Abstract
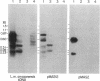
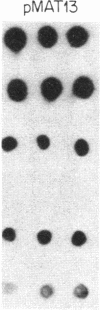
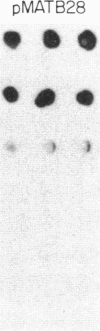
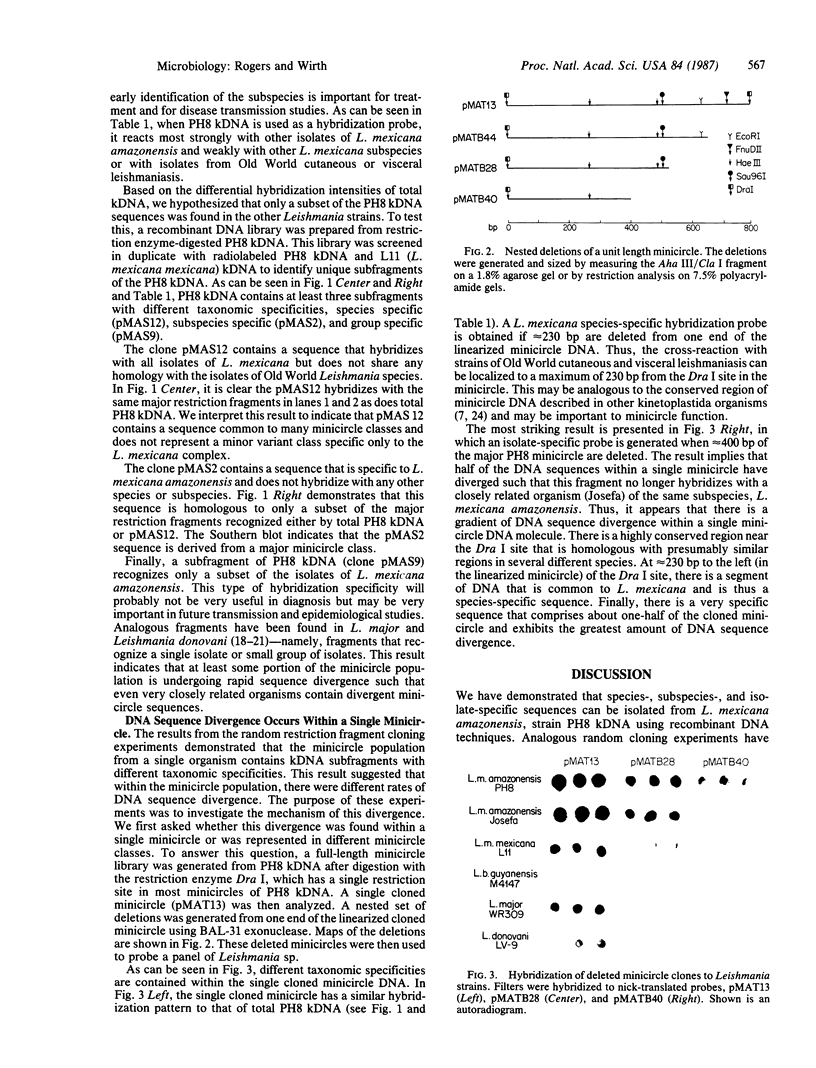
Previous work has shown that the kinetoplast minicircle DNA of Leishmania species exhibits species-specific sequence divergence and this observation has led to the development of a DNA probe-based diagnostic test for leishmaniasis. In the work reported here, we demonstrate that the minicircle is composed of three types of DNA sequences with differing specificities reflecting different rates of DNA sequence change. A library of cloned fragments of kinetoplast DNA (kDNA) from Leishmania mexicana amazonensis was prepared and the cloned subfragments were found to contain DNA sequences with different taxonomic specificities based on hybridization analysis with various species of Leishmania. Four groups of subfragments were found, those that hybridized with a large number of Leishmania sp. as well as sequences unique to the species, subspecies, or isolate. Analysis of nested deletions of a single, full-length minicircle demonstrates that these different taxonomic specificities are contained within a single minicircle. This implies that different regions of a single minicircle have DNA sequences that diverge at different rates. These sequences represent potentially valuable tools in diagnostic, epidemiologic, and ecological studies of leishmaniasis and provide the basis for a model of kDNA sequence evolution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barker D. C. Biochemical identification of cutaneous leishmanias by analysis of kinetoplast DNA. II. Sequence homologies in Leishmania kDNA. Mol Biochem Parasitol. 1981 May;3(1):47–56. doi: 10.1016/0166-6851(81)90076-1. [DOI] [PubMed] [Google Scholar]
- Barker D. C., Arnot D. E. Biochemical identification of cutaneous leishmanias by analysis of kinetoplast DNA. I. Ultrastructural and buoyant density analysis. Mol Biochem Parasitol. 1981 May;3(1):33–46. doi: 10.1016/0166-6851(81)90075-x. [DOI] [PubMed] [Google Scholar]
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Chen K. K., Donelson J. E. Sequences of two kinetoplast DNA minicircles of Tryptanosoma brucei. Proc Natl Acad Sci U S A. 1980 May;77(5):2445–2449. doi: 10.1073/pnas.77.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch A. C., Goijman S. G., Cazzulo J. J., Stoppani A. O. Constant and variable regions in DNA mini-circles from Trypanosoma cruzi and Trypanosoma rangeli: application to species and stock differentiation. Mol Biochem Parasitol. 1981 Dec;4(3-4):163–170. doi: 10.1016/0166-6851(81)90015-3. [DOI] [PubMed] [Google Scholar]
- Hendricks L. D., Wood D. E., Hajduk M. E. Haemoflagellates: commercially available liquid media for rapid cultivation. Parasitology. 1978 Jun;76(3):309–316. doi: 10.1017/s0031182000048186. [DOI] [PubMed] [Google Scholar]
