Abstract
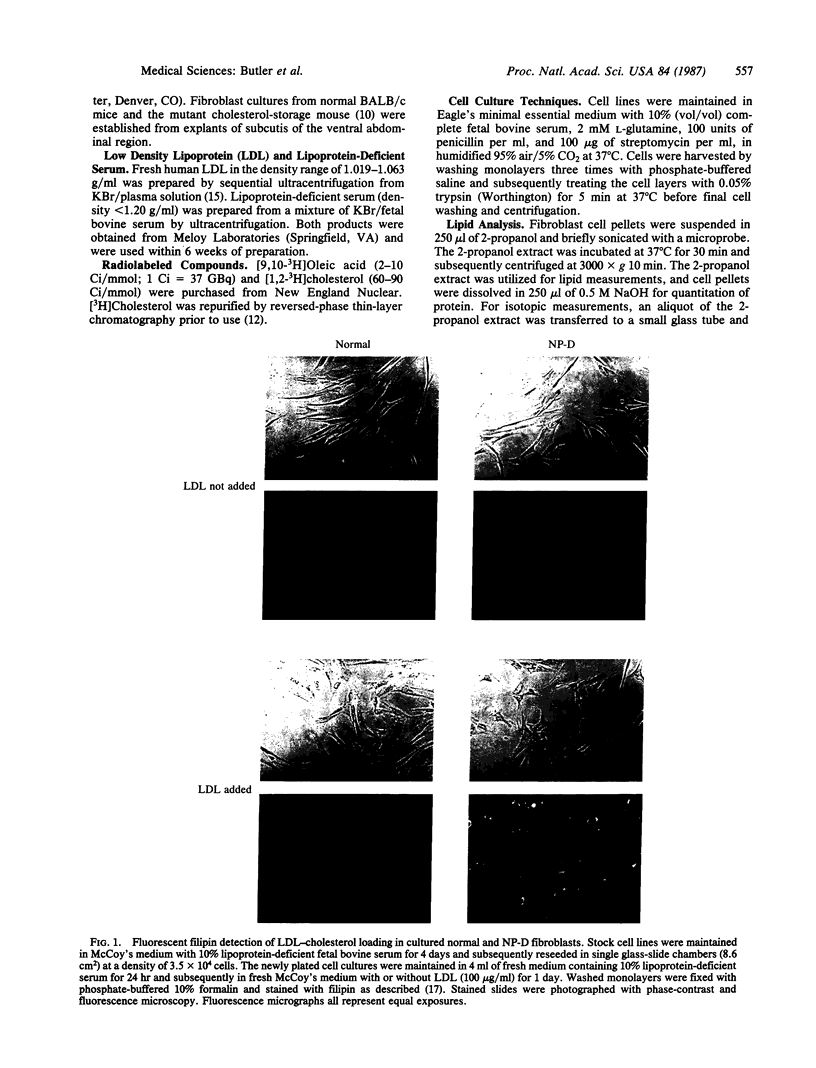
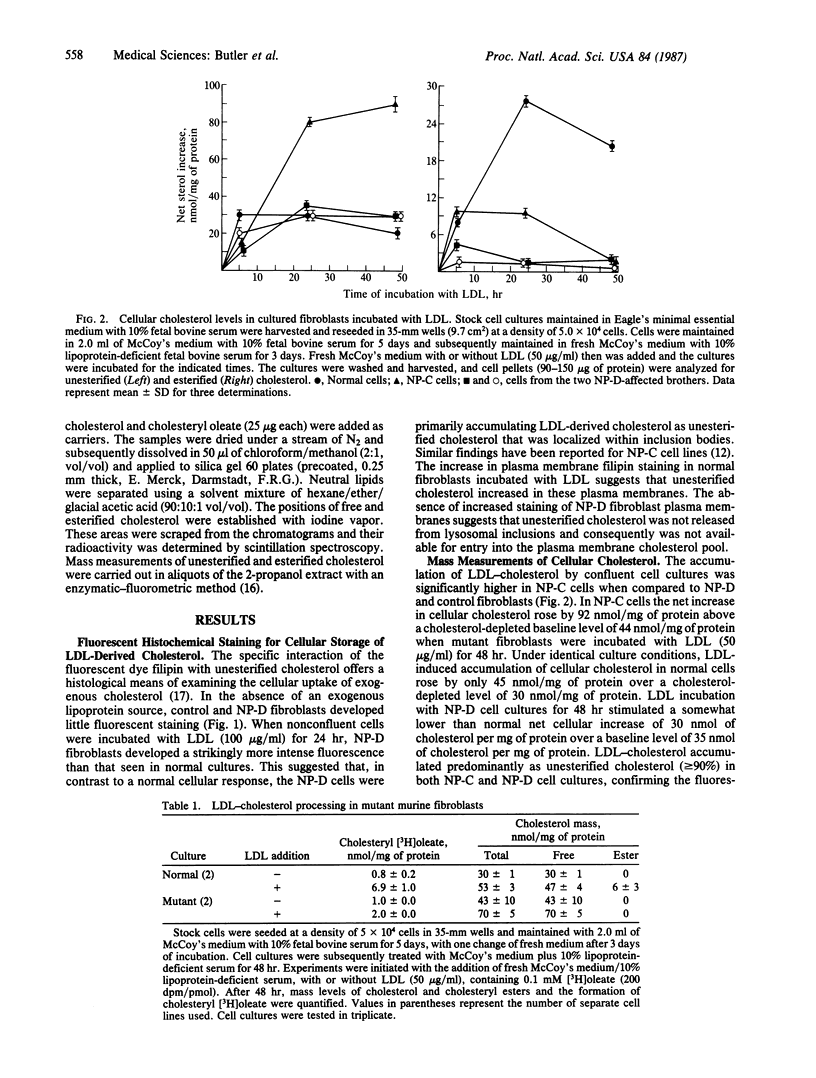
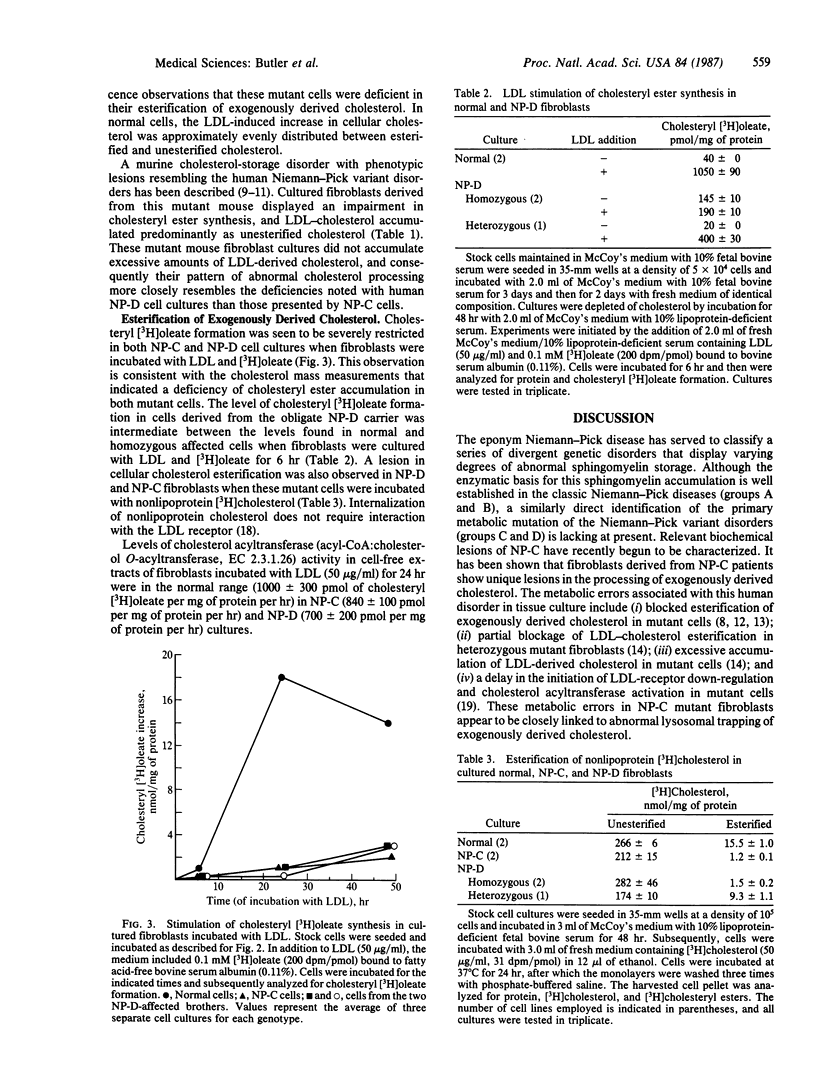
Fluorescence microscopic examination of filipin-stained cultured skin fibroblasts derived from two brothers with group D Niemann-Pick disease revealed abnormal storage of low density lipoprotein (LDL)-derived cholesterol. LDL stimulation of intracellular cholesteryl ester synthesis was severely compromised in the Niemann-Pick D fibroblasts, as it also was in fibroblasts obtained from Niemann-Pick C patients. Cholesteryl ester synthesis was intermediately deficient in cells derived from an obligate group-D heterozygous carrier. Activity of acyl-CoA:cholesterol acyltransferase was within the normal range in cell-free extracts of both LDL-depleted and LDL-supplemented cultures of Niemann-Pick C and D fibroblasts. Incubation of Niemann-Pick D fibroblasts with LDL did not lead to as high a level of intracellular cholesterol accumulation as the excessive storage observed with Niemann-Pick C fibroblasts. These findings suggest that the Niemann-Pick variant disorders may represent a family of specific and possibly individual mutations that disrupt cellular cholesterol homeostasis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady R. O., Kanfer J. N., Mock M. B., Fredrickson D. S. