Abstract
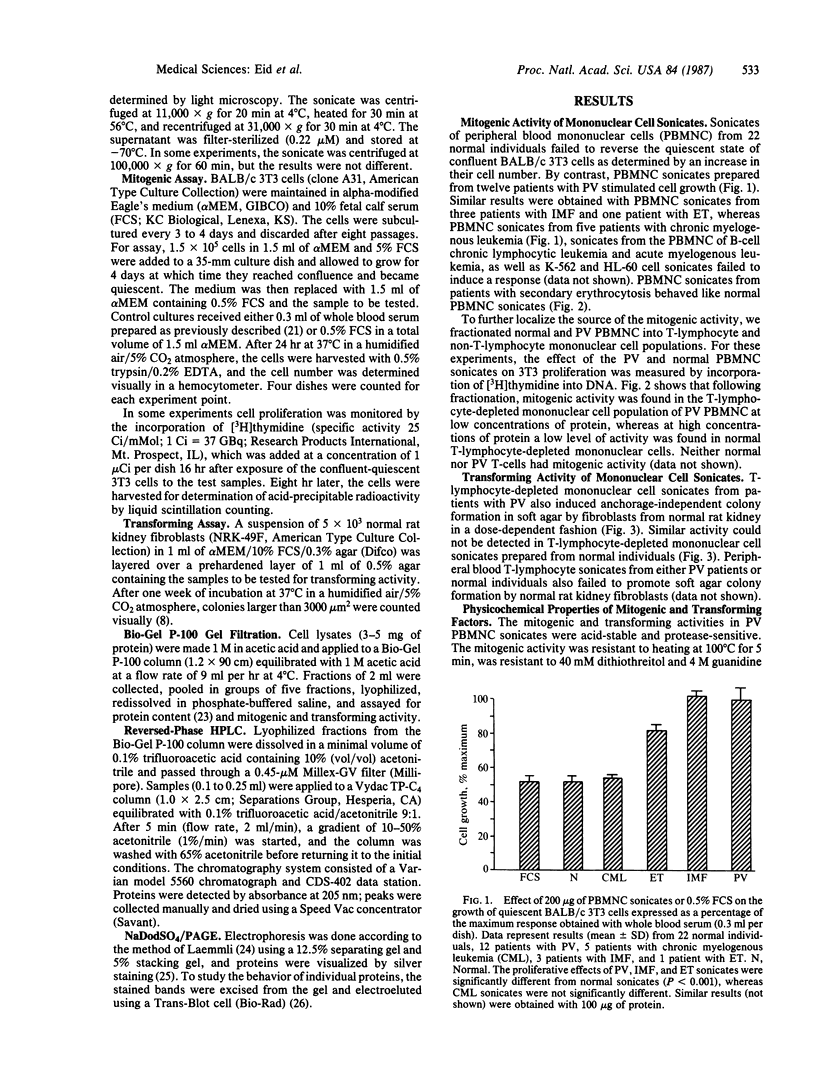
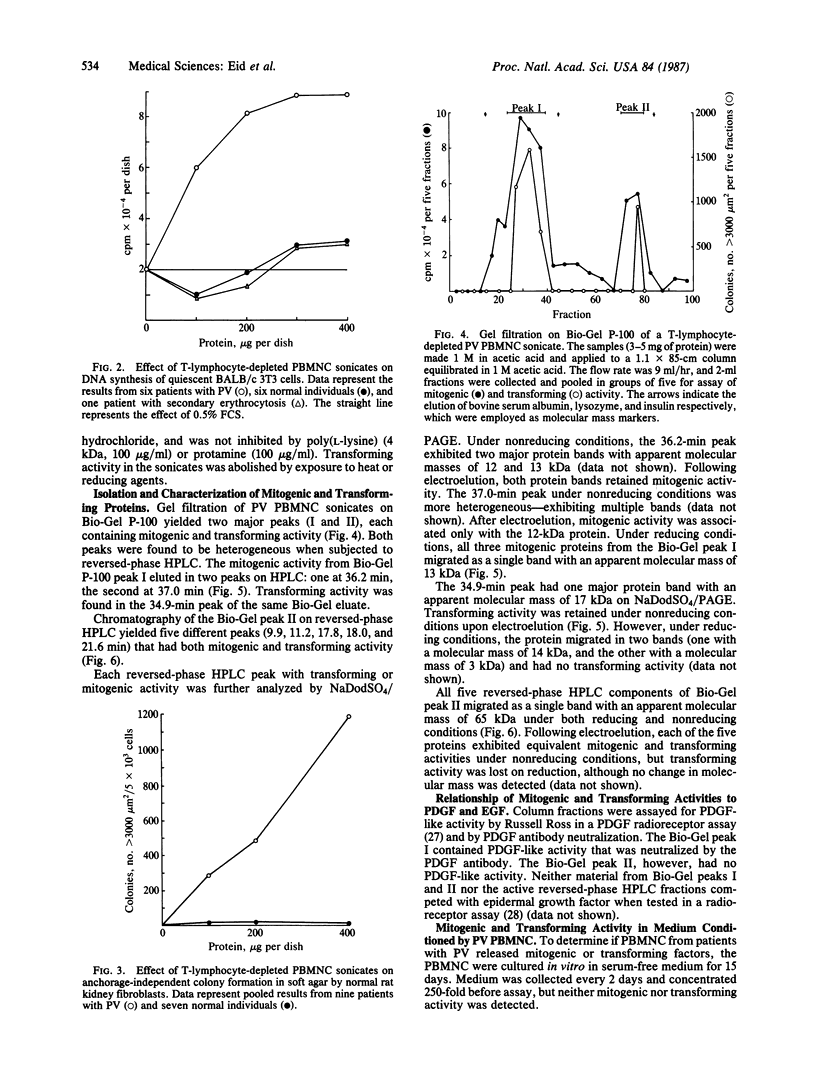
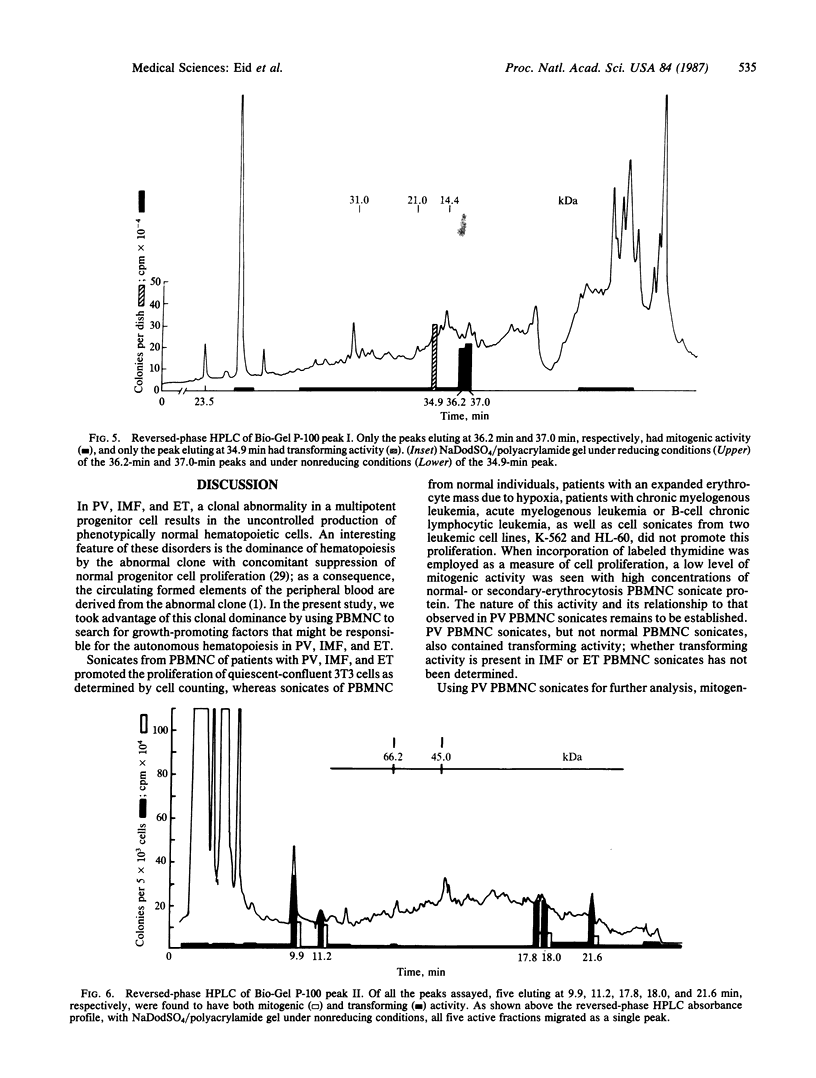
In polycythemia vera, idiopathic myelofibrosis, and essential thrombocytosis, hematopoietic cell proliferation is increased in the absence of a recognizable stimulus, suggesting the autonomous production of growth factors in these disorders. Sonicates of peripheral blood mononuclear cells (PBMNC) from patients with polycythemia vera, idiopathic myelofibrosis, and essential thrombocytosis contained soluble factors that stimulated the proliferation of quiescent-confluent 3T3 cells. PBMNC sonicates from normal individuals; from patients with secondary erythrocytosis, chronic myelogenous leukemia, B-cell chronic lymphocytic leukemia, and acute myelogenous leukemia; and from K-562 and HL-60 cells did not stimulate proliferation. Polycythemia vera PBMNC sonicates also induced anchorage-independent colony formation in soft agar by normal rat kidney fibroblasts. Both the mitogenic and transforming activities of the polycythemia vera PBMNC sonicates resided in the T-lymphocyte-depleted mononuclear fraction of the PBMNC and were not secreted. By gel filtration, reversed-phase HPLC and NaDodSO4/PAGE, the mitogenic and transforming activities in the polycythemia vera PBMNC were localized to three proteins with molecular masses of 13-, 17-, and 65-kDa. The 13-kDa protein was only mitogenic, and the 17-kDa protein was only transforming, whereas the 65-kDa protein had both mitogenic and transforming activity. These proteins may be involved in the autonomous hematopoiesis that characterizes polycythemia vera, idiopathic myelofibrosis, and essential thrombocytosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Fialkow P. J., Murphy S., Prchal J. F., Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976 Oct 21;295(17):913–916. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- Adamson J. W., Singer J. W., Catalano P., Murphy S., Lin N., Steinmann L., Ernst C., Fialkow P. J. Polycythemia vera. Further in vitro studies of hematopoietic regulation. J Clin Invest. 1980 Dec;66(6):1363–1368. doi: 10.1172/JCI109989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson J. W. The erythropoietin-hematocrit relationship in normal and polycythemic man: implications of marrow regulation. Blood. 1968 Oct;32(4):597–609. [PubMed] [Google Scholar]
- Betsholtz C., Heldin C. H., Nister M., Ek B., Wasteson A., Westermark B. Synthesis of a PDGF-like growth factor in human glioma and sarcoma cells suggests the expression of the cellular homologue to the transforming protein of simian sarcoma virus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):176–182. doi: 10.1016/0006-291x(83)91557-7. [DOI] [PubMed] [Google Scholar]
- Bhown A. S., Mole J. E., Hunter F., Bennett J. C. High-sensitivity sequence determination of proteins quantitatively recovered from sodium dodecyl sulfate gels using an improved electrodialysis procedure. Anal Biochem. 1980 Mar 15;103(1):184–190. doi: 10.1016/0003-2697(80)90254-7. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Malpass T. W., Foster D. M., Ross R. Platelet-derived growth factor in vivo: levels, activity, and rate of clearance. Blood. 1984 Aug;64(2):458–469. [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Eaves C. J., Eaves A. C. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978 Dec;52(6):1196–1210. [PubMed] [Google Scholar]
