Abstract
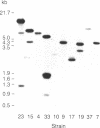
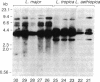
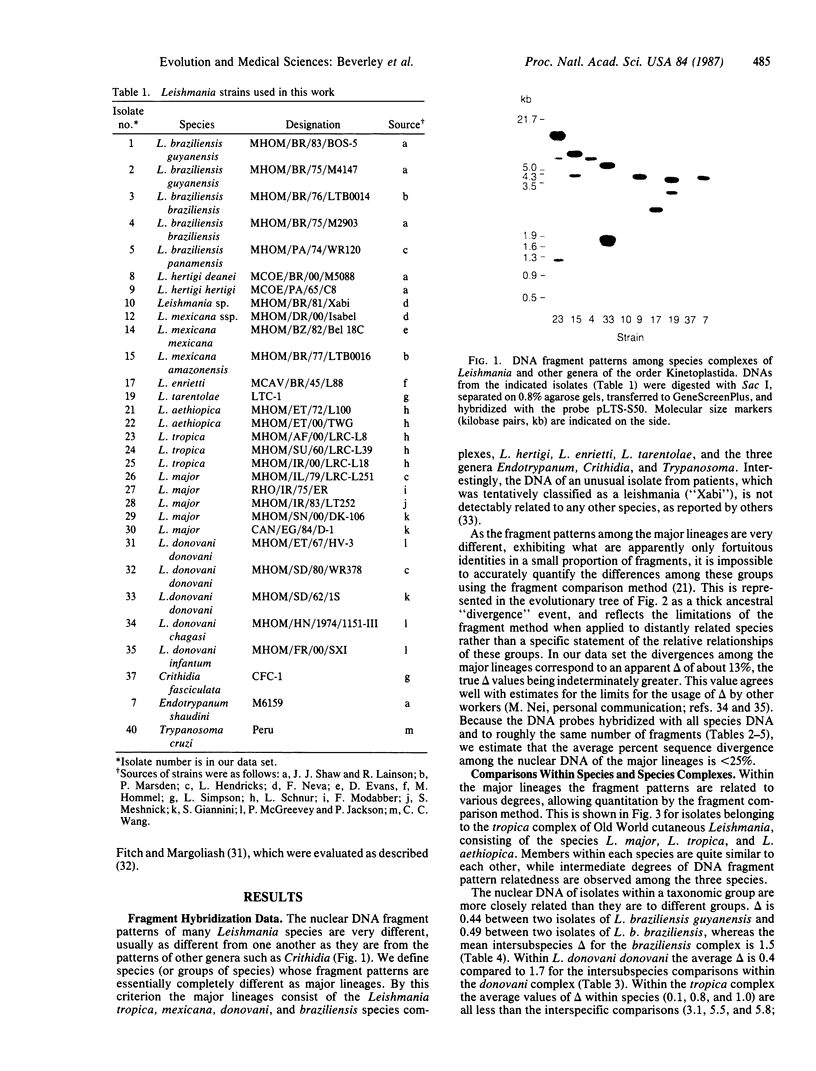
Restriction endonuclease DNA fragment patterns have been used to examine the relationships among 28 isolates of Leishmania as well as Crithidia, Endotrypanum, and Trypanosoma cruzi. Fragments of nuclear DNA were generated with six restriction enzymes, and blots were hybridized with probes from three loci. Among the major lineages the fragment patterns are essentially completely different, while within the major lineages various degrees of divergence are found. Molecular evolutionary trees were constructed using the method of Nei and Li to estimate the percent nucleotide sequence divergence among strains from the fraction of fragments shared. Defined groups, such as species or subspecies within the major lineages, are also grouped by nuclear DNA comparisons. Within the donovani complex, we find Leishmania donovani chagasi and Leishmania donovani infantum to be as similar as strains within Leishmania donovani donovani, consistent with the proposal by other workers that New World visceral leishmaniasis originated quite recently.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony R. L., Williams K. M., Sacci J. B., Rubin D. C. Subcellular and taxonomic specificity of monoclonal antibodies to New World Leishmania. Am J Trop Med Hyg. 1985 Nov;34(6):1085–1094. doi: 10.4269/ajtmh.1985.34.1085. [DOI] [PubMed] [Google Scholar]
- Arnot D. E., Barker D. C. Biochemical identification of cutaneous leishmanias by analysis of kinetoplast DNA. II. Sequence homologies in Leishmania kDNA. Mol Biochem Parasitol. 1981 May;3(1):47–56. doi: 10.1016/0166-6851(81)90076-1. [DOI] [PubMed] [Google Scholar]
- Barker D. C., Gibson L. J., Kennedy W. P., Nasser A. A., Williams R. H. The potential of using recombinant DNA species-specific probes for the identification of tropical Leishmania. Parasitology. 1986;92 (Suppl):S139–S174. doi: 10.1017/s0031182000085747. [DOI] [PubMed] [Google Scholar]
- Belazzoug S., Lanotte G., Maazoun R., Pratlong F., Rioux J. A. Un nouveau variant enzymatique de Leishmania infantum Nicolle, 1908, agent de la leishmaniose cutanée du nord de l'Algérie. Ann Parasitol Hum Comp. 1985;60(1):1–3. doi: 10.1051/parasite/19856011. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Britten R. J. Rates of DNA sequence evolution differ between taxonomic groups. Science. 1986 Mar 21;231(4744):1393–1398. doi: 10.1126/science.3082006. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Beverley S. M., Schimke R. T. Relationship of amplified dihydrofolate reductase genes to double minute chromosomes in unstably resistant mouse fibroblast cell lines. Mol Cell Biol. 1981 Dec;1(12):1077–1083. doi: 10.1128/mcb.1.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Schmidtke J. DNA restriction fragment length polymorphisms and heterozygosity in the human genome. Hum Genet. 1984;66(1):1–16. doi: 10.1007/BF00275182. