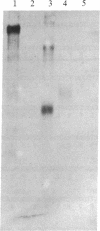
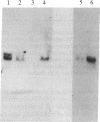
Abstract
Myelin-associated glycoprotein (MAG) may play a role in the cellular interactions leading to myelination. Using monoclonal antibodies and conventional antisera against MAG, we have isolated a cDNA clone from an expression library prepared from rat brain mRNA. The identity of the clone was confirmed by the exact match between its nucleotide sequence and two peptide sequences of 13 and 9 amino acids that we obtained by Edman degradation of two CNBr fragments of MAG. The cDNA clone hybridized to two size species of mRNA in rat approximately 3.5 kilobases in length. These mRNAs were present in brain but not liver and were expressed most abundantly at the time of active myelination (day 14). The mRNA for MAG was present at barely detectable levels in hypomyelinating jimpy mice compared to normal littermate controls. Therefore the MAG cDNA clone is both brain and myelin specific. DNA sequence analