Abstract
BACKGROUND AND PURPOSE
Melanocortins reverse circulatory shock and improve survival by counteracting the systemic inflammatory response, and through the activation of the vagus nerve-mediated cholinergic anti-inflammatory pathway. To gain insight into the potential therapeutic value of melanocortins against multiple organ damage following systemic inflammatory response, here we investigated the effects of the melanocortin analogue [Nle4, D-Phe7]α-MSH (NDP-α-MSH) in a widely used murine model of multiple organ dysfunction syndrome (MODS).
EXPERIMENTAL APPROACH
MODS was induced in mice by a single intraperitoneal injection of lipopolysaccharide followed, 6 days later (= day 0), by zymosan. After MODS or sham MODS induction, animals were randomized to receive intraperitoneally NDP-α-MSH (340 µg·kg−1 day) or saline for up to 16 days. Additional groups of MODS mice were concomitantly treated with the melanocortin MC4 receptor antagonist HS024, or the nicotinic acetylcholine receptor antagonist chlorisondamine, and NDP-α-MSH.
KEY RESULTS
At day 7, in the liver and lung NDP-α-MSH, significantly reduced mRNA expression of tumour necrosis factor-α (TNF-α), increased mRNA expression of interleukin-10 and improved the histological picture, as well as reduced TNF-α plasma levels; furthermore, NDP-α-MSH dose-dependently increased survival rate, as assessed throughout the 16 day observation period. HS024 and chlorisondamine prevented all the beneficial effects of NDP-α-MSH in MODS mice.
CONCLUSIONS AND IMPLICATIONS
These data indicate that NDP-α-MSH protects against experimental MODS by counteracting the systemic inflammatory response, probably through brain MC4 receptor-triggered activation of the cholinergic anti-inflammatory pathway. These findings reveal previously undescribed effects of melanocortins and could have clinical relevance in the MODS setting.
Keywords: multiple organ dysfunction syndrome, melanocortin peptides, cholinergic anti-inflammatory pathway, melanocortin MC4 receptors, nicotinic acetylcholine receptors, mouse
Introduction
Multiple organ dysfunction syndrome (MODS) is the leading cause of morbidity and mortality in the intensive care unit. MODS may occur after several pathological conditions, including circulatory shock by various causes, injury by accident or surgery, hypermetabolism and pancreatitis, and there is a need for novel therapeutically effective approaches (Baue, 1997; 2006; Wheeler and Bernard, 1999; Volman et al., 2005; van Meurs et al., 2008; Dewar et al., 2009; Rosas-Ballina and Tracey, 2009). Recently, remarkable advances have been made in understanding the cellular mechanisms underlying organ dysfunction and recovery relating to these conditions. Although several new therapeutic approaches have improved outcome in these patients, including septic patients, the far-reaching potential of these new insights into the mechanisms of the development of multiple organ failure is only beginning to be realized (Cohen, 2002; Volman et al., 2005; Tracey, 2007; Tsukamoto et al., 2010). Current understanding of the pathophysiology of MODS highlights the multiple cell populations and cell-signalling pathways involved in this complex condition characterized by generalized inflammation. The intricate cross-talk provided by temporal changes in mediators, hormones, metabolites, neural signalling, alterations in oxygen delivery and utilization, underlies adaptive and coordinated processes beyond the initial clinical condition (Schlag et al., 1991; Baue, 1997; 2006; Cohen, 2002; Tracey, 2002; 2007; Volman et al., 2005; Dewar et al., 2009; Tsukamoto et al., 2010).
Melanocortins are endogenous peptides of the adrenocorticotropin/melanocyte-stimulating hormone (ACTH/MSH) group. To date, melanocortin peptides have been shown to possess a multitude of actions including modulation of the host inflammatory response in acute and chronic inflammation (Wikberg et al., 2000; Catania et al., 2004; Getting, 2006; Catania, 2007). Indeed, several melanocortins including ACTH-(1–24), α-MSH, shorter fragments and synthetic analogues such as [Nle4, D-Phe7]α-MSH (NDP-α-MSH) are effective in restoring cardiovascular and respiratory functions, and improving survival, in animal and human pathological conditions characterized by systemic inflammatory response such as circulatory shock (Bertolini et al., 1986a,b; Guarini et al., 1987; 1990; 1999; 2004; Squadrito et al., 1999; Noera et al., 2001; Jochem, 2004; Bertuglia and Giusti, 2004; Giuliani et al., 2007). Our previous data showed that melanocortins reduce early morphological and immunocytochemical alterations in the heart, lung, liver and kidney following experimental haemorrhagic shock (Giuliani et al., 2007), suggesting a possible protective effect against late damage. These neuropeptides are likewise protective in other severe hypoxic, inflammatory conditions, including prolonged respiratory arrest (Guarini et al., 1997a), myocardial ischaemia (Bazzani et al., 2001; Vecsernyes et al., 2003; Mioni et al., 2005; Ottani et al., 2010) and ischaemic stroke (Giuliani et al., 2006a,b, 2009; Ottani et al., 2009). The beneficial effects of melanocortins are associated with blunted nuclear factor-kB (NF-κB) activation, and a marked reduction in the levels of free radicals, tumour necrosis factor-α (TNF-α) and other pro-inflammatory cytokines, as well as of intercellular adhesion molecule expression by vascular endothelium (for reviews see: Wikberg et al., 2000; Catania et al., 2004; Getting, 2006; Catania, 2007; Giuliani et al., 2010). Moreover, it is well known that melanocortins increase the production and release of the potent anti-inflammatory cytokine interleukin-10 (IL-10) (Catania et al., 2004; Giuliani et al., 2010).
Recent findings indicate that during endotoxic/septic, haemorrhagic and splanchnic artery occlusion shock the central nervous system (CNS) modulates the systemic inflammatory response through the rapid activation of efferent vagal nerve fibres (the ‘cholinergic anti-inflammatory pathway’), and these effects are mediated by acetylcholine – the major vagus nerve neurotransmitter – which interacts with nicotinic receptors located on inflammatory cells (Borovikova et al., 2000; Tracey, 2002; 2007; Guarini et al., 2003; 2004; Altavilla et al., 2006; Rosas-Ballina and Tracey, 2009; Giuliani et al., 2010). Furthermore, we previously demonstrated that the anti-shock effect of melanocortins is due to the activation of the cholinergic anti-inflammatory pathway, by stimulation of melanocortin MC4 receptors within the CNS (Guarini et al., 2004).
To gain insight into the potential therapeutic value of melanocortins against multiple organ damage following systemic inflammatory response, we investigated the effects of NDP-α-MSH in a murine model of generalized inflammation that leads to MODS, recognized as the one that best resembles human MODS.
Methods
Animals and induction of multiple organ dysfunction
C57BL/6 male mice, 9 weeks old and weighing 20–25 g (Charles River, Calco, Italy) were used. They were kept in air conditioned colony rooms (temperature 21 ± 1°C; humidity 60%) on a natural light/dark cycle, with food in pellets and tap water available ad libitum. Animals were used 6 days after arrival. Housing conditions and experimental procedures were in strict accordance with the Declaration of Helsinki and European Community regulations on the use and care of animals for scientific purposes (CEE Council 89/609; Italian D.L.22-1-92 no. 116), and were approved by the Committee on Animal Health and Care of Messina University.
