13 – 8.17 (m, 1H), 7.93 – 7.98 (m, 1H), 7.87 (td, J = 1.28, 7.69 Hz, 1H), 7.61 (s, 1H), 7.56 (dd, J = 2.20, 8.42 Hz, 1H), 7.27 – 7.34 (m, 1H), 7.03 (d, J = 8.42 Hz, 1H), 2.23 (s, 3H); LCMS (ESI) m/z 269 (MH+)
(5-Methylpyridin-2-yl)-(4-phenylthiazol-2-yl)-amine (25)
Intermediate 52 was reacted with commercially available 2-bromo-1-phenyl-ethanone according to the general procedure to afford the title compound in 82% yield; mp 260–264 °C. 1H NMR (400 MHz, methanol-d4) δ 8.32 – 8.37 (m, 1H), 8.17 (dd, J = 2.01, 8.97 Hz, 1H), 7.93 – 8.01 (m, 2H), 7.54 (s, 1H), 7.45 – 7.52 (m, 2H), 7.33 – 7.45 (m, 2H), 2.43 (s, 3H); LCMS (ESI) m/z 268 (MH+)
(5-Methylpyridin-2-yl)-[4-(5-pyridin-2-yl-thiophen-2-yl)-thiazol-2-yl]-amine (26)
Intermediate 52 was reacted with commercially available 2-bromo-1-(5-pyridin-2-yl-thiophen-2-yl)-ethanone according to the general procedure to afford the title compound in 73% yield; mp 197–199 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.37 (s, 1H, NH), 8.52 (d, J = 5.86 Hz, 1H), 8.13 (s, 1H), 7.88 – 7.92 (m, 1H), 7.77 – 7.86 (m, 1H), 7.73 – 7.77 (m, 1H), 7.55 (dd, J = 2.20, 8.42 Hz, 1H), 7.50 (d, J = 3.85 Hz, 1H), 7.33 (s, 1H), 7.25 (dd, J = 4.76, 7.33 Hz, 1H), 7.02 (d, J = 8.42 Hz, 1H), 2.22 (s, 3H); LCMS (ESI) m/z 351 (MH+)
(5-Methylpyridin-2-yl)-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (27)
Intermediate 52 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 63% yield; mp 224–227 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.56 (s, 1H, NH), 8.17 (s, 1H), 7.88 – 7.99 (m, 2H), 7.65 (s, 1H), 7.51 – 7.61 (m, 4H), 7.19 (s, 1H), 7.02 (d, J = 8.42 Hz, 1H), 2.24 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.9, 162.9, 161.3, 150.1, 146.4, 139.7, 137.9, 131.0, 129.8 (2), 129.2, 127.4 (2), 125.8, 112.1, 111.2, 99.0, 17.9; LCMS (ESI) m/z 335 (MH+)
[4-(5-Methyl-3-phenylisoxazol-4-yl)-thiazol-2-yl]-(5-methylpyridin-2-yl)-amine (28)
Intermediate 52 was reacted with commercially available 2-bromo-1-(5-methyl-3-phenylisoxazol-4-yl)-ethanone according to the general procedure to afford the title compound in 54% yield; mp 267–269 °C. 1H NMR (400 MHz, methanol-d4) δ 8.12 (dd, J = 2.11, 8.88 Hz, 1H), 7.71 (s, 1H), 7.51 – 7.57 (m, 2H), 7.40 – 7.48 (m, 3H), 7.31 (d, J = 8.97 Hz, 1H), 7.22 (s, 1H), 2.64 (s, 3H), 2.37 (s, 3H); LCMS (ESI) m/z 349 (MH+)
(5-Methylpyridin-2-yl)-(4-methylthiazol-2-yl)-amine (29)
Intermediate 52 was reacted with commercially available 1-bromo-propan-2-one according to the general procedure to afford the title compound in 64% yield; mp 212–214 °C. 1H NMR (400 MHz, DMSO-d6) δ d 11.01 (br. s., 1H, NH), 8.09 (s, 1H), 7.51 (dd, J = 2.01, 8.42 Hz, 1H), 6.94 (d, J = 8.42 Hz, 1H), 6.48 (s, 1H), 2.21 (s, 3H), 2.20 (s, 3H); LCMS (ESI) m/z 206 (MH+)
Isoquinolin-3-yl-[4-(4-methoxyphenyl)-thiazol-2-yl]-amine (30)
Intermediate 59 was reacted with commercially available 2-bromo-1-(4-methoxyphenyl)-ethanone according to the general procedure to afford the title compound in 57% yield; mp 224–228 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.37 (s, 1H, NH), 9.19 (s, 1H), 8.02 (d, J = 8.06 Hz, 1H), 7.84 – 7.92 (m, 2H), 7.81 (d, J = 8.24 Hz, 1H), 7.61 – 7.71 (m, 1H), 7.54 (br. s., 1H), 7.38 – 7.47 (m, 1H), 7.19 – 7.28 (m, 1H), 6.93 – 7.05 (m, 2H), 3.79 (s, 3H); LCMS (ESI) m/z 334 (MH+)
Isoquinolin-3-yl-[4-(4-trifluoromethoxyphenyl)-thiazol-2-yl]-amine (31)
Intermediate 59 was reacted with commercially available 2-bromo-1-(4-trifluoromethoxyphenyl)-ethanone according to the general procedure to afford the title compound in 67% yield; mp 269–271 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.46 (s, 1H), 9.20 (s, 1H), 8.00 – 8.09 (m, 3H), 7.83 (d, J = 8.42 Hz, 1H), 7.66 (t, J = 7.60 Hz, 1H), 7.54 (s, 1H), 7.50 (s, 1H), 7.37 – 7.47 (m, 3H); LCMS (ESI) m/z 388 (MH+)
Isoquinolin-3-yl-[4-pyridin-4-yl)-thiazol-2-yl]-amine (32)
Intermediate 59 was reacted with commercially available 2-bromo-1-pyridin-4-yl-ethanone according to the general procedure to afford the title compound in 52% yield; mp 259–260 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.54 (s, 1H, NH), 9.22 (s, 1H), 8.58 – 8.70 (m, 2H), 8.05 (d, J = 8.24 Hz, 1H), 7.87 – 7.91 (m, 2H), 7.85 (d, J = 8.24 Hz, 1H), 7.80 (s, 1H), 7.66 (ddd, J = 1.10, 6.91, 8.29 Hz, 1H), 7.55 (s, 1H), 7.41 – 7.49 (m, 1H); LCMS (ESI) m/z 305 (MH+)
Isoquinolin-3-yl-(4-phenylthiazol-2-yl)-amine (33)
