Abstract
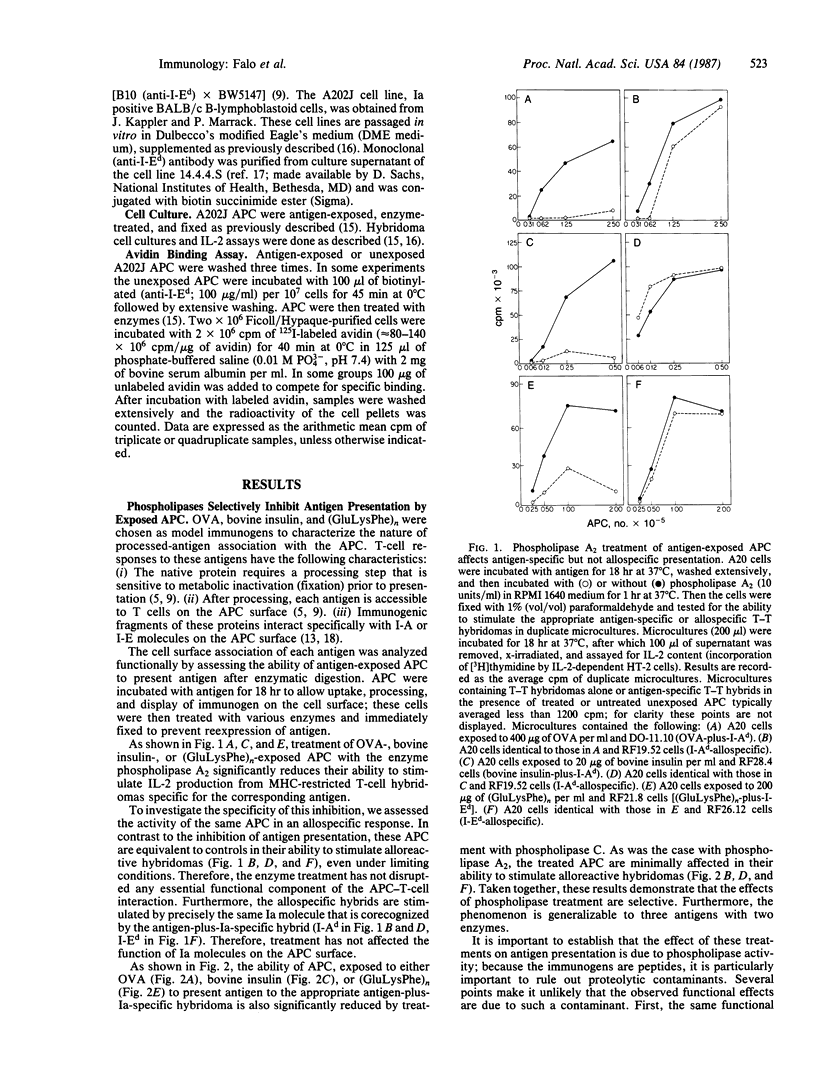
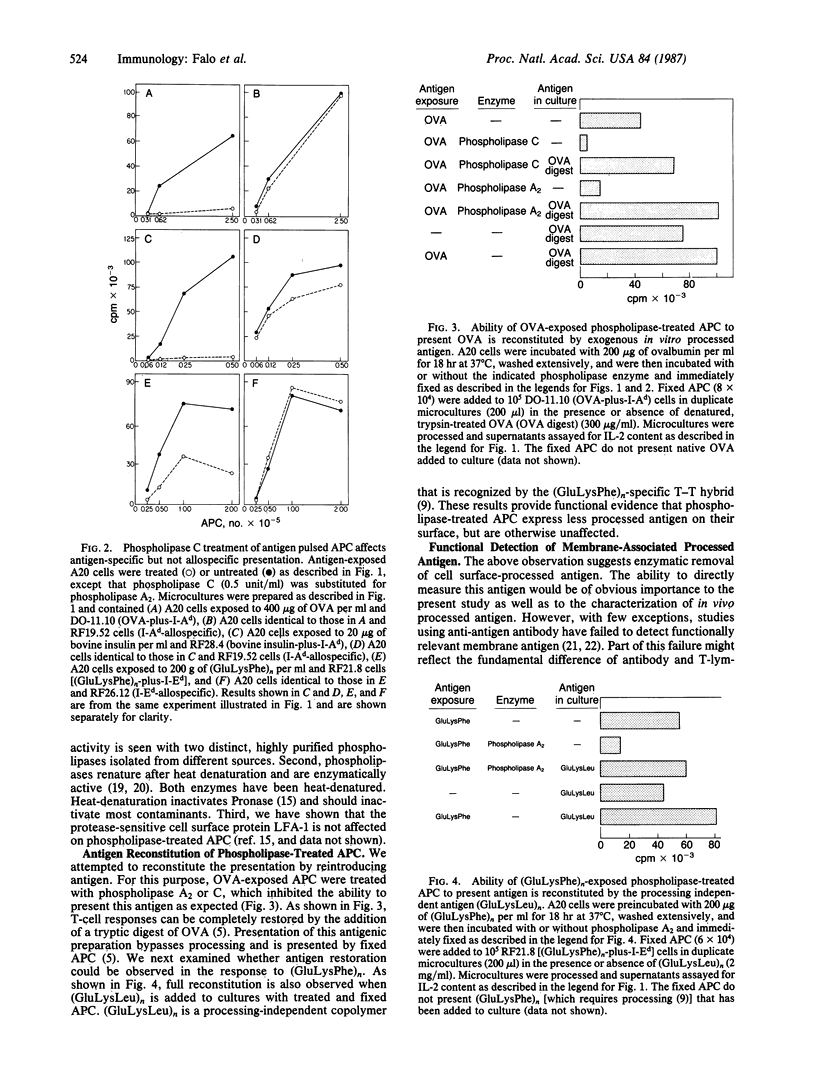
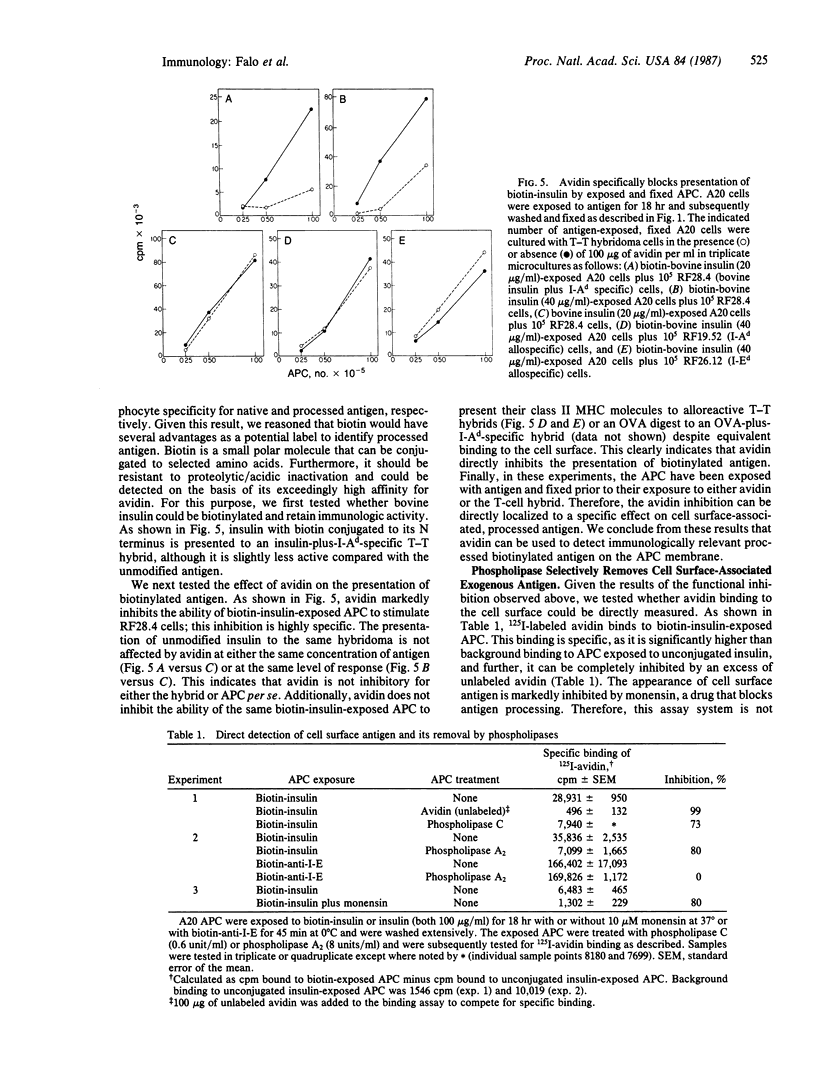
To characterize the basis for the cell surface association of processed antigen with the antigen-presenting cell (APC) we analyzed its sensitivity to enzymatic digestion. Antigen-exposed APC that are treated with phospholipase and then immediately fixed lose their ability to stimulate antigen-plus-Ia-specific T-T hybridomas. This effect is seen with highly purified phospholipase A2 and phospholipase C. In addition it is observed with three distinct antigens--ovalbumin, bovine insulin, and poly(LGlu56LLys35LPhe9) [(GluLysPhe)n]. The effect of phospholipases is highly specific. Identically treated APC are equivalent to controls in their ability to stimulate alloreactive hybridomas specific for precisely the same Ia molecule that is corecognized by antigen-plus-Ia-specific hybrids. Furthermore, the antigen-presenting function of enzyme-treated, fixed APC can be reconstituted by the addition of exogenous in vitro processed or "processing independent" antigens. In parallel studies 125I-labeled avidin was shown to specifically bind to APC that were previously exposed and allowed to process biotin-insulin. Biotin-insulin-exposed APC that are pretreated with phospholipase bind significantly less 125I-labeled avidin than do untreated, exposed APC. Identical enzyme treatment does not reduce the binding of avidin to a biotinylated antibody already bound to class II major histocompatibility complex molecules of APC. At least some