The zymosan-induced generalized inflammation model for MODS has been already extensively described (Goris et al., 1986; Jansen et al., 1997; Cuzzocrea et al., 2001; Volman et al., 2002; 2004;). Briefly, mice received an aseptic i.p. injection of 40 µg lipopolysaccharide (LPS; Escherichia coli; Sigma, St Louis, MO) dissolved in 200 µL of phosphate-buffered saline, followed 6 days later by i.p. administration of zymosan A (Sigma) – that consists of protein-carbohydrate complexes derived from cell wall of Saccharomyces cerevisiae– dissolved in warm 0.9% NaCl solution (day 0). Zymosan dose was 8 mg·kg−1 body weight. Sham MODS animals received only phosphate-buffered saline.
Experimental design and treatments
Mice with zymosan-induced generalized inflammation were randomly allocated to the following groups (Figure 1). Group 1: MODS (LPS and zymosan) treated daily with saline (1 mL·kg−1 day i.p.). Group 2: MODS treated daily with NDP-α-MSH (a dose-response study: 85–340 µg·kg−1 day i.p.). Group 3: MODS treated daily with the melanocortin MC4 receptor antagonist HS024 (130 µg·kg−1 i.p.) followed by NDP-α-MSH (340 µg·kg−1 day i.p.). Group 4: MODS treated daily with the nicotinic acetylcholine receptor antagonist chlorisondamine (0.25 mg·kg−1, s.c.) followed by NDP-α-MSH (340 µg·kg−1 day i.p.). Group 5: MODS treated daily with the MC4 receptor antagonist HS024 followed by saline. Group 6: MODS treated daily with the nicotinic acetylcholine receptor antagonist chlorisondamine followed by saline. Sham MODS animals were randomly divided into two groups. Group 1: sham MODS receiving daily saline i.p. Group 2: sham MODS receiving daily NDP-α-MSH (340 µg·kg−1 i.p.). Pharmacological treatments (and/or with saline) started after zymosan administration (day 0) and were performed for 16 days, or until death. Biomolecular and histological parameters were evaluated at day 7 following zymosan administration, when all animals were still alive. Clinical signs of systemic inflammation were evaluated from day 1 to day 7 and scored on a subjective scale ranging from 0 to 3; 0 = absence, 1 = mild, 2 = moderate, 3 = severe (Cuzzocrea et al., 2001). The scale was used for each of the signs (ruffled fur, diarrhoea, lethargy, hypothermia, weight loss and dyspnoea) observed in animals. Climbing, chewing and digging are considered normal behaviours for mice and indicate a good health status. Survival was recorded for 16 days after zymosan administration (Volman et al., 2004).
Figure 1.
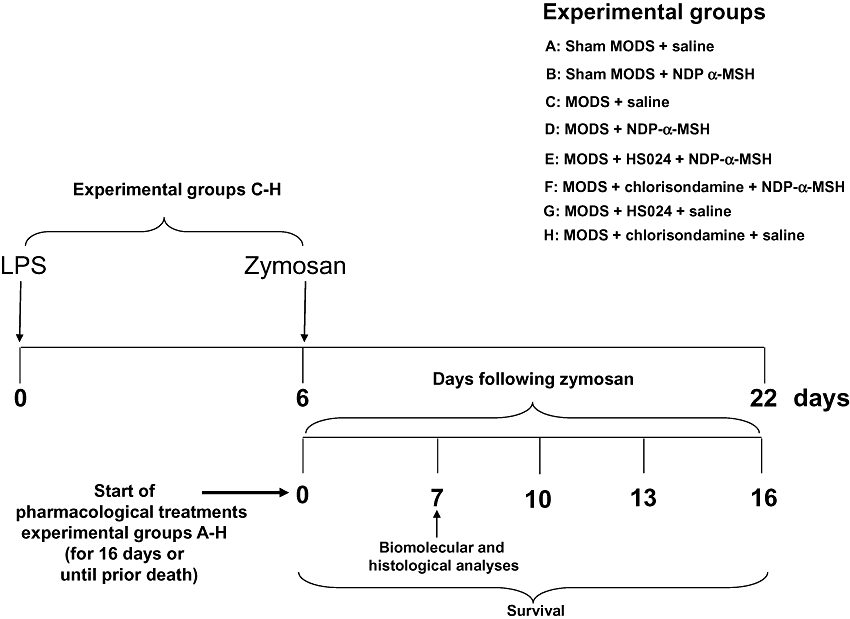
Experimental schedule and treatments.
NDP-α-MSH, HS024 and chlorisondamine were dissolved in saline immediately before use, in a volume of 1 mL·kg−1. NDP-α-MSH was kindly supplied by Prof Paolo Grieco (University of Naples, Italy). Pretreatment with chlorisondamine diiodide and HS024 (supplied by Tocris; Bristol, United Kingdom) was performed 1 min before treatment with NDP-α-MSH (or saline). For some drugs, doses and times were chosen on the basis of previous experiments performed in our laboratory (Bazzani et al., 2001; Guarini et al., 2003; 2004; Altavilla et al., 2006; Giuliani et al., 2006a,b; 2007;).
Drug/molecular target nomenclature conforms to ‘British Journal Pharmacology's Guide to Receptors and Channels’ (Alexander et al., 2009).
TNF-α plasma level assay
At day 7 after zymosan injection, blood was drawn in six mice randomly assigned for each group by cardiac puncture under general anaesthesia. Blood samples were collected in EDTA tube and centrifuged for 10 min at 2600×g; plasma was then removed and stored at −20°C until the analysis. The day of the assay, after reconstituting the reagents, the amount of TNF-α was measured with a specific enzyme-linked immunosorbent assay kit (Genzyme, Modena, Italy) and compared with a TNF-α standard curve (Guarini et al., 2003; 2004;).
TNF-α and IL-10 mRNA expression assay
At day 7 after zymosan injection, after blood withdrawal, samples of lung and liver (30 mg each) from the same animals were used for total RNA extraction. Total RNA was isolated by using Trizol Reagent (Invitrogen, Milan, Italy), and the procedure was performed according to the protocol provided by the manufacturer. RNA (5 µg) from each sample was reverse transcribed using High-Capacity cDNA Archive Kit according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). cDNA from each sample (5 ng) was amplified by real-time PCR with 2X TaqMan universal PCR Mastermix (Applied Biosystems), 20X target primer and probe. β-Actin was used as the control, due to its constitutive expression as a house-keeping gene. Each sample was analysed in duplicate using SDS 7300 (Applied Biosystems). The results are expressed as an n-fold difference relative to β-actin (relative expression levels).