Intermediate 59 was reacted with commercially available 2-bromo-1-phenyl-ethanone according to the general procedure to afford the title compound in 72% yield; mp 265–267 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.44 (br. s., 1H, NH), 9.21 (s, 1H), 8.04 (d, J = 8.42 Hz, 1H), 7.91 – 7.97 (m, 2H), 7.83 (d, J = 8.61 Hz, 1H), 7.66 (dd, J = 7.05, 8.15 Hz, 1H), 7.55 (s, 1H), 7.39 – 7.48 (m, 4H), 7.28 – 7.35 (m, 1H); LCMS (ESI) m/z 304 (MH+)
Isoquinolin-3-yl-[4-(5-pyridin-2-yl-thiophen-2-yl)-thiazol-2-yl]-amine (34)
Intermediate 59 was reacted with commercially available 2-bromo-1-(5-pyridin-2-yl-thiophen-2-yl)-ethanone according to the general procedure to afford the title compound in 63% yield; mp 246–248 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.54 (br. s., 1H, NH), 9.20 (s, 1H), 8.52 – 8.57 (m, 1H), 8.03 (d, J = 8.24 Hz, 1H), 7.90 – 7.95 (m, 1H), 7.79 – 7.87 (m, 2H), 7.78 (d, J = 4.03 Hz, 1H), 7.66 (ddd, J = 1.10, 6.91, 8.29 Hz, 1H), 7.54 (d, J = 3.85 Hz, 1H), 7.49 (s, 1H), 7.43 (ddd, J = 0.92, 6.91, 8.10 Hz, 1H), 7.36 (s, 1H), 7.27 (ddd, J = 1.10, 4.90, 7.37 Hz, 1H); LCMS (ESI) m/z 387 (MH+)
Isoquinolin-3-yl-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (35)
Intermediate 59 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 59% yield; mp decomposition 260 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.68 (s, 1H, NH), 9.21 (s, 1H), 8.04 (d, J = 8.42 Hz, 1H), 7.94 (dd, J = 1.74, 6.32 Hz, 2H), 7.85 (d, J = 8.24 Hz, 1H), 7.62 – 7.70 (m, 2H), 7.48 – 7.58 (m, 4H), 7.39 – 7.48 (m, 1H), 7.24 (s, 1H); LCMS (ESI) m/z 371 (MH+)
Isoquinolin-3-yl-(4-methylthiazol-2-yl)-amine (36)
Intermediate 59 was reacted with commercially available 1-bromo-propan-2-one according to the general procedure to afford the title compound in 73% yield; mp 212–215 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.16 (br. s., 1H, NH), 9.15 (s, 1H), 8.00 (d, J = 7.87 Hz, 1H), 7.77 (d, J = 8.24 Hz, 1H), 7.57 – 7.70 (m, 1H), 7.47 (s, 1H), 7.34 – 7.45 (m, 1H), 6.51 (s, 1H), 2.26 (s, 3H); LCMS (ESI) m/z 242 (MH+)
Isoquinolin-3-yl-(4-trifluoromethylthiazol-2-yl)-amine (37)
Intermediate 59 was reacted with commercially available 3-bromo-1,1,1-trifluoro-propan-2-one according to the general procedure to afford the title compound in 67% yield; mp 200–203 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.81 (s, 1H), 9.23 (s, 1H), 8.02 – 8.09 (m, 1H), 7.80 – 7.87 (m, 1H), 7.64 – 7.72 (m, 2H), 7.46 (ddd, J = 1.01, 6.96, 8.15 Hz, 1H), 7.37 (s, 1H); LCMS (ESI) m/z 296 (MH+)
(8H-Indeno[1,2-d]thiazol-2-yl)-(5-methylpyridin-2-yl)-amine (38)
A solution of intermediate 52 (1 mmol) and commercially available 2-bromoindan-1-one (1 mmol, 1 eq.) in EtOH (10 mL) was heated to 60 °C for 3 h, after which time the reaction was judged complete. The reaction mixture was then poured into water-ice (20 mL) and stirred for 30 minutes. Aqueous 1N Na2CO3 was then added to this solution until a pH~ 8 was reached. The product precipitated from this solution and was collected by filtration and washed with water. The crude product was purified by column chromatography (40–80% ethyl acetate-hexane). Relevant fractions were collected and evaporated to afford the product in 69% yield; mp decomposition 282 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.85 (s, 1H), 8.18 – 8.23 (m, 1H), 7.74 (d, J = 8.24 Hz, 1H), 7.63 (d, J = 7.33 Hz, 1H), 7.54 (d, J = 7.51 Hz, 1H), 7.37 (t, J = 7.42 Hz, 1H), 7.20 – 7.27 (m, 1H), 7.13 (d, J = 8.42 Hz, 1H), 3.85 (s, 2H), 2.28 (s, 3H); LCMS (ESI) m/z 280 (MH+)
(5,6-Dimethoxy-8H-indeno[1,2-d]thiazol-2-yl)-(5-methylpyridin-2-yl)-amine (39)
A solution of intermediate 52 (1 mmol) and bromo-5,6-dimethoxy-indan-1-one (65, 1 mmol, 1 eq.) in EtOH (10 mL) was heated to 60 °C for 3 h, after which time the reaction was judged complete. The reaction mixture was then poured into water-ice (20 mL) and stirred for 30 minutes. Aqueous 1N Na2CO3 was then added to this solution until a pH~ 8 was reached. The product precipitated from this solution and was collected by filtration and washed with water. The crude product was purified by column chromatography (40–80% ethyl acetate-hexane). Relevant fractions were collected and evaporated to afford the product in 72% yield; mp decomposition 265 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.60 (s, 1H), 8.18 (s, 1H), 7.68 (d, J = 8.06 Hz, 1H), 7.23 (s, 1H), 7.18 (s, 1H), 7.10 (d, J = 8.79 Hz, 1H), 3.83 (s, 3H), 3.79 (s, 3H), 3.74 (s, 2H), 2.26 (s, 3H); LCMS (ESI) m/z 340 (MH+)
(5-Methoxypyridin-2-yl)-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (40)
Intermediate 56 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 53% yield; mp 203–205 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H, NH), 8.06 (d, J = 3.11 Hz, 1H), 7.88 – 7.97 (m, 2H), 7.62 (s, 1H), 7.50 – 7.59 (m, 3H), 7.47 (dd, J = 2.93, 8.97 Hz, 1H), 7.19 (s, 1H), 7.09 (d, J = 8.97 Hz, 1H), 3.81 (s, 3H); LCMS (ESI) m/z 351 (MH+)