- Fialkow P. J., Faguet G. B., Jacobson R. J., Vaidya K., Murphy S. Evidence that essential thrombocythemia is a clonal disorder with origin in a multipotent stem cell. Blood. 1981 Nov;58(5):916–919. [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Jacobson R. J., Salo A., Fialkow P. J. Agnogenic myeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosis. Blood. 1978 Feb;51(2):189–194. [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- LAWRENCE J. H., ELMLINGER P. J., FULTON G. Oxygen and the control of red cell production in primary and secondary polycythemia; effects on the iron turnover patterns with Fe59 as tracer. Cardiologia. 1952;21(4):337–346. doi: 10.1159/000165213. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MURRAY J. F. ARTERIAL STUDIES IN PRIMARY AND SECONDARY POLYCYTHEMIC DISORDERS. Am Rev Respir Dis. 1965 Sep;92:435–449. doi: 10.1164/arrd.1965.92.3.435. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J. Human transforming growth factor. Production by a melanoma cell line, purification, and initial characterization. J Biol Chem. 1982 May 10;257(9):5220–5225. [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Massagué J. Type beta transforming growth factor from feline sarcoma virus-transformed rat cells. Isolation and biological properties. J Biol Chem. 1984 Aug 10;259(15):9756–9761. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Pantazis P., Pelicci P. G., Dalla-Favera R., Antoniades H. N. Synthesis and secretion of proteins resembling platelet-derived growth factor by human glioblastoma and fibrosarcoma cells in culture. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2404–2408. doi: 10.1073/pnas.82.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Surface bound immunoglobulins as a cell marker in human lymphoproliferative diseases. Blood. 1972 Dec;40(6):777–794. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman L. R. The management of polycythaemia vera. Br J Haematol. 1971 Oct;21(4):371–376. doi: 10.1111/j.1365-2141.1971.tb02698.x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]