Histological examination
For light microscopy, at day 7 after zymosan in the previously mentioned animals, a sample from the central body of the liver and lung was rapidly removed and fixed in 10% buffered formalin. Subsequently, it was embedded in paraffin, cut and stained with Haematoxylin-Eosin (Fluka Chemie, Buchs SG, Switzerland). The histology of organ sections (6 µm thick) was evaluated in five seriated slices for each organ using a Leica photomicroscope (Milan, Italy). The histological study of the liver was based on the following parameters: infiltration of inflammatory cells, steatosis, necrosis and ballooning degeneration; they were evaluated by the following score scale of values: 0, absent; 1, mild; 2, moderate; 3, severe. The histological study of the lung was based on the following parameters: infiltration of inflammatory cells, vascular congestion and interstitial oedema; they were scored from 0 (absent) to 3 (severe).
Statistics
Data are expressed as means ± SEM. Comparison of TNF-α and IL-10 mRNA values, as well as TNF-α plasma levels, was performed using one way analysis of variance (ANOVA) followed by a multiple comparison test (Student-Newman-Keuls). Statistical analysis of histological data and clinical signs was performed using the Kruskal-Wallis test followed by the Dunn multiple comparison post test. Survival rates were analysed by the Kaplan-Meier method, followed by the Log Rank test. P < 0.05 was considered significant.
Results
NDP-α-MSH improves clinical conditions and survival in MODS animals
After LPS injection, control animals had a transient loss in body weight that completely recovered when they received zymosan (day 0). Zymosan injection induced an acute peritonitis as indicated by ruffled fur, diarrhoea, lethargy, hypothermia and decrease in body weight (first 2 days) (Table S1). During days 3 to 5, the general conditions of the surviving mice appeared recovered, as reflected by spontaneous locomotor exploration, grooming, smooth fur, disappearance of diarrhoea and hypothermia, and weight gain. After approximately 6 days, animals entered the third phase, characterized by lethargy, dyspnoea, weight loss and decrease in body temperature, with 50% mortality at day 16 (Figure 2). This is in agreement with previous descriptions of the clinical conditions of this MODS model (Cuzzocrea et al., 2001; Volman et al., 2002; 2005;).
Figure 2.
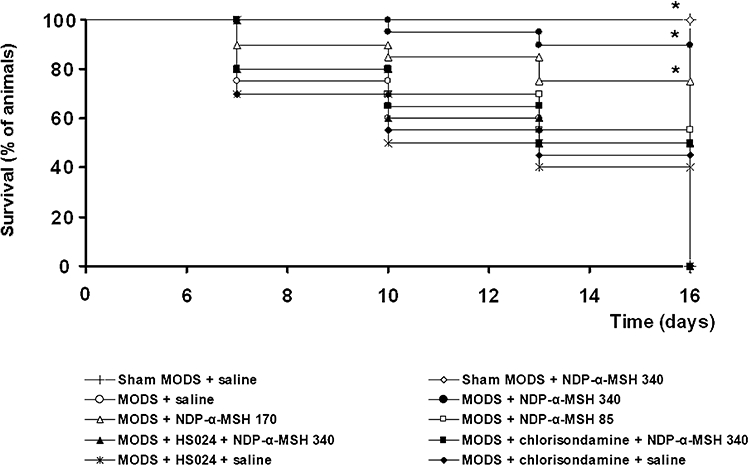
NDP-α-MSH improved survival rate in MODS mice. HS024 and chlorisondamine counteracted the life-saving effect of NDP-α-MSH. Kaplan-Meier curves. At day 0, mice were 20 per group; each time point represents percentage of animals surviving. NDP-α-MSH: daily administration of 85, 170 or 340 µg·kg−1 i.p. throughout the observation period (up to 16 days after zymosan, where indicated); saline: 1 mL·kg−1 i.p.; HS024: 130 µg·kg−1 i.p. 1 min before each NDP-α-MSH or saline administration; chlorisondamine: 0.25 mg·kg−1 s.c. 1 min before each NDP-α-MSH or saline administration. *P < 0.01 vs. MODS + saline (Log Rank test).
In MODS mice daily treated with NDP-α-MSH, starting after zymosan injection, there was a dose-dependent improvement in the general conditions of animals that with the highest dose of NDP-α-MSH (340 µg·kg−1) appeared close to normality, as indicated by the lack of appreciable alterations (Table S1). In these MODS mice treated with NDP-α-MSH, survival rate dose-dependently improved and, with the highest dose, at day 16 after zymosan increased up to 90% (Figure 2).
NDP-α-MSH reduces mRNA expression of TNF-α in the liver and lung
To investigate systemic inflammatory reactions in LPS-zymosan injected mice, we assessed TNF-α mRNA expression in the liver and lung. RT-PCR analysis showed that the hepatic and pulmonary TNF-α mRNA levels increased in saline-treated control animals, when detected 7 days after zymosan administration (Figure 3A,B).
Figure 3.
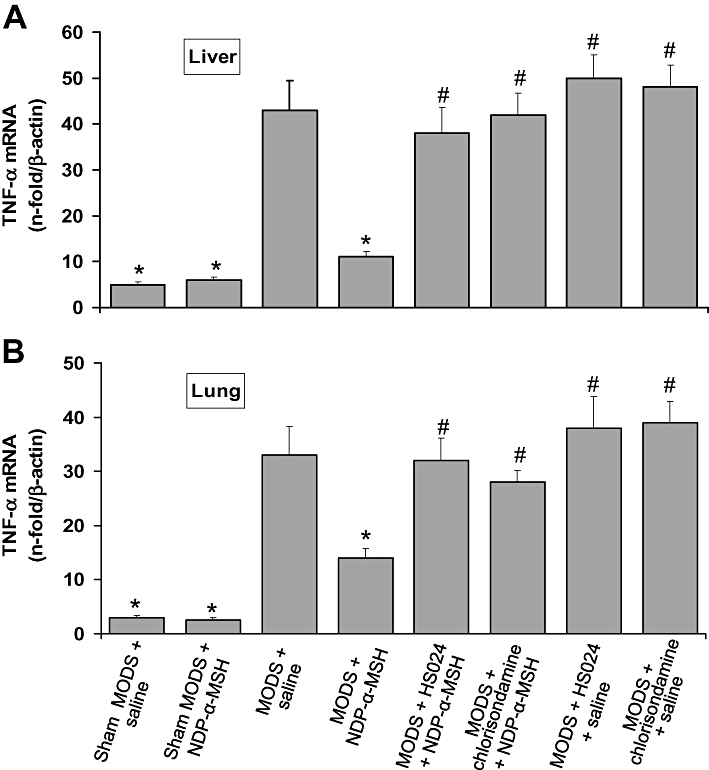
Treatment with NDP-α-MSH reduced TNF-α mRNA expression in the liver (A) and lung (B) of MODS mice. Pretreatment with the melanocortin MC4 receptor antagonist HS024 and the nicotinic acetylcholine receptor antagonist chlorisondamine prevented the effect of NDP-α-MSH. Values are means ± SEM for 6 mice per group at day 7 after zymosan. NDP-α-MSH: 340 µg·kg−1 i.p. for 7 days after zymosan; saline: 1 mL·kg−1 i.p. for 7 days after zymosan; HS024: 130 µg·kg−1 i.p. 1 min before each NDP-α-MSH or saline administration; chlorisondamine: 0.25 mg·kg−1 s.c. 1 min before each NDP-α-MSH or saline administration. *P < 0.01 vs. MODS + saline; #P < 0.01 vs. MODS + NDP-α-MSH (Student-Newman-Keuls test).