- Hudspeth M. E., Vincent R. D., Perlman P. S., Shumard D. S., Treisman L. O., Grossman L. I. Expandable var1 gene of yeast mitochondrial DNA: in-frame insertions can explain the strain-specific protein size polymorphisms. Proc Natl Acad Sci U S A. 1984 May;81(10):3148–3152. doi: 10.1073/pnas.81.10.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy W. P. Novel identification of differences in the kinetoplast DNA of Leishmania isolates by recombinant DNA techniques and in situ hybridisation. Mol Biochem Parasitol. 1984 Jul;12(3):313–325. doi: 10.1016/0166-6851(84)90088-4. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Lawrie J. M., Jackson P. R., Stiteler J. M., Hockmeyer W. T. Identification of pathogenic Leishmania promastigotes by DNA: DNA hybridization with kinetoplast DNA cloned into E. coli plasmids. Am J Trop Med Hyg. 1985 Mar;34(2):257–265. doi: 10.4269/ajtmh.1985.34.257. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Howe C. J., Stern D. B. Maize mitochondrial DNA contains a sequence homologous to the ribulose-1,5-bisphosphate carboxylase large subunit gene of chloroplast DNA. Cell. 1983 Oct;34(3):1007–1014. doi: 10.1016/0092-8674(83)90558-5. [DOI] [PubMed] [Google Scholar]
- Lopes U. G., Momen H., Grimaldi G., Jr, Marzochi M. C., Pacheco R. S., Morel C. M. Schizodeme and zymodeme characterization of Leishmania in the investigation of foci of visceral and cutaneous leishmaniasis. J Parasitol. 1984 Feb;70(1):89–98. [PubMed] [Google Scholar]
- Lopes U. G., Wirth D. F. Identification of visceral Leishmania species with cloned sequences of kinetoplast DNA. Mol Biochem Parasitol. 1986 Jul;20(1):77–84. doi: 10.1016/0166-6851(86)90144-1. [DOI] [PubMed] [Google Scholar]
- Marsden P. D. Current concepts in parasitology. Leishmaniasis. N Engl J Med. 1979 Feb 15;300(7):350–352. doi: 10.1056/NEJM197902153000706. [DOI] [PubMed] [Google Scholar]
- Masuda H., Simpson L., Rosenblatt H., Simpson A. M. Restriction map, partial cloning and localization of 9S and 12S kinetoplast RNA genes on the maxicircle component of the kinetoplast DNA of Leishmania tarentolae. Gene. 1979 May;6(1):51–73. doi: 10.1016/0378-1119(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Morel C., Chiari E., Camargo E. P., Mattei D. M., Romanha A. J., Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C., Simpson L. Characterization of pathogenic trypanosomatidae by restriction endonuclease fingerprinting of kinetoplast DNA minicircles. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1070–1074. doi: 10.4269/ajtmh.1980.29.1070. [DOI] [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA, mitochondrial DNA with a difference. Mol Biochem Parasitol. 1983 Oct;9(2):93–104. doi: 10.1016/0166-6851(83)90103-2. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Pratt D. M. Rapid identification of Leishmania species by specific hybridization of kinetoplast DNA in cutaneous lesions. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6999–7003. doi: 10.1073/pnas.79.22.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth D. F., Rogers W. O., Barker R., Jr, Dourado H., Suesebang L., Albuquerque B. Leishmaniasis and malaria: new tools for epidemiologic analysis. Science. 1986 Nov 21;234(4779):975–979. doi: 10.1126/science.3535070. [DOI] [PubMed] [Google Scholar]