Abstract
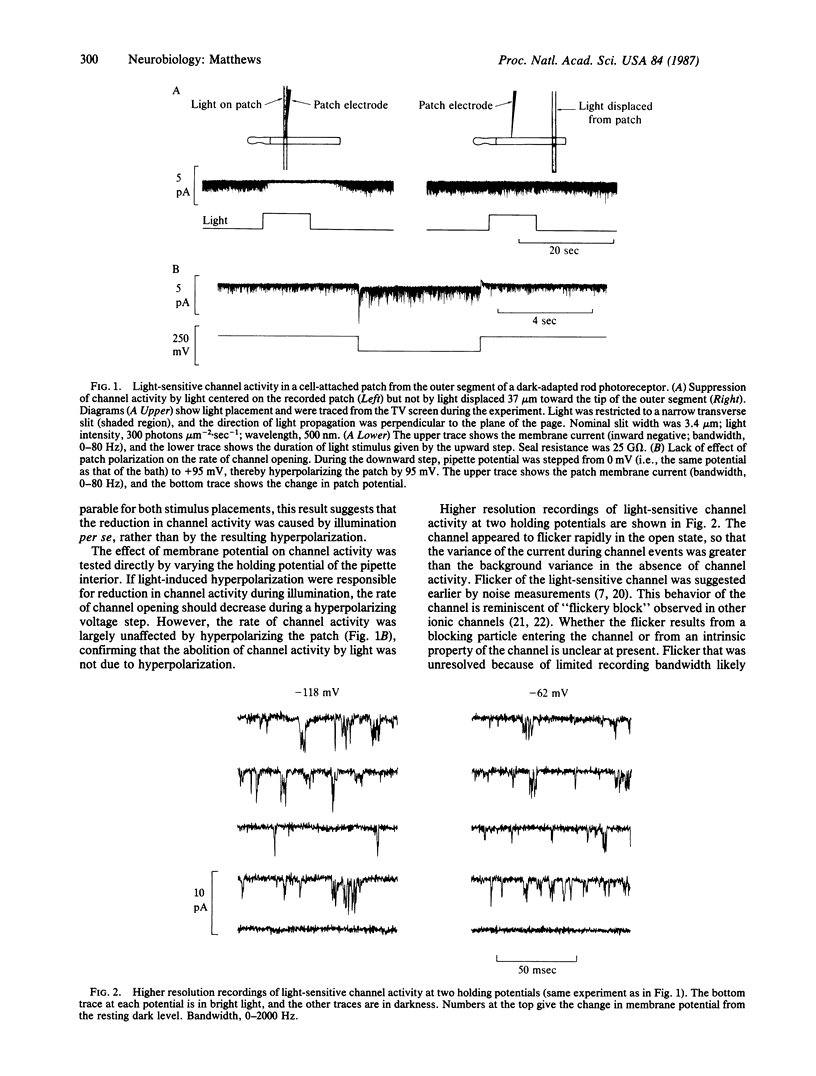
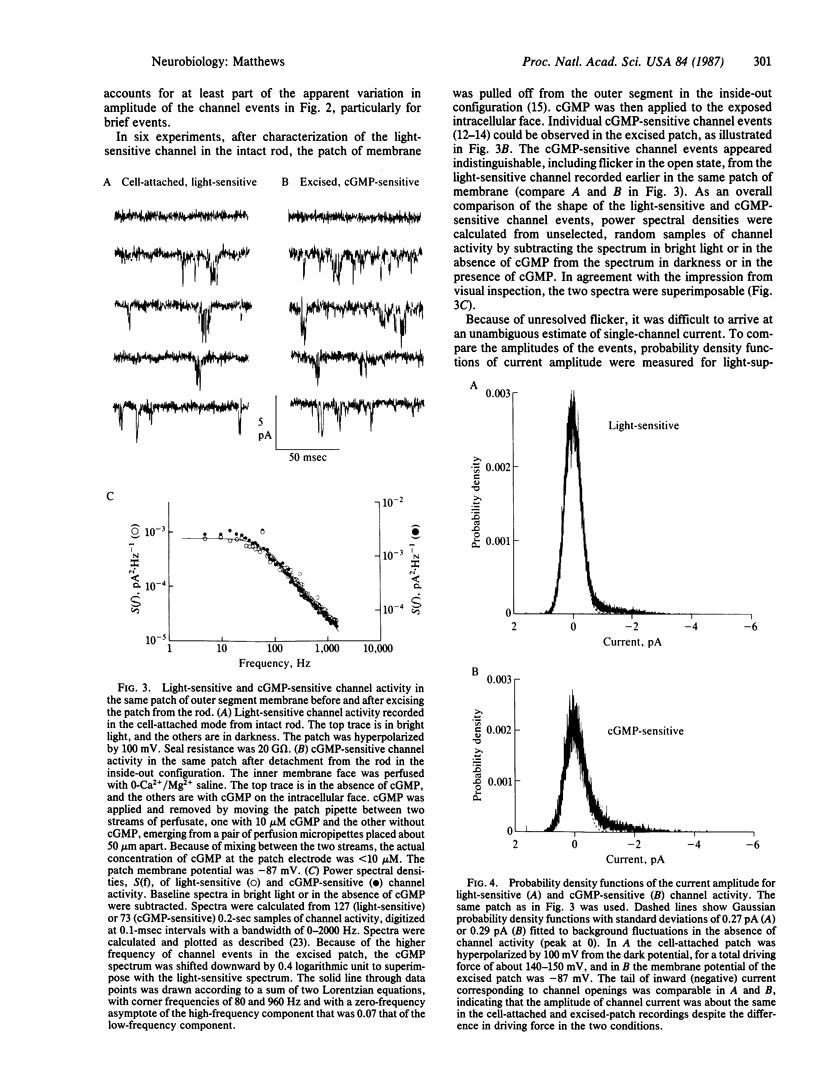
Patch-clamp recordings were made from outer segments of single dark-adapted rod photoreceptors from toad retina. When patch pipettes were filled with a solution free of divalent cations, inward current through individual light-sensitive ion channels was sufficiently large to allow single-channel recording. Channel activity was suppressed by illumination within the normal response range of the dark-adapted rod. When illumination was restricted to a portion of the outer segment, the effect of light on channel activity was spatially localized to the illuminated region. Hyperpolarization of the recorded patch did not reduce the frequency of channel opening, indicating that the suppression of channel activity by light was due to illumination itself and not to light-induced hyperpolarization of the rod. After recording single light-sensitive channel activity in the intact cell, the patch of membrane was detached to form an excised, inside-out patch. Cyclic GMP applied to the intracellular face of the excised patch then opened a channel that appeared to be the same as the light-sensitive channel recorded earlier from the same membrane in the intact, functioning rod. This provides direct evidence that cyclic GMP acts as an internal transmitter that opens the light-sensitive channel of the vertebrate photoreceptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Membrane current noise in toad retinal rods exposed to low external calcium. J Physiol. 1985 Apr;361:205–217. doi: 10.1113/jphysiol.1985.sp015641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Spatial spread of light-induced sensitization in rod photoreceptors exposed to low external calcium. Vision Res. 1985;25(6):733–740. doi: 10.1016/0042-6989(85)90180-4. [DOI] [PubMed] [Google Scholar]
- Matthews G. Spread of the light response along the rod outer segment: an estimate from patch-clamp recordings. Vision Res. 1986;26(4):535–541. doi: 10.1016/0042-6989(86)90002-7. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]