Daily treatment with NDP-α-MSH (340 µg·kg−1) for 7 days greatly reduced TNF-α mRNA levels in both organs (Figure 3A,B).
NDP-α-MSH increases mRNA expression of IL-10 in the liver and lung
At day 7 after zymosan, we also investigated mRNA expression of the anti-inflammatory cytokine IL-10 in the liver and lung. LPS-zymosan injection caused a compensatory increase in the mRNA levels of this cytokine, both in the liver and lung of saline-treated control mice (Figure 3A,B).
Consistent with the established ability of melanocortins to increase the production and release of IL-10 from monocytes (Catania et al., 2004), treatment with NDP-α-MSH (340 µg·kg−1) produced a marked increase in the mRNA expression of this potent anti-inflammatory cytokine, both in the liver and lung (Figure 4A,B).
Figure 4.
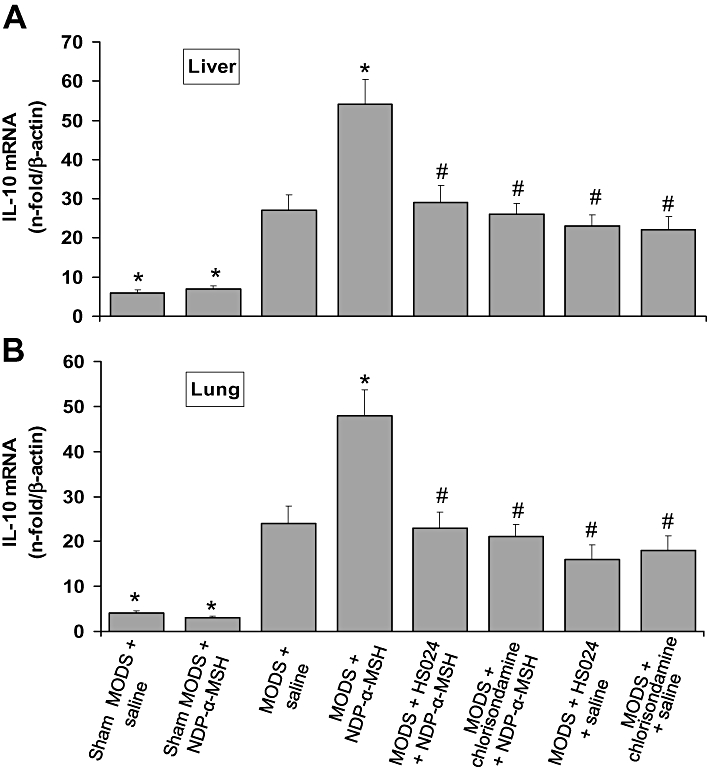
Treatment with NDP-α-MSH increased IL-10 mRNA expression in the liver (A) and lung (B) of MODS mice. Pretreatment with the melanocortin MC4 receptor antagonist HS024 and the nicotinic acetylcholine receptor antagonist chlorisondamine prevented the effect of NDP-α-MSH. Values are means ± SEM for 6 mice per group at day 7 after zymosan. NDP-α-MSH: 340 µg·kg−1 i.p. for 7 days after zymosan; saline: 1 mL·kg−1 i.p. for 7 days after zymosan; HS024: 130 µg·kg−1 i.p. 1 min before each NDP-α-MSH or saline administration; chlorisondamine: 0.25 mg·kg−1 s.c. 1 min before each NDP-α-MSH or saline administration. *P < 0.01 vs. MODS + saline; #P < 0.01 vs. MODS + NDP-α-MSH (Student-Newman-Keuls test).
NDP-α-MSH reduces TNF-α plasma levels
Plasma TNF-α levels were markedly increased at day 7 after zymosan (Figure 3). NDP-α-MSH treatment (340 µg·kg−1), as expected, significantly reduced TNF-α levels (Figure 5).
Figure 5.
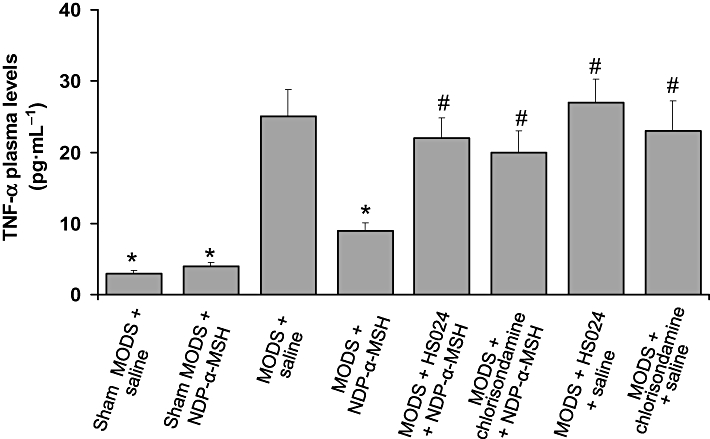
Treatment with NDP-α-MSH reduced TNF-α plasma levels. Pretreatment with the melanocortin MC4 receptor antagonist HS024 and the nicotinic acetylcholine receptor antagonist chlorisondamine prevented the effect of NDP-α-MSH. Values are means ± SEM for 6 mice per group at day 7 after zymosan. NDP-α-MSH: 340 µg·kg−1 i.p. for 7 days after zymosan; saline: 1 mL·kg−1 i.p. for 7 days after zymosan; HS024: 130 µg·kg−1 i.p. 1 min before each NDP-α-MSH or saline administration; chlorisondamine: 0.25 mg·kg−1 s.c. 1 min before each NDP-α-MSH or saline administration. *P < 0.01 versus MODS + saline; #P < 0.01 vs. MODS + NDP-α-MSH (Student-Newman-Keuls test).
NDP-α-MSH reduces histological damage
Seven days after zymosan we further evaluated histological grading of the severity of LPS-zymosan-induced organ damage by a score scale of values (Table 1). In MODS mice, hepatic damage was characterized by a high degree of inflammatory infiltrate, steatosis, necrosis and ballooning degeneration (Figure 6B). Moreover, lung histological examination again showed a great inflammatory infiltrate, as well as marked vascular congestion and interstitial oedema (Figure 6F).
Table 1.