(4-Methoxypyridin-2-yl)-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (41)
Intermediate 63 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 58% yield; mp 177–179 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.54 (s, 1H), 8.16 (d, J = 6.23 Hz, 1H), 7.88 – 8.01 (m, 2H), 7.67 (s, 1H), 7.47 – 7.59 (m, 3H), 7.20 (s, 1H), 6.57 – 6.66 (m, 2H), 3.82 (s, 3H); LCMS (ESI) m/z 351 (MH+)
[4-(3-Phenylisoxazol-5-yl)-thiazol-2-yl]-pyridin-2-yl-amine (42)
Intermediate 62 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 52% yield; mp 211–215 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.68 (s, 1H, NH), 8.34 (dd, J = 0.92, 5.13 Hz, 1H), 7.90 – 7.99 (m, 2H), 7.75 (ddd, J = 1.83, 7.05, 8.52 Hz, 1H), 7.69 (s, 1H), 7.51 – 7.59 (m, 3H), 7.21 (s, 1H), 7.10 (d, J = 8.24 Hz, 1H), 6.98 (ddd, J = 0.92, 5.63, 6.64 Hz, 1H); LCMS (ESI) m/z 321 (MH+)
(4-Methylpyridin-2-yl)-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (43)
Intermediate 53 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 62% yield; mp decomposition 296 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.70 (br. s., 1H, NH), 8.22 (d, J = 5.31 Hz, 1H), 7.88 – 7.98 (m, 2H), 7.70 (s, 1H), 7.49 – 7.60 (m, 3H), 7.25 (s, 1H), 6.93 (s, 1H), 6.86 (d, J = 5.31 Hz, 1H), 2.32 (s, 3H); LCMS (ESI) m/z 335 (MH+)
(6-Methylpyridin-2-yl)-[4-(3-phenylisoxazol-5-yl)-thiazol-2-yl]-amine (44)
Intermediate 51 was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 58% yield; mp 291–293 °C. 1H NMR (400 MHz, methanol-d4) δ 8.17 – 8.26 (m, 1H), 7.86 – 7.95 (m, 3H), 7.48 – 7.55 (m, 3H), 7.26 – 7.34 (m, 3H), 2.85 (s, 3H); LCMS (ESI) m/z 335 (MH+)
[4-(3-Phenylisoxazol-5-yl)-thiazol-2-yl]-pyridin-4-yl-amine (45)
Pyridin-4-yl-thiourea (Alfa Aesar) was reacted with commercially available 2-bromo-1-(3-phenylisoxazol-5-yl)-ethanone according to the general procedure to afford the title compound in 57% yield; mp 269–270 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.92 (br. s., 1H), 8.39 – 8.46 (m, 2H), 7.95 – 8.04 (m, 2H), 7.66 – 7.73 (m, 3H), 7.51 – 7.61 (m, 3H), 7.45 (s, 1H); LCMS (ESI) m/z 321 (MH+)
2-(5-Methylpyridin-2-ylamino)-thiazole-4-carboxylic acid (3,4-dimethoxyphenyl)-amide (46)
A solution of commercially available 3,4-dimethoxyphenylamine (0.13 mmol, 1 eq.) and triethylamine (0.65 mmol, 5eq.) in THF (2 mL) and was added to a solution of 67 (0.13 mmol) and HATU (0.13 mmol) in THF (2 mL). The reaction mixture was stirred for 3 h at 60°C. After cooling, the mixture was concentrated and the crude product taken into 100 mL ethyl acetate and washed with water. The organic phase was dried (MgSO4), filtered, and concentrated. The crude residue was purified by column chromatography on silica gel (30–60% ethyl acetate-hexane) and relevant fractions collected and concentrated to afford the desired product as a white powder in 76% yield; mp 212–214 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.36 (s, 1H), 9.55 (s, 1H), 8.15 – 8.17 (m, 1H), 7.69 (s, 1H), 7.58 (dd, J = 2.20, 8.61 Hz, 1H), 7.45 (d, J = 2.38 Hz, 1H), 7.30 (dd, J = 2.38, 8.61 Hz, 1H), 7.05 (d, J = 8.42 Hz, 1H), 6.93 (d, J = 8.79 Hz, 1H), 3.76 (s, 3H), 3.74 (s, 3H), 2.24 (s, 3H); LCMS (ESI) m/z 371 (MH+)
Isoquinoline-3-carboxylic acid [4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-amide (47)
A solution of 70 (30 mg, 0.13 mmol, 1eq) and triethylamine (0.65 mmol, 5 eq.) in THF (2 mL) was added to a solution of commercially available isoquinoline-3-carboxylic acid (22 mg, 0.13 mmol) and HATU (50 mg, 0.13 mmol) in THF (2 mL). The reaction was stirred for 3h at 60°C, then cooled to room temperature and poured into water-ice. The product was extracted with EtOAc, dried (MgSO4), filtered and concentrated. The crude product was purified by column chromatography on silica gel (ethyl acetate-hexanes) to afford the product as a white powder in 39% yield; mp 228–230 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.99 (s, 2H), 9.51 (s, 1H), 8.77 (s, 1H), 8.34 (d, J = 7.33 Hz, 1H), 8.29 (d, J = 8.06 Hz, 1H), 7.87 – 7.99 (m, 2H), 7.66 (s, 1H), 7.56 (d, J = 2.01 Hz, 1H), 7.53 (dd, J = 1.92, 8.33 Hz, 1H), 7.03 (d, J = 8.42 Hz, 1H), 3.85 (s, 3H), 3.80 (s, 3H); LCMS (ESI) m/z 392 (MH+)
4-(3,4-dimethoxyphenyl)-thiazole-2-carboxylic acid isoquinolin-3-ylamide (48)