of the biotin-insulin surface sites are immunologically relevant, because the presentation of processed biotin-insulin by fixed APC is blocked by avidin. This effect is specific. Avidin binding to biotin-insulin-exposed APC does not inhibit allospecific stimulation nor the presentation of unconjugated insulin. These studies demonstrate that phospholipase effectively removes processed cell surface antigen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Beller D. I., Braun J., Unanue E. R. The handling of Listeria monocytogenes by macrophages: the search for an immunogenic molecule in antigen presentation. J Immunol. 1984 Jan;132(1):323–331. [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deems R. A., Dennis E. A. Phospholipase A2 from cobra venom (Naja naja naja). Methods Enzymol. 1981;71(Pt 100):703–710. doi: 10.1016/0076-6879(81)71083-8. [DOI] [PubMed] [Google Scholar]
- Falo L. D., Jr, Benacerraf B., Rock K. L. Phospholipase treatment of accessory cells that have been exposed to antigen selectively inhibits antigen-specific Ia-restricted, but not allospecific, stimulation of T lymphocytes. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6994–6997. doi: 10.1073/pnas.83.18.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falo L. D., Jr, Sullivan K., Benacerraf B., Mescher M. F., Rock K. L. Analysis of antigen presentation by metabolically inactive accessory cells and their isolated membranes. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6647–6651. doi: 10.1073/pnas.82.19.