NDP-α-MSH reduced histological damage in MODS mice; HS024 and chlorisondamine prevented the protective effect of NDP-α-MSH
| Parameter | Sham MODS + saline | Sham MODS + NDP-α-MSH | MODS + saline | MODS + NDP-α-MSH | MODS + HS024 + NDP-α-MSH | MODS + chlorisondamine + NDP-α-MSH | MODS + HS024 + saline | MODS + chlorisondamine + saline |
|---|---|---|---|---|---|---|---|---|
| Liver histological score at day 7 after zymosan | ||||||||
| Inflammatory infiltrate | 0 | 0 | 2.67 ± 0.21 | 1.00 ± 0.26* | 2.50 ± 0.22# | 2.50 ± 0.34# | 3.00 ± 0.00# | 3.00 ± 0.00# |
| Steatosis | 0 | 0 | 2.50 ± 0.34 | 0.83 ± 0.17* | 2.33 ± 0.33# | 2.33 ± 0.42# | 2.83 ± 0.17# | 2.67 ± 0.33# |
| Necrosis | 0 | 0 | 2.83 ± 0.17 | 0.33 ± 0.21* | 2.50 ± 0.34# | 2.33 ± 0.21# | 3.00 ± 0.00# | 3.00 ± 0.00# |
| Ballooning degeneration | 0 | 0 | 2.83 ± 0.17 | 0.33 ± 0.33* | 2.67 ± 0.21# | 2.67 ± 0.33# | 3.00 ± 0.00# | 2.83 ± 0.17# |
| Lung histological score at day 7 after zymosan | ||||||||
| Inflammatory infiltrate | 0 | 0 | 2.67 ± 0.21 | 1.17 ± 0.17* | 2.50 ± 0.15# | 2.50 ± 0.22# | 2.83 ± 0.17# | 3.00 ± 0.00# |
| Vascular congestion | 0 | 0 | 2.83 ± 0.17 | 0.33 ± 0.21* | 2.33 ± 0.21# | 2.33 ± 0.33# | 3.00 ± 0.00# | 2.83 ± 0.17# |
| Interstitial oedema | 0 | 0 | 2.50 ± 0.22 | 0.17 ± 0.17* | 2.50 ± 0.34# | 2.67 ± 0.33# | 2.83 ± 0.17# | 2.67 ± 0.21# |
NDP-α-MSH: 340 µg·kg−1 i.p. for 7 days; saline: 1 mL·kg−1 i.p. for 7 days; HS024: 130 µg·kg−1 i.p 1 min before each NDP-α-MSH or saline administration; chlorisondamine: 0.25 mg·kg−1 s.c. 1 min before each.
NDP-α-MSH or saline administration. Values shown are mean ± SEM for 6 mice per group.
P < 0.01 versus MODS + saline;
P < 0.01 versus MODS + NDP-α-MSH (Dunn test).
Figure 6.
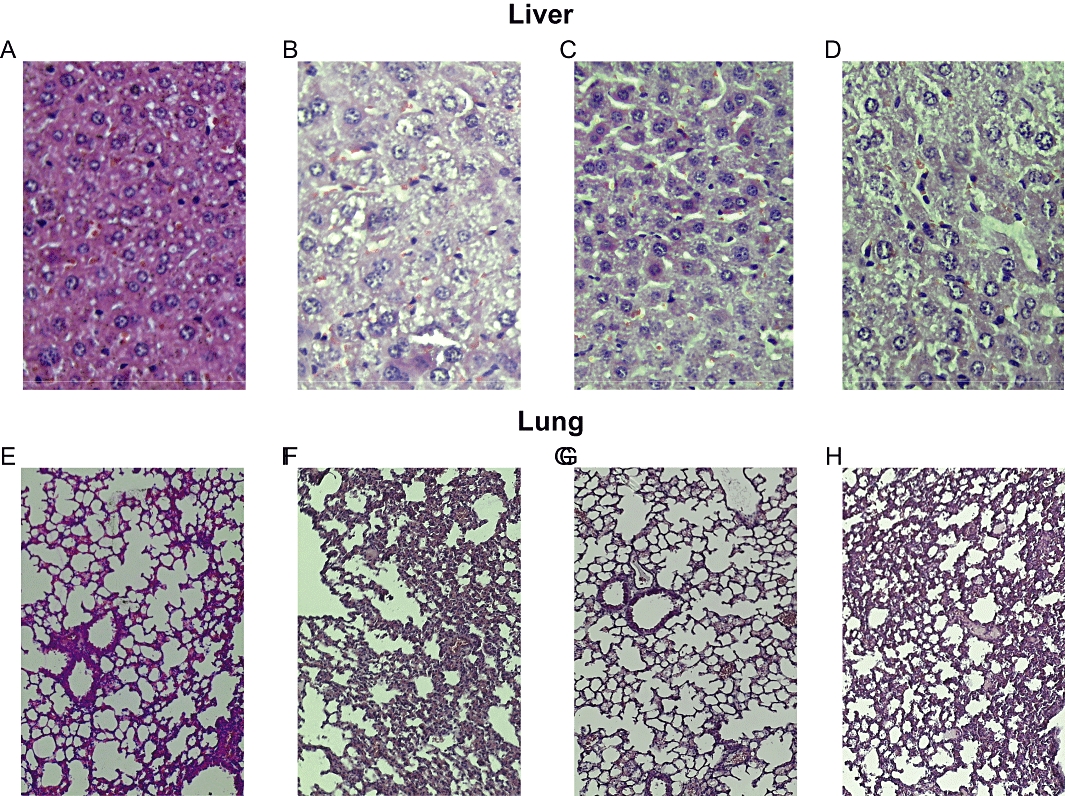
Treatment with NDP-α-MSH (340 µg·kg−1 i.p. for 7 days after zymosan) reduced histological damage in the liver and lung of MODS mice. Representative light microscopy images of experiments of Table 1. (A) Normal liver histology at day 7 from sham MODS mice. (B) Representative liver from saline-treated MODS mice: presence of inflammatory cell infiltrates, steatosis, necrosis and ballooning degeneration. (C) Representative liver from NDP-α-MSH-treated MODS mice: few inflammatory cells, reduced steatosis, absence of necrosis and/or ballooning degeneration. (D) Representative liver from MODS mice treated with the nicotinic receptor antagonist chlorisondamine 1 min before each administration of NDP-α-MSH: massive necrosis, cellular degeneration, strong presence of inflammatory cells. (E) Normal lung histology at day 7 from sham MODS mice. (F) Representative lung from saline-treated MODS mice: presence of a sustained inflammatory cells infiltrate, vascular congestion and interstitial oedema. (G) Representative lung from NDP-α-MSH-treated MODS mice: reduction of inflammatory cells infiltrate, vascular congestion and interstitial oedema. (H) Representative lung from MODS mice treated with the nicotinic receptor antagonist chlorisondamine 1 min before each administration of NDP-α-MSH: strong inflammatory cells infiltrate, vascular congestion and interstitial oedema. Haematoxylin-Eosin staining; original magnification: liver X 40 and lung X 20.
Daily treatment with NDP-α-MSH (340 µg·kg−1) for 7 days significantly reduced the histological alterations in MODS mice (Table 1). Indeed, the histological picture showed that the inflammatory infiltrate and steatosis were less noticeable, and the absence of necrosis and cellular ballooning degeneration in the liver (Figure 6C). Lung histology revealed a reduced inflammatory infiltrate, vascular congestion and interstitial oedema following melanocortin treatment (Figure 6G).