A solution of commercially available isoquinolin-3-ylamine (0.06 g, 0.19 mmol, 1 eq) and triethylamine (0.95 mmol, 5 eq.) in THF (3 mL) was added to a mixture of 69 (0.10 g, 0.19 mmol) and HATU (0.15g, 0.19 mmol) in THF (3mL). The reaction mixture was stirred for 3 h at 60°C, then cooled to room temperature and poured into water-ice. This solution was extracted with EtOAc, and the organic phase dried (MgSO4), filtered and concentrated. The crude product was purified by column chromatography on silica gel (ethyl acetate-hexanes) to afford the product as a white powder in 55% yield; mp 196–202 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H), 9.27 (s, 1H), 8.59 (s, 1H), 8.46 (s, 1H), 8.14 (d, J = 8.06 Hz, 1H), 8.01 (d, J = 8.24 Hz, 1H), 7.78 (ddd, J = 1.19, 6.91, 8.20 Hz, 1H), 7.68 – 7.75 (m, 2H), 7.56 – 7.66 (m, 1H), 7.07 (d, J = 8.24 Hz, 1H), 3.90 (s, 3H), 3.83 (s, 3H); LCMS (ESI) m/z 392 (MH+)
[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-isoquinolin-3-yl-methyl-amine (49)
To a solution of 17 (50 mg, 0.138 mmol) in THF (3 mL) was added NaH (60% in mineral oil; 8mg, 0.21 mmol, 1.5eq) at 0°C. After stirring for 15 min at 0°C, the reaction mixture was treated with methyl iodide (39 mg, 17.2 µL, 0.28 mmol, 2eq) and stirred for 2 h at room temperature. The reaction was quenched by the addition of aqueous NH4Cl and the product was extracted with ether (2 × 50 mL). Combined organic phases were dried (MgSO4), filtered and concentrated. The crude product was purified by column chromatography (10–50% ethyl acetate-hexane) to provide the product in 77% yield; mp 165–166 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.27 (s, 1H), 8.09 (d, J = 8.06 Hz, 1H), 7.94 (d, J = 8.42 Hz, 1H), 7.69 – 7.78 (m, 1H), 7.65 (s, 1H), 7.43 – 7.58 (m, 3H), 7.35 (s, 1H), 7.00 (d, J = 8.97 Hz, 1H), 3.94 (s, 3H), 3.84 (s, 3H), 3.78 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ 162.9, 150.4, 149.5, 149.2, 148.9, 148.9, 138.5, 131.0, 128.9, 127.9, 126.3, 125.0, 125.0, 118.7, 111.5, 109.7, 105.0, 102.8, 56.2, 56.1, 36.3; LCMS (ESI) m/z 378 (MH+)
N-[4-(3,4-dimethoxyphenyl)-thiazol-2-yl]-N-isoquinolin-3-yl-acetamide (50)
Acetic anhydride (5 mL) was added to 17 (50 mg, 0.138 mmol) and the reaction mixture heated to 100 °C for 4 hrs. The solution was allowed to cool to room temperature and then poured into water-ice. The product was extracted with EtOAc and the organic phase dried (MgSO4), filtered and concentrated. The crude product was purified by column chromatography (30–60% ethyl acetate-hexane) to provide the product as a white powder in 81% yield; mp 164–165 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.46 (s, 1H), 8.32 (d, J = 8.24 Hz, 1H), 8.25 (s, 1H), 8.13 (d, J = 7.69 Hz, 1H), 7.88 – 7.95 (m, 1H), 7.84 (ddd, J = 1.19, 6.91, 8.19 Hz, 1H), 7.64 (s, 1H), 7.17 (d, J = 2.01 Hz, 1H), 7.02 (dd, J = 1.92, 8.33 Hz, 1H), 6.82 (d, J = 8.61 Hz, 1H), 3.67 (s, 3H), 3.59 (s, 3H), 2.15 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ 170.0, 160.1, 153.2, 149.4, 149.0, 148.9, 147.8, 137.6, 131.4, 128.7, 128.6, 128.0, 127.9, 127.2, 121.0, 118.7, 111.3, 109.6, 107.4, 56.1, 55.7, 24.0; LCMS (ESI) m/z 406 (MH+)
(6-Methylpyridin-2-yl)-thiourea (51)
Commercially available 6-methylpyridin-2-ylamine was reacted according to the general procedure to afford the product in 58% yield; mp 188–190 °C. 1H NMR (DMSO-d6) δ 10.59 (s, 1H), 10.38 (s, 1H), 8.78 (s, 1H), 7.59 (t, J = 8.0 Hz, 1H), 6.90 (d, J = 8.3 Hz, 1H), 6.84 (d, J = 7.6 Hz, 1H), 2.35 (s, 3H); LCMS (ESI) m/z 168 (MH+)
(5-Methylpyridin-2-yl)-thiourea (52)
Commercially available 5-methylpyridin-2-ylamine was reacted according to the general procedure to afford the product in 60% yield; mp 178–181 °C. 1H NMR (DMSO-d6) δ 10.46 (s, 1H), 10.38 (s, 1H), 8.73 (s, 1H), 8.00 (d, J = 2.2 Hz, 1H), 7.54 (dd, J = 8.45, 2.3 Hz, 1H), 7.02 (d, J = 8.6 Hz, 1H), 2.17 (s, 3H); LCMS (ESI) m/z 168 (MH+)
(4-Methylpyridin-2-yl)-thiourea (53)
Commercially available 4-methylpyridin-2-ylamine was reacted according to the general procedure to afford the product in 60% yield; mp 210–212 °C. 1H NMR (DMSO-d6) δ 10.57 (s, 1H), 10.38 (s, 1H), 8.77 (s, 1H), 8.03 (d, J = 5.3 Hz, 1H), 6.91 (s, 1H), 6.83 (d, J = 4.75 Hz, 1H), 2.21 (s, 3H); LCMS (ESI) m/z 168 (MH+)
(3-Methylpyridin-2-yl)-thiourea (54)
Commercially available 3-methylpyridin-2-ylamine was reacted according to the general procedure to afford the product in 52% yield; mp 152–154 °C. 1H NMR (DMSO-d6) δ 10.33 (s, 1H), 8.87 (s, 1H), 8.73 (s, 1H), 8.08 (d, J = 4.9 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.00 (dd, J = 7.5, 5.0 Hz, 1H), 2.25 (s, 3H); LCMS (ESI) m/z 168 (MH+)
(5-Trifluoromethylpyridin-2-yl)-thiourea (55)