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Distinguishing homologous from analogous proteins. Syst Zool. 1970 Jun;19(2):99–113. [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm-Bychowski K. M., Wilson A. C. Rates of nuclear DNA evolution in pheasant-like birds: evidence from restriction maps. Proc Natl Acad Sci U S A. 1986 Feb;83(3):688–692. doi: 10.1073/pnas.83.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. L., Roberts B. E., Pratt D. M., David J. R., Miller J. S. Structure and arrangement of the beta-tubulin genes of Leishmania tropica. Mol Cell Biol. 1984 Jul;4(7):1372–1383. doi: 10.1128/mcb.4.7.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler L. G., Avise J. C. A comparative description of mitochondrial DNA differentiation in selected avian and other vertebrate genera. Mol Biol Evol. 1985 Mar;2(2):109–125. doi: 10.1093/oxfordjournals.molbev.a040339. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R., Molyneux D. H., Rioux J. A., Lanotte G., Leaney A. J. Possible origins of Leishmania chagasi. Ann Trop Med Parasitol. 1980 Oct;74(5):563–565. doi: 10.1080/00034983.1980.11687385. [DOI] [PubMed] [Google Scholar]
- King M. C., Wilson A. C. Evolution at two levels in humans and chimpanzees. Science. 1975 Apr 11;188(4184):107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- Kreutzer R. D., Christensen H. A. Characterization of Leishmania spp. by isozyme electrophoresis. Am J Trop Med Hyg. 1980 Mar;29(2):199–208. doi: 10.4269/ajtmh.1980.29.199. [DOI] [PubMed] [Google Scholar]
- Kreutzer R. D., Semko M. E., Hendricks L. D., Wright N. Identification of Leishmania spp. by multiple isozyme analysis. Am J Trop Med Hyg. 1983 Jul;32(4):703–715. doi: 10.4269/ajtmh.1983.32.703. [DOI] [PubMed] [Google Scholar]
- Lanotte G., Rioux J. A., Maazoun R., Pasteur N., Pratlong F., Lepart J. Application de la méthode numérique à la taxonomie du genre Leishmania Ross, 1903. A propos de 146 souches originaires de l'Ancien Monde. Utilisation des allozymes. Corollaires épidémilogiques et phylétiques. Ann Parasitol Hum Comp. 1981;56(6):575–591. [PubMed] [Google Scholar]
- Le Blancq S. M., Peters W. Leishmania in the Old World: 2. Heterogeneity among L. tropica zymodemes. Trans R Soc Trop Med Hyg. 1986;80(1):113–119. doi: 10.1016/0035-9203(86)90208-7. [DOI] [PubMed] [Google Scholar]
- Le Blancq S. M., Schnur L. F., Peters W. Leishmania in the Old World: 1. The geographical and hostal distribution of L. major zymodemes. Trans R Soc Trop Med Hyg. 1986;80(1):99–112. doi: 10.1016/0035-9203(86)90206-3. [DOI] [PubMed] [Google Scholar]
- Maazoun R., Lanotte G., Pasteur N., Rioux J. A., Kennou M. F., Pratlong F. Ecologie des Leishmanioses dans le sud de la France. 16. Contribution á l'analyse chimiotaxonomique des parasites de la leishmaniose viscérale méditerranéenne. A propos de 55 souches isolées en Cévennes, Cote d'Azur, Corse et Tunisie. Ann Parasitol Hum Comp. 1981;56(2):131–146. [PubMed] [Google Scholar]