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proc Natl Acad Sci U S A. 1966 Feb;55(2):366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCKER A. C. The cerebral defect in Tay-Sachs disease and Niemann-Pick disease. J Neurochem. 1961 Apr;7:69–80. doi: 10.1111/j.1471-4159.1961.tb13499.x. [DOI] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth H. S., Vaughan M. Quantification of low density lipoprotein binding and cholesterol accumulation by single human fibroblasts using fluorescence microscopy. J Lipid Res. 1980 Jan;21(1):123–130. [PubMed] [Google Scholar]
- Pentchev P. G., Boothe A. D., Kruth H. S., Weintroub H., Stivers J., Brady R. O. A genetic storage disorder in BALB/C mice with a metabolic block in esterification of exogenous cholesterol. J Biol Chem. 1984 May 10;259(9):5784–5791. [PubMed] [Google Scholar]
- Pentchev P. G., Comly M. E., Kruth H. S., Patel S., Proestel M., Weintroub H. The cholesterol storage disorder of the mutant BALB/c mouse. A primary genetic lesion closely linked to defective esterification of exogenously derived cholesterol and its relationship to human type C Niemann-Pick disease. J Biol Chem. 1986 Feb 25;261(6):2772–2777. [PubMed] [Google Scholar]
- Pentchev P. G., Comly M. E., Kruth H. S., Vanier M. T., Wenger D. A., Patel S., Brady R. O. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8247–8251. doi: 10.1073/pnas.82.23.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentchev P. G., Gal A. E., Booth A. D., Omodeo-Sale F., Fouks J., Neumeyer B. A., Quirk J. M., Dawson G., Brady R. O. A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim Biophys Acta. 1980 Sep 8;619(3):669–679. doi: 10.1016/0005-2760(80)90116-2. [DOI] [PubMed] [Google Scholar]
- Schneider P. B., Kennedy E. P. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J Lipid Res. 1967 May;8(3):202–209. [PubMed] [Google Scholar]
- Sloan H. R., Uhlendorf B. W., Kanfer J. N., Brady R. O., Fredrickson D. S. Deficiency of sphingomyelin-cleaving enzyme activity in tissue cultures derived from patients with Niemann-Pick disease. Biochem Biophys Res Commun. 1969 Mar 10;34(5):582–588. doi: 10.1016/0006-291x(69)90777-3. [DOI] [PubMed] [Google Scholar]