Commercially available 5-trifluoromethylpyridin-2-ylamine was reacted according to the general procedure to afford the product in 62% yield; mp 203–205 °C. 1H NMR (DMSO-d6) δ 10.88 (s, 1H), 10.36 (s, 1H), 9.16 (s, 1H), 8.60 (s, 1H), 8.11 (dd, J = 9.0, 2.4 Hz, 1H), 7.31 (d, J = 8.8 Hz, 1H); LCMS (ESI) m/z 222 (MH+)
(5-Methoxypyridin-2-yl)-thiourea (56)
Commercially available 5-methoxypyridin-2-ylamine was reacted according to the general procedure to afford the product in 58% yield; mp 131–135 °C. 1H NMR (DMSO-d6) δ 10.40 (s, 1H), 10.29 (s, 1H), 8.68 (s, 1H), 7.94 – 7.96 (m, 1H), 7.46 (dd, J = 3.11, 9.16 Hz, 1H), 7.15 (d, J = 8.97 Hz, 1H), 3.79 (s, 3H); LCMS (ESI) m/z 184 (MH+)
Pyridin-3-yl-thiourea (57)
Commercially available pyridin-3-ylamine was reacted according to the general procedure to afford the product in 64% yield; mp 165–167 °C. 1H NMR (DMSO-d6) δ 9.77 (s, 1H), 8.55 (d, J = 2.56 Hz, 1H), 8.30 (dd, J = 1.46, 4.76 Hz, 1H), 7.91 – 7.99 (m, 2H), 7.31 – 7.39 (m, 2H); LCMS (ESI) m/z 154 (MH+)
Pyrazin-2-yl-thiourea (58)
Commercially available pyrazin-2-ylamine was reacted according to the general procedure to afford the product in 64% yield; mp 238–240 °C. 1H NMR (DMSO-d6) δ 10.79 (s, 1H), 9.90 (s, 1H), 9.04 (s, 1H), 8.49 (s, 1H), 8.19 (d, J = 0.7 Hz, 2H); LCMS (ESI) m/z 155 (MH+)
Isoquinolin-3-yl-thiourea (59)
Commercially available isoquinolin-3-ylamine was reacted according to the general procedure to afford the product in 74% yield; mp 225–227 °C. 1H NMR (DMSO-d6) d 10.60 (s, 1H), 10.25 (s, 1H), 9.13 (s, 1H), 8.72 (s, 1H), 8.07 (d, J = 8.24 Hz, 1H), 7.81 (d, J = 8.42 Hz, 1H), 7.70 (ddd, J = 1.28, 6.87, 8.33 Hz, 1H), 7.56 (s, 1H), 7.52 (ddd, J = 1.10, 6.96, 8.24 Hz, 1H); LCMS (ESI) m/z 204 (MH+)
Quinolin-2-yl-thiourea (60)
Commercially available quinolin-2-ylamine was reacted according to the general procedure to afford the product in 68% yield; mp 179–180 °C. 1H NMR (DMSO-d6) d 11.10 (s, 1H), 10.80 (s, 1H), 9.17 (s, 1H), 8.29 (d, J = 8.97 Hz, 1H), 7.85 (dt, J = 1.56, 8.06 Hz, 2H), 7.64 – 7.73 (m, 1H), 7.44 – 7.52 (m, 1H), 7.34 (d, J = 8.79 Hz, 1H); LCMS (ESI) m/z 204 (MH+)
Pyrimidin-2-yl-thiourea (61)
Commercially available pyrimidin-2-ylamine was reacted according to the general procedure to afford the product in 77% yield; mp 262–264 °C. 1H NMR (DMSO-d6) δ 10.53 (s, 1H), 10.16 (s, 1H), 9.09 (s, 1H), 8.60 (d, J = 5.0 Hz, 2H), 7.11 (t, J = 5.0 Hz, 1H); LCMS (ESI) m/z 155 (MH+)
Pyridin-2-yl-thiourea (62)
Commercially available pyridin-2-ylamine was reacted according to the general procedure to afford the product in 67% yield; mp 144–146 °C. 1H NMR (DMSO-d6) d 10.57 (br. s., 1H), 10.52 (s, 1H), 8.87 (br. s., 1H), 8.23 (dd, J = 1.28, 5.13 Hz, 1H), 7.69 – 7.82 (m, 1H), 7.16 (d, J = 8.42 Hz, 1H), 7.04 (ddd, J = 0.73, 5.17, 7.28 Hz, 1H); LCMS (ESI) m/z 154 (MH+)
(4-Methoxypyridin-2-yl)-thiourea (63)
Commercially available 4-methoxypyridin-2-ylamine was reacted according to the general procedure to afford the product in 52% yield; mp 214–217 °C. 1H NMR (DMSO-d6) d 10.66 (br. s., 1H), 10.34 (s, 1H), 8.83 (br. s., 1H), 8.06 (d, J = 6.04 Hz, 1H), 6.75 (d, J = 2.20 Hz, 1H), 6.67 (dd, J = 2.38, 6.04 Hz, 1H), 3.78 (s, 3H); LCMS (ESI) m/z 184 (MH+)
Naphthalen-2-yl-thiourea (64)
Commercially available naphthalen-2-ylamine was reacted according to the general procedure to afford the product in 65% yield; mp 190–192 °C. 1H NMR (DMSO-d6) d 9.88 (s, 1H), 7.95 (s, 1H), 7.82 – 7.89 (m, 3H), 7.38 – 7.56 (m, 4H); LCMS (ESI) m/z 203 (MH+)
Bromo-5,6-dimethoxy-indan-1-one (65)
A solution of bromine (1.0 g, 330 µL, 6.30 mmol, 1.2 eq.) in Et2O (5 mL) was added dropwise to a solution of commercially available 5,6-dimethoxy-indan-1-one (1.0 g, 5.2 mmol) in Et2O (30 mL). The reaction mixture was stirred at room temperature overnight, solvent evaporated, and the resulting crude product re-crystallized from MeOH (10mL) to afford 0.65 g (46%) of the desired intermediate 65 a yellow solid; mp 162–163 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.15 (s, 1H), 7.13 (s, 1H), 4.96 (ddd, J = 0.92, 2.75, 7.14 Hz, 1H), 3.88 – 3.90 (m, 3H), 3.81 – 3.83 (m, 3H), 3.75 – 3.81 (m, 1H), 3.21 (dd, J = 2.75, 17.95 Hz, 1H); LCMS (ESI) m/z 272 (MH+)
2-(5-Methylpyridin-2-ylamino)-thiazole-4-carboxylic acid ethyl ester (66)
A solution of ethyl bromopyruvate (0.5g, 0.32 mL, 2.6 mmol) and intermediate 52 (2.6 mmol, 1 eq.) in EtOH (10 mL) were stirred at 60°C for 1 hour. The solvent was then evaporated to afford crude 66, which was used directly in the next step; mp 198–200 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 7.92 (s, 1H), 7.56 (d, J = 8.25 Hz, 1H), 7.17 (d, J = 8.25 Hz, 1H), 4.33 (q, J = 6.95 Hz, 2H), 2.24 (s, 3H), 1.37 (t, J = 6.95 Hz, 3H); LCMS (ESI) m/z 264 (MH+)