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELL P. G., BENACERRAF B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in guinea pigs. Immunology. 1959 Jan;2(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Schroer J. A., Chan C., Shevach E. M. Fine specificity of cloned insulin-specific T cell hybridomas: evidence supporting a role for tertiary conformation. J Immunol. 1983 Dec;131(6):2868–2874. [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. Evidence of specific competition for accessory cell antigen presentation. J Exp Med. 1983 May 1;157(5):1618–1634. doi: 10.1084/jem.157.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. II. Competitive inhibition of I-E-restricted, antigen-specific T cell responses. J Exp Med. 1984 Dec 1;160(6):1864–1879. doi: 10.1084/jem.160.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Selective modification of a private I-A allo-stimulating determinant(s) upon association of antigen with an antigen-presenting cell. J Exp Med. 1984 Apr 1;159(4):1238–1252. doi: 10.1084/jem.159.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L. The role of Ia molecules in the activation of T lymphocytes. I. The activation of an IL 1-dependent IL 2-producing T cell hybridoma by Con A requires an interaction, which is not H-2-restricted, with an Ia-bearing accessory cell. J Immunol. 1982 Oct;129(4):1360–1366. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Sugahara T., Ohsaka A. Phospholipase C from Clostridium perfringens. Methods Enzymol. 1981;71(Pt 100):710–725. doi: 10.1016/0076-6879(81)71084-x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]