The blockade of central melanocortin MC4 receptors and peripheral acetylcholine nicotinic receptors prevents the protective effects of NDP-α-MSH
Next, we investigated whether a brain MC4 receptor-triggered efferent vagal cholinergic pathway is involved in the protective effect of melanocortins against damage following generalized inflammation induced by a LPS-zymosan treatment. To evaluate this hypothesis, a group of MODS mice was concomitantly treated for 7 days with both the selective melanocortin MC4 receptor antagonist HS024 and the highest dose of NDP-α-MSH assessed (340 µg·kg−1), while another group received the nicotinic acetylcholine receptor antagonist chlorisondamine together with the same dose of NDP-α-MSH. As occurs in circulatory shock, HS024 and chlorisondamine − at a dose able to block central MC4 and peripheral nicotinic receptors, respectively (Guarini et al., 2003; 2004; Altavilla et al., 2006; Giuliani et al., 2007) − counteracted the effect of NDP-α-MSH on the mRNA expression of TNF-α and IL-10 (Figures 3 and 4), and on the histological picture (Table 1 and Figure 6D,H), both in the liver and lung, as well as on TNF-α plasma levels (Figure 5), as detected at day 7 after zymosan. HS024 and chlorisondamine also blunted the favourable effects of NDP-α-MSH on survival rate, as found throughout the 16 day observation period (Figure 2). HS024 and chlorisondamine alone worsened, although in a statistically non-significant manner, all biomolecular and histological parameters assessed in MODS mice (Figures 3–5), as well as survival rate (Figure 2).
Discussion
MODS encompasses a heterogeneous population with a variety of clinical conditions where serious abnormalities in multiple organs occur hours to days after an event like haemorrhage, infection, trauma, severe burns, pancreatitis, etc. (Volman et al., 2005; Baue, 2006; Dewar et al., 2009; Tsukamoto et al., 2010). The costs of MODS are very high; these include economic for health care and decrease in quality of life, as well as for disability to work. Activation of the systemic inflammatory response, including the increase in oxidative and nitrosative stress, are important pathophysiological components of MODS, and mortality rate closely depends on the number of failing organs (Schlag et al., 1991; Redl et al., 1993; Baue, 1997; 2006; Wheeler and Bernard, 1999; Cohen, 2002; Tracey, 2002; 2007; Guarini et al., 2004; Cui et al., 2005; Volman et al., 2005; Altavilla et al., 2006; van Meurs et al., 2008; Tsukamoto et al., 2010). Almost 40 years after MODS was first described (Tilney et al., 1973), mortality still remains practically unchanged, thus the demand for novel and effective therapeutic approaches.
The present data provide evidence that the melanocortin analogue NDP-α-MSH protects against experimental MODS by counteracting the systemic inflammatory response. Indeed, NDP-α-MSH inhibited progression of the generalized inflammation by reducing mRNA expression of TNF-α, and by increasing that of IL-10, both in the liver and lung. Furthermore, NDP-α-MSH reduced TNF-α plasma levels, prevented late histological damage of the liver and lung – inflammatory infiltrate, steatosis, necrosis, ballooning degeneration, vascular congestion and interstitial oedema – and dose-dependently increased survival rate. These protective effects occurred at nanomolar doses, and they appear to be due to a central action of melanocortins and mediated by efferent vagal fibres, the so-called ‘cholinergic anti-inflammatory pathway’: indeed, the peripheral nicotinic acetylcholine receptor antagonist chlorisondamine blunted the favourable and life-saving effects of NDP-α-MSH.
Consistently, experimental evidence indicates that melanocortins reach the CNS after systemic injection (Guarini et al., 1987; 1999; 2004; Banks and Kastin, 1995; Wikberg et al., 2000; Catania et al., 2004; Mioni et al., 2005; Getting, 2006; Giuliani et al., 2006a; 2009; 2010; Ottani et al., 2009; 2010;). Moreover, we previously demonstrated that melanocortins reverse haemorrhagic shock through the rapid activation of an efferent vagal anti-inflammatory pathway, after stimulation of melanocortin MC4 receptors within the brain (Guarini et al., 2004). Our present data suggest that the mechanism by which NDP-α-MSH protects mice against MODS following LPS-zymosan injection could also involve direct activation of brain melanocortin MC4 receptors, which would then trigger the vagus nerve-mediated cholinergic anti-inflammatory pathway, as occurs in circulatory shock (Guarini et al., 2004; Giuliani et al., 2007; 2010;). Indeed, melanocortin MC4 receptors are mainly expressed in the CNS and are much more widely expressed than other brain MC receptor subtypes (Wikberg et al., 2000; Catania et al., 2004; Getting, 2006). Consistently, the selective MC4 receptor antagonist HS024 prevented the protective and life-saving effects of NDP-α-MSH against MODS. Obviously, a full demonstration of the involvement of the vagus nerve in the protective effects of NDP-α-MSH reported here would need experiments with vagotomized animals: cervical vagotomy, in fact, prevents the protective effects of melanocortins in short-lasting experimental models, such as haemorrhagic shock, myocardial ischaemia and, very surprisingly, ischaemic stroke (Guarini et al., 2004; Mioni et al., 2005; Giuliani et al., 2010; Ottani et al., 2009; 2010;). However, vagotomy is incompatible with long-term survival, thus unsuitable for a MODS long-lasting study such as ours.
The exact mechanism leading to multiple organ failure is unclear, and it appears that MODS is unlikely to be the result of overexpression of a single mediator. Anyway, the increase in cytokine and adhesion molecule production immediately after the inflammatory insult, and the rapid decrease in IL-10 levels in patients who develop multiple organ failure suggest a defect in endogenous anti-inflammatory mechanisms (Simons et al., 1996; Seekamp et al., 1998; Cohen, 2002; Volman et al., 2005; Tracey, 2007; Rosas-Ballina and Tracey, 2009; Giuliani et al., 2010; Tsukamoto et al., 2010). Indeed, IL-10 is an important anti-inflammatory cytokine, and an inadequate IL-10 response after systemic injury can have detrimental consequences. Consistent with this idea, an increase in IL-10 levels seems to exert protective effects in rats subjected to haemorrhage shock (Cui et al., 2005), as well as in a MODS model (present data) that resembles human MODS. Reduced concentrations of circulating α-MSH have been detected in critically injured trauma patients (Todd et al., 2009), as well as in patients with septic shock (Catania et al., 2000) and brain injury (Magnoni et al., 2003) with a worse outcome, thus suggesting a physiological, anti-inflammatory role of endogenous melanocortin peptides. Accordingly, in severe inflammatory/ischaemic conditions, including haemorrhagic shock, splanchnic artery occlusion shock, septic peritonitis induced by cecal ligation and puncture, ischaemic stroke, and other pathologies, melanocortins inhibit nuclear NF-κB activation, reduce pro-inflammatory cytokine and adhesion molecule production, up-regulate IL-10 modulating the inflammatory cascade and increase survival (Lipton et al., 1994; Altavilla et al., 1998; Squadrito et al., 1999; Wikberg et al., 2000; Catania et al., 2004; Guarini et al., 2004; Getting, 2006; Giuliani et al., 2006b; 2010; Catania, 2007; Ottani et al., 2009). Reduction of free radical discharge may likewise contribute to the prevention of organ damage (Schlag et al., 1991; Redl et al., 1993; Volman et al., 2005), and melanocortins reduce blood levels of free radicals, including nitric oxide, in circulatory shock and myocardial ischaemia (Guarini et al., 1996; 1997b; Bazzani et al., 2001; Bertuglia and Giusti, 2004; Mioni et al., 2005; Giuliani et al., 2007).