2-(5-Methylpyridin-2-ylamino)-thiazole-4-carboxylic acid (67)
Crude 66 (0.7 g, 2.66 mmol) and aqueous 5N HCl (3mL) were heated in a sealed tube in a CEM microwave for 10 min at 130°C. After cooling, the precipitate was filtered and washed with water and acetone to afford 67 as a white powder in 83% yield. This material was used in the next step without further purification; mp decomposition 280 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.16 (s, 1H), 7.78–7.81 (m, 1H), 7.61–7.68 (m, 1H), 7.02–7.08 (m, 1H), 2.24 (s, 3H); LCMS (ESI) m/z 236 (MH+)
4-(3,4-dimethoxyphenyl)-thiazole-2-carboxylic acid ethyl ester (68)
A solution of 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone (0.5 g, 0.96 mmol) and ethyl thiooxamate (0.25 g, 0.96 mmol) in EtOH (3mL) was stirred at room temperature overnight. The solvent was then evaporated and the crude product purified by column chromatography on silica (ethyl acetate-hexanes) to afford the product as an orange powder in 97% yield; mp 100–102 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.43 (s, 1H), 7.52 – 7.58 (m, 2H), 7.05 (d, J = 8.42 Hz, 1H), 4.41 (q, J = 7.14 Hz, 2H), 3.84 (s, 3H), 3.80 (s, 3H), 1.35 (t, J = 7.14 Hz, 3H); LCMS (ESI) m/z 294 (MH+)
4-(3,4-dimethoxyphenyl)-thiazole-2-carboxylic acid (69)
To a solution of 68 (0.55 g, 1.87 mmol) in MeOH, was added 5N NaOH (1mL). The reaction was stirred at room temperature overnight, then the mixture was poured into water-ice and 1N HCl added to until the solution reached pH~ 2. This solution was extracted with EtOAc, and the organic phase dried (MgSO4), filtered and concentrated to afford the desired product as an orange solid in ~90% yield. This material could be used in the next step without further purification; mp 105–106 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.38 (s, 1H), 7.57 – 7.59 (m, 1H), 7.56 (s, 1H), 7.06 (d, J = 8.42 Hz, 1H), 3.86 (s, 3H), 3.81 (s, 3H); LCMS (ESI) m/z 266 (MH+)
4-(3,4-dimethoxyphenyl)-thiazol-2-ylamine (70)
A solution of 2-bromo-1-(3,4-dimethoxyphenyl)-ethanone (0.10 g, 0.39 mmol) and thiourea (0.03 g, 0.39 mmol) in EtOH was stirred at 80°C overnight. The solvent was evaporated and the residue was partitioned between EtOAc and sat. NaHCO3. The organic layer was washed with water, dried (MgSO4), filtered and concentrated. The solid residue was crystallized from ethyl acetate-hexanes to afford the product as a light yellow powder in 60% yield; mp 198–201 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.30 – 7.38 (m, 2H), 7.00 (s, 2H), 6.93 (d, J = 8.42 Hz, 1H), 6.87 (s, 1H), 3.78 (s, 3H), 3.76 (s, 3H); LCMS (ESI) m/z 237 (MH+)
Assays of Antiprion Activity and Cell Viability
The methods employed to evaluate the effects of compounds on PrPSc levels and cell viability were similar to previously published protocols20 with the following modifications. ScN2a cells (N2a cells infected with the Rocky Mountain Laboratory prion strain) were seeded into black wall, clear bottom, tissue culture treated plates (Greiner) at either 40,000 cells/well (in 100 µL of assay medium: MEM supplemented with 10% FBS, GlutaMax and 500 µg/mL geneticin) for dividing cell assays or 150,000 cells/well (in assay medium + 7 mM sodium butyrate to arrest cell division) for non-dividing cell assays. Compounds were dissolved in 100% DMSO and diluted in assay medium at 2X final concentration before addition to the assay plates (0.5% final DMSO concentration). Compound addition occurred 4 hours (dividing cells) or 24 hours (non-dividing cells) after cell seeding into the assay plates. After 5 days incubation at 37 °C in a humidified and 5% CO2-enriched environment, lysates were generated as previously described20 and transferred to high binding ELISA plates (Greiner) coated with D18 primary antibody for overnight incubation at 4 °C. The next day, the plates were washed 3 times with TBST before addition of 100 µL of a 1:1000 dilution of HRP-conjugated D13 antibody in 1%BSA/PBS for a 1 hour incubation at room temperature. After incubation with the D13 antibody, the plates were washed seven times with TBST, 100 µL of ABTS was added to each well for 10 minutes and absorbance at 405 nm was read using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). Calcein cell viability assays were run on separately seeded 96 well black wall plates as previously described.1
In vitro ADME studies
Permeability (MDR1-MDCK) and microsome stability data was generated at ADMETRx, Inc. (Kalamazoo, MI) as described below.