- McCutchan T. F., Dame J. B., Miller L. H., Barnwell J. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science. 1984 Aug 24;225(4664):808–811. doi: 10.1126/science.6382604. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Lainson R., Shaw J. J., Póvoa M., de Souza A. A. Leishmaniasis in Brazil: XV. Biochemical distinction of Leishmania mexicana amazonensis, L. braziliensis braziliensis and L. braziliensis guyanensis--aetiological agents of cutaneous leishmaniasis in the Amazon Basin of Brazil. Trans R Soc Trop Med Hyg. 1981;75(4):524–529. doi: 10.1016/0035-9203(81)90191-7. [DOI] [PubMed] [Google Scholar]
- Morel C., Simpson L. Characterization of pathogenic trypanosomatidae by restriction endonuclease fingerprinting of kinetoplast DNA minicircles. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1070–1074. doi: 10.4269/ajtmh.1980.29.1070. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Standard error of immunological dating of evolutionary time. J Mol Evol. 1977 May 13;9(3):203–211. doi: 10.1007/BF01796109. [DOI] [PubMed] [Google Scholar]
- Nei M., Tajima F. DNA polymorphism detectable by restriction endonucleases. Genetics. 1981 Jan;97(1):145–163. doi: 10.1093/genetics/97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. Maximum likelihood estimation of the number of nucleotide substitutions from restriction sites data. Genetics. 1983 Sep;105(1):207–217. doi: 10.1093/genetics/105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinov R. Strong doublet preferences in nucleotide sequences and DNA geometry. J Mol Evol. 1984;20(2):111–119. doi: 10.1007/BF02257371. [DOI] [PubMed] [Google Scholar]
- Paindavoine P., Pays E., Laurent M., Geltmeyer Y., Le Ray D., Mehlitz D., Steinert M. The use of DNA hybridization and numerical taxonomy in determining relationships between Trypanosoma brucei stocks and subspecies. Parasitology. 1986 Feb;92(Pt 1):31–50. doi: 10.1017/s0031182000063435. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. Construction of phylogenetic trees for proteins and nucleic acids: empirical evaluation of alternative matrix methods. J Mol Evol. 1978 Jun 20;11(2):129–142. doi: 10.1007/BF01733889. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Saravia N. G., Holguín A. F., McMahon-Pratt D., D'Alessandro A. Mucocutaneous leishmaniasis in Colombia: Leishmania braziliensis subspecies diversity. Am J Trop Med Hyg. 1985 Jul;34(4):714–720. doi: 10.4269/ajtmh.1985.34.714. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Collings J. C., Gray M. W. Structure and evolution of the small subunit ribosomal RNA gene of Crithidia fasciculata. Curr Genet. 1986;10(5):405–410. doi: 10.1007/BF00418414. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol. 1984;20(1):2–15. doi: 10.1007/BF02101980. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Pratt D. M. Rapid identification of Leishmania species by specific hybridization of kinetoplast DNA in cutaneous lesions. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6999–7003. doi: 10.1073/pnas.79.22.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ibarra A. A., Howard J. G., Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982 Dec;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]