In conclusion, the present data provide clear evidence for the first time that melanocortins prevent late multiple organ damage following a severe and generalized inflammation, and improve survival, seemingly by counteracting the release of key mediators involved in the generation of MODS: these effects are dose-dependent and probably occur through the brain MC4 receptor-triggered activation of the vagus nerve-mediated cholinergic anti-inflammatory pathway. A pharmacological approach to preventing MODS with melanocortins could be more physiological and very promising. Indeed, our previous findings on the protective effects of melanocortins in experimental conditions characterized by local and generalized inflammation (circulatory shock, myocardial ischaemia and ischaemic stroke) have recently been drawn to the attention of clinicians by Corander et al. (2009) because of their potential therapeutic value. Our present findings therefore has provided further insights into the potential therapeutic value of melanocortins and could be of relevance clinically.
Acknowledgments
This work was supported in part by grants from University of Messina, Italy.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- CNS
central nervous system
- IL-10
interleukin-10
- LPS
lipopolysaccharide
- MODS
multiple organ dysfunction syndrome
- NDP-α-MSH
[Nle4, D-Phe7]α-melanocyte-stimulating hormone
- NF-κB
nuclear factor-κB
- TNF-α
tumour necrosis factor-α
Conflict of interests
The authors state no conflict of interests.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 NDP-α-MSH reduced clinical signs of severity in MODS mice; HS024 and chlorisondamine prevented the protective effect of NDP-α-MSH
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. 4th edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altavilla D, Cainazzo MM, Squadrito F, Guarini S, Bertolini A, Bazzani C. Tumour necrosis factor-a as a target of melanocortins in haemorrhagic shock, in the anaesthetized rat. Br J Pharmacol. 1998;124:1587–1590. doi: 10.1038/sj.bjp.0702038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, Bigiani A, et al. Activation of the cholinergic anti-infiammatory pathway reduces NF-kB activation, blunts TNF-α production, and protects against splanchnic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Permeability of the blood-brain barrier to melanocortins. Peptides. 1995;16:1157–1161. doi: 10.1016/0196-9781(95)00043-j. [DOI] [PubMed] [Google Scholar]
- Baue AE. Multiple organ failure, multiple organ dysfunction syndrome and systemic inflammatory response syndrome. Why no magic bullets? Arch Surg. 1997;132:703–707. doi: 10.1001/archsurg.1997.01430310017002. [DOI] [PubMed] [Google Scholar]
- Baue AE. MOF, MODS, and SIRS: what is in a name or an acronym? Shock. 2006;26:438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- Bazzani C, Guarini S, Botticelli AR, Zaffe D, Tomasi A, Bini A, et al. Protective effect of melanocortin peptides in rat myocardial ischemia. J Pharmacol Exp Ther. 2001;297:1082–1087. [PubMed] [Google Scholar]
- Bertuglia S, Giusti A. Influence of ACTH-(1-24) and plasma hyperviscosity on free radical production and capillary perfusion after hemorrhagic shock. Microcirculation. 2004;11:227–238. doi: 10.1080/10739680490425930. [DOI] [PubMed] [Google Scholar]
- Bertolini A, Guarini S, Ferrari W. Adrenal-independent, anti-shock effect of ACTH-(1-24) in rats. Eur J Pharmacol. 1986a;122:387–388. doi: 10.1016/0014-2999(86)90424-3. [DOI] [PubMed] [Google Scholar]
- Bertolini A, Guarini S, Rompianesi E, Ferrari W. MSH and other ACTH fragments improve cardiovascular function and survival in experimental hemorrhagic shock. Eur J Pharmacol. 1986b;130:19–26. doi: 10.1016/0014-2999(86)90179-2. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81:383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- Catania A, Cutuli M, Garofalo L, Airaghi L, Valenza F, Lipton JM, et al. Plasma concentrations and anti-cytokine effects of α-melanocyte stimulating hormone in septic patients. Crit Care Med. 2000;28:1403–1407. doi: 10.1097/00003246-200005000-00024. [DOI] [PubMed] [Google Scholar]
- Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Corander MP, Fenech M, Coll AP. Science of self-preservation: how melanocortin action in the brain modulates body weight, blood pressure, and ischemic damage. Circulation. 2009;120:2260–2268. doi: 10.1161/CIRCULATIONAHA.109.854612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Wu R, Zhou M, Dong W, Ulloa L, Yang H, et al. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit Care Med. 2005;33:391–398. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, McDonald MC, Mazzon E, Filipe HM, Centorrino T, Lepore V, et al. Beneficial effects of tempol, a membrane-permeable radical scavenger, on the multiple organ failure induced by zymosan in the rat. Crit Care Med. 2001;29:102–111. doi: 10.1097/00003246-200101000-00022. [DOI] [PubMed] [Google Scholar]
- Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912–918. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Getting SJ. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. 2006;111:1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Leone S, Mioni C, Bazzani C, Zaffe D, Botticelli AR, et al. Broad therapeutic treatment window of [Nle4, D-Phe7]α-melanocyte-stimulating hormone for long-lasting protection against ischemic stroke, in Mongolian gerbils. Eur J Pharmacol. 2006a;538:48–56. doi: 10.1016/j.ejphar.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Mioni C, Altavilla D, Leone S, Bazzani C, Minutoli L, et al. Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology. 2006b;147:1126–1135. doi: 10.1210/en.2005-0692. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Mioni C, Bazzani C, Zaffe D, Botticelli AR, Capolongo S, et al. Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. Br J Pharmacol. 2007;150:595–603. doi: 10.1038/sj.bjp.0707115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani D, Ottani A, Minutoli L, Di Stefano V, Galantucci M, Bitto A, et al. Functional recovery after delayed treatment of ischemic stroke with melanocortins is associated with overexpression of the activity-dependent gene Zif268. Brain Behav Immun. 2009;23:844–850. doi: 10.1016/j.bbi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Ottani A, Altavilla D, Bazzani C, Squadrito F, Guarini S. Melanocortins and the cholinergic anti-inflammatory pathway. In: Catania A, editor. Advances in Experimental Medicine and Biology – Melanocortins, Multiple Actions and Therapeutic Potential. Vol. 681. Austin, TX, New York, NY: Landes Bioscience and Springer Science + Business Media; 2010. pp. 71–87. [DOI] [PubMed] [Google Scholar]
- Goris RJ, Boekholtz WK, van Bebber IP, Nuytinck JK, Schillings PH. Multiple-organ failure and sepsis without bacteria. An experimental model. Arch Surg. 1986;121:897–901. doi: 10.1001/archsurg.1986.01400080039006. [DOI] [PubMed] [Google Scholar]