Bidirectional MDR1-MDCK Cell Permeability
MDR1-MDCK cells were grown to confluence for 5–10 days on 1 µm filters in 24-well plates. Aliquots of DMSO solute stocks were diluted into Hank’s balanced salt solution (HBSS) pH 7.4 containing 25mM +0.05% PS80 to give 10 µM solute concentration. The solute containing donor solutions were transferred to either the apical or basolateral chamber of the permeability diffusion apparatus. Receiver solutions consisted of Hank’s balanced salt solution (HBSS) pH 7.4 containing 25mM HEPES + 0.05% PS80. Sequential samples of transported solute were taken at 20 minute intervals using an automated liquid handling platform. The concentration of transported solute during each sampling interval was determined by HPLC/UV/MS. Permeability coefficients were calculated for each sampling interval. The average and standard deviation from the intervals are reported. Mass balance in the system was ascertained by comparing the sum of total transported solute and remaining donor solute with the starting mass of solute and is expressed as a percentage of donor solute at time zero. Significant deviations from 100% (generally less than 70%) suggest solute adsorption to the apparatus or monolayer, or chemical or metabolic instability during the course of the experiment. Mass balance values for compounds reported herein were between 67% and 86%. In the event of mass balance less than 70%, the cell monolayers were extracted with acetonitrile and analyzed for the solute of interest. Determinates were conducted in duplicate.
Hepatic Microsome Stability
Aliquots of DMSO solute stock were diluted into acetonitrile and then into assay buffer. Assay buffer was pH 7.4 phosphate buffered saline (PBS). Final experimental solute concentrations were 1µM (0.6 % acetonitrile, 0.01 % DMSO). Commercially available rat human hepatic microsomes (approx 0.3 mg/ml final concentration) and NADPH (1mM) with 4mM UDPGA or PBS were added. The resulting mixture was incubated at 37°C with aliquots removed at 0, 1, 10, 30 and 60 minutes and quenched with acetonitrile containing 2 µM carbamazepine (internal standard), centrifuged and supernatant analyzed by LCMS for remaining starting material. Duplicate incubations were run at each timepoint. Control incubations were conducted with Midazolam (T1/2 = 11 min) and 4-Nitrophenol (T1/2 = 20 min).
In vivo Studies
Aminothiazole analog (e.g., 4.5 g of 27) was added to 15 mL of pure PEG400, vortexed and sonicated to ensure dissolution, then stored at −4 °C until needed. This highly concentrated PEG400 solution was subsequently diluted to final dosing concentrations, where the volume added was composed of homogenized solid rodent feed, cocoa (taste masking agent), and water. Wild-type FVB mice weigh ~25 gm and typically drink 20 mL of liquid diet per day, allowing an estimate of daily drug consumption. A single dosing cohort consisted of three mice in a shared cage and liquid diet was provided at the start of the study in sufficient volume to last for the entire three-day trial (~200 mL). At the end of the three-day dosing period, animals were euthanized by CO2, followed by collection of plasma (cardiac puncture) and removal of whole brain. Heparinized blood was centrifuged to separate plasma and both plasma and brain samples were stored at −80 °C prior to analysis. Plasma samples were prepared for analysis by precipitating proteins and reconstituting the remaining fraction with HPLC mobile phase. Brain samples were typically prepared by four-fold dilution with water after weighing, then homogenized using bead-beater or Polytron™ resulting in a highly concentrated solution that was further diluted with mobile phase as appropriate in preparation for bioanalytical analysis using LC-MS. Analysis was by LC-MS (Shimadzu dual-HPLC pumps, C18 analytical column, with detection using an Applied Biosystems API-4000 triple quadrupole mass spectrometer). Specific LC-MS methods were developed for each compound analyzed and the stability of the compounds in brain and plasma were demonstrated for the time period of sample handling, workup, and LC-MS analysis.
Supplementary Material
Acknowledgement
This work was funded by federal grants (NIH AG021601 and NIH AG031220) as well as by gifts from the Sherman Fairchild Foundation, Lincy Foundation, and Robert Galvin. We thank Drs. S. Ghaemmaghami, M. Jacobson, J. Wells, F.E. Cohen, Z. Li, and M. Arkin for helpful discussions and suggestions. We acknowledge Drs. B. C. H. May, and P.-W. Phuan for experimental contributions made in the early stages of this project.
Footnotes
Abbreviations: SAR, structure-activity relationships; ScN2a-cl3, Neuroblastoma cells infected with ‘clone-3’ Rocky Mountain Laboratory prions; PrPC, Endogenous form of the prion protein; PrPSc, misfolded and pathogenic form of the prion protein; BBB, blood-brain barrier; clogP, calculated octonal/water partition coefficient; P-gp, P-glycoprotein; MDR1-MDCK, Multidrug resistance-1 Madin-Darby canine kidney cell line; FVB mice, mice inbred for the Friend leukemia virus B (Fv1b) allele.