- Guarini S, Vergoni AV, Bertolini A. Anti-shock effect of ACTH-(1-24): comparison between intracerebroventricular and intravenous route of administration. Pharmacol Res Commun. 1987;19:255–260. doi: 10.1016/0031-6989(87)90068-3. [DOI] [PubMed] [Google Scholar]
- Guarini S, Tagliavini S, Bazzani C, Ferrari W, Bertolini A. Early treatment with ACTH-(1-24) in a rat model of hemorrhagic shock prolongs survival and extends the time-limit for blood reinfusion to be effective. Crit Care Med. 1990;18:862–865. doi: 10.1097/00003246-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Guarini S, Bazzani C, Mattera Ricigliano G, Bini A, Tomasi A, Bertolini A. Influence of ACTH-(1-24) on free radical levels in the blood of haemorrhage-shocked rats: direct ex vivo detection by electron spin resonance spectrometry. Br J Pharmacol. 1996;119:29–34. doi: 10.1111/j.1476-5381.1996.tb15673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarini S, Bazzani C, Bertolini A. Resuscitating effect of melanocortin peptides after prolonged respiratory arrest. Br J Pharmacol. 1997a;121:1454–1460. doi: 10.1038/sj.bjp.0701264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarini S, Bini A, Bazzani C, Mattera Ricigliano G, Cainazzo MM, Tomasi A, et al. Adrenocorticotropin normalizes the blood levels of nitric oxide in hemorrhage-shocked rats. Eur J Pharmacol. 1997b;336:15–21. doi: 10.1016/s0014-2999(97)01210-7. [DOI] [PubMed] [Google Scholar]
- Guarini S, Bazzani C, Cainazzo MM, Mioni C, Ferrazza G, Vergoni AV, et al. Evidence that melanocortin 4 receptor mediates hemorrhagic shock reversal caused by melanocortin peptides. J Pharmacol Exp Ther. 1999;291:1023–1027. [PubMed] [Google Scholar]
- Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, et al. Efferent vagal fibre stimulation blunts NF-kB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Jansen MJ, Hendriks T, Verhofstad AA, Lange W, Geeraedts LMJ, Goris RJ. Gradual development of organ damage in the murine zymosan-induced multiple organ dysfunction syndrome. Shock. 1997;8:261–267. doi: 10.1097/00024382-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Jochem J. Involvement of proopiomelanocortin-derived peptides in endogenous central istamine-induced reversal of critical haemorrhagic hypotension in rats. J Physiol Pharmacol. 2004;55:57–71. [PubMed] [Google Scholar]
- Lipton JM, Ceriani G, Macaluso A, McCoy D, Carnes K, Biltz J, et al. Antiinflammatory effects of the neuropeptide α-MSH in acute, chronic, and systemic inflammation. Ann NY Acad Sci. 1994;741:137–148. doi: 10.1111/j.1749-6632.1994.tb39654.x. [DOI] [PubMed] [Google Scholar]
- Magnoni S, Stocchetti N, Colombo G, Carlin A, Colombo A, Lipton JM, et al. α-Melanocyte-stimulating hormone is decreased in plasma of patients with acute brain injury. J Neurotrauma. 2003;20:251–260. doi: 10.1089/089771503321532833. [DOI] [PubMed] [Google Scholar]
- van Meurs M, Wulfert FM, Knol AJ, De Haes A, Houwertjes M, Aarts LP, et al. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock. 2008;29:291–299. doi: 10.1097/SHK.0b013e318145a7c1. [DOI] [PubMed] [Google Scholar]
- Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- Noera G, Lamarra M, Guarini S, Bertolini A. Survival rate after early treatment for acute type-A aortic dissection with ACTH-(1-24) Lancet. 2001;358:469–470. doi: 10.1016/S0140-6736(01)05631-8. [DOI] [PubMed] [Google Scholar]
- Ottani A, Giuliani D, Mioni C, Galantucci M, Minutoli L, Bitto A, et al. Vagus nerve mediates the protective effects of melanocortins against cerebral and systemic damage after ischemic stroke. J Cereb Blood Flow Metab. 2009;29:512–523. doi: 10.1038/jcbfm.2008.140. [DOI] [PubMed] [Google Scholar]
- Ottani A, Giuliani D, Galantucci M, Spaccapelo L, Novellino E, Grieco P, et al. Melanocortins counteract inflammatory and apoptotic responses to prolonged myocardial ischemia/reperfusion through a vagus nerve-mediated mechanism. Eur J Pharmacol. 2010;637:124–130. doi: 10.1016/j.ejphar.2010.03.052. [DOI] [PubMed] [Google Scholar]
- Redl H, Gasser H, Schlag G, Marzi I. Involvement of oxygen radicals in shock related cell injury. Br Med Bull. 1993;49:556–565. doi: 10.1093/oxfordjournals.bmb.a072630. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag G, Redl H, Hallström S. The cell in shock: the origin of multiple organ failure. Resuscitation. 1991;21:137–180. doi: 10.1016/0300-9572(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Seekamp AJ, Jochum M, Ziegler M, Micheal M, Gerd R. Cytokine and adhesion molecules in elective and accidental trauma-related ischaemia/reperfusion. J Trauma. 1998;44:874–882. doi: 10.1097/00005373-199805000-00022. [DOI] [PubMed] [Google Scholar]
- Simons RK, Hoyt DB, Winchell RJ, Rose RM, Holbrook T. Elevated selectin levels after severe trauma: a marker for sepsis and organ failure and a potential target for immunomodulatory therapy. J Trauma. 1996;41:653–662. doi: 10.1097/00005373-199610000-00010. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Guarini S, Altavilla D, Squadrito G, Campo GM, Arlotta M, et al. Adrenocorticotropin reverses vascular dysfunction and protects against splanchnic artery occlusion shock. Br J Pharmacol. 1999;128:816–822. doi: 10.1038/sj.bjp.0702848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney NL, Bailey GL, Morgan AP. Sequential system failure after rupture of abdominal aortic aneurysms: an unsolved problem in postoperative care. Ann Surg. 1973;178:117–122. doi: 10.1097/00000658-197308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd SR, Kao LS, Catania A, Mercer DW, Adams SD, Moore FA. α-Melanocyte stimulating hormone in critically injured trauma patients. J Trauma. 2009;66:465–469. doi: 10.1097/TA.0b013e31818b1e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010;41:21–26. doi: 10.1016/j.injury.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Vecsernyes M, Juhasz B, Der P, Kocsan R, Feher P, Bacskay I, et al. The administration of α-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur J Pharmacol. 2003;470:177–183. doi: 10.1016/s0014-2999(03)01780-1. [DOI] [PubMed] [Google Scholar]
- Volman TJ, Goris RJ, van der Jagt M, van de Loo FA, Hendriks T. Organ damage in zymosan-induced multiple organ dysfunction syndrome in mice is not mediated by inducible nitric oxide synthase. Crit Care Med. 2002;30:1553–1559. doi: 10.1097/00003246-200207000-00026. [DOI] [PubMed] [Google Scholar]
- Volman TJ, Goris RJ, van der Meer JW, Hendriks T. Tissue- and time-dependent upregulation of cytokine mRNA in a murine model for the multiple organ dysfunction syndrome. Ann Surg. 2004;240:142–150. doi: 10.1097/01.sla.0000130725.52373.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman TJ, Hendriks T, Goris RJ. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock. 2005;23:291–297. doi: 10.1097/01.shk.0000155350.95435.28. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- Wikberg JES, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, et al. New aspects on the melanocortins and their receptors. Pharmacol Res. 2000;42:393–420. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