Supporting Information Available: Supplemental synthetic schemes for new 2-aminothiazole analogs 4–50 and synthetic intermediates 51 to 70. Triplicate data set for compounds 3–50 in the ScN2a-cl3 dividing cell assay (EC50 and pEC50 values), standard deviation (SD), and percent coefficient of variation (CV) values. Mean values (three determinations) for 3–50 in the ScN2a-cl3 non-dividing cell assay and calcein-AM cell viability assay. 1D NOESY data that supports the assigned site of methylation and acylation for compounds 49 and 50, respectively. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Ghaemmaghami S, May BC, Renslo AR, Prusiner SB. Discovery of 2-aminothiazoles as potent antiprion compounds. J Virol. 2010;84:3408–3412. doi: 10.1128/JVI.02145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller G. Neurodegeneration. Could they all be prion diseases? Science. 2009;326:1337–1339. doi: 10.1126/science.326.5958.1337. [DOI] [PubMed] [Google Scholar]
- 4.Race RE, Fadness LH, Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987;68:1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- 5.Butler DA, Scott MR, Bockman JM, Borchelt DR, Taraboulos A, Hsiao KK, Kingsbury DT, Prusiner SB. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988;62:1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A. 2001;98:9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May BC, Witkop J, Sherrill J, Anderson MO, Madrid PB, Zorn JA, Prusiner SB, Cohen FE, Guy RK. Structure-activity relationship study of 9-aminoacridine compounds in scrapie-infected neuroblastoma cells. Bioorg Med Chem Lett. 2006;16:4913–4916. doi: 10.1016/j.bmcl.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 8.May BC, Fafarman AT, Hong SB, Rogers M, Deady LW, Prusiner SB, Cohen FE. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc Natl Acad Sci U S A. 2003;100:3416–3421. doi: 10.1073/pnas.2627988100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dollinger S, Lober S, Klingenstein R, Korth C, Gmeiner P. A chimeric ligand approach leading to potent antiprion active acridine derivatives: design, synthesis, and biological investigations. J Med Chem. 2006;49:6591–6595. doi: 10.1021/jm060773j. [DOI] [PubMed] [Google Scholar]
- 10.Kempster S, Bate C, Williams A. Simvastatin treatment prolongs the survival of scrapie-infected mice. NeuroReport. 2007;18:479–482. doi: 10.1097/WNR.0b013e328058678d. [DOI] [PubMed] [Google Scholar]
- 11.Heal W, Thompson MJ, Mutter R, Cope H, Louth JC, Chen B. Library Synthesis and Screening:2,4-Diphenylthiazoles and 2,4-Diphenyloxazoles as Potential Novel Prion Disease Therapeutics. J Med Chem. 2007;50:1347–1353. doi: 10.1021/jm0612719. [DOI] [PubMed] [Google Scholar]
- 12.Kimata A, Nakagawa H, Ohyama R, Fukuuchi T, Ohta S, Suzuki T, Miyata N. New Series of Antiprion Compounds: Pyrazolone Derivatives Have the Potent Activity of Inhibiting Protease-Resistant Prion Protein Accumulation. J Med Chem. 2007;50:5053–5056. doi: 10.1021/jm070688r. [DOI] [PubMed] [Google Scholar]
- 13.Kimata A, Nakagawa H, Ohyama R, Fukuuchi T, Ohta S, Doh-ura K, Suzuki T, Miyata N. Additions and Corrections to New Series of Antiprion Compounds: Pyrazolone Derivatives Have the Potent Activity of Inhibiting Protease-Resistant Prion Protein Accumulation. J Med Chem. 2008;51:1503. doi: 10.1021/jm070688r. [DOI] [PubMed] [Google Scholar]
- 14.Thompson MJ, Borsenberger V, Louth JC, Judd KE, Chen B. Design, Synthesis, and Structure-Activity Relationship of Indole-3-glyoxylamide Libraries Possessing Highly Potent Activity in a Cell Line Model of Prion Disease. J Med Chem. 2009;52:7503–7511. doi: 10.1021/jm900920x. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki Y, Kawagoe K, Chen CJ, Teruya K, Sakasegawa Y, Doh-ura K. Orally administered amyloidophilic compound is effective in prolonging the incubation periods of animals cerebrally infected with prion diseases in a prion strain-dependent manner. J Virol. 2007;81:12889–12898. doi: 10.1128/JVI.01563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yudovin-Farber I, Azzam T, Metzer E, Taraboulos A, Domb AJ. Cationic polysaccharides as antiprion agents. J Med Chem. 2005;48:1414–1420. doi: 10.1021/jm049378o. [DOI] [PubMed] [Google Scholar]
- 17.Trevitt CR, Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129:2241–2265. doi: 10.1093/brain/awl150. [DOI] [PubMed] [Google Scholar]
- 18.Perrier V, Wallace AC, Kaneko K, Safar J, Prusiner SB, Cohen FE. Mimicking dominant negative inhibition of prion replication through structure-based drug design. Proc Natl Acad Sci U S A. 2000;97:6073–6078. doi: 10.1073/pnas.97.11.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaemmaghami S, Ullman J, Ahn M, St Martin S, Prusiner SB. Chemical induction of misfolded prion protein conformers in cell culture. J Biol Chem. 2010;285:10415–10423. doi: 10.1074/jbc.M109.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May BC, Zorn JA, Witkop J, Sherrill J, Wallace AC, Legname G, Prusiner SB, Cohen FE. Structure-activity relationship study of prion inhibition by 2-aminopyridine-3,5-dicarbonitrile-based compounds: parallel synthesis, bioactivity, and in vitro pharmacokinetics. J Med Chem. 2007;50:65–73. doi: 10.1021/jm061045z. [DOI] [PubMed] [Google Scholar]
- 21.Hitchcock SA, Pennington LD. Structure-brain exposure relationships. J Med Chem. 2006;49:7559–7583. doi: 10.1021/jm060642i. [DOI] [PubMed] [Google Scholar]
- 22.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun A, Hammerle S, Suda K, Rothen-Rutishauser B, Gunthert M, Kramer SD, Wunderli-Allenspach H. Cell cultures as tools in biopharmacy. Eur J Pharm Sci. 2000;11 Suppl 2:S51–S60. doi: 10.1016/s0928-0987(00)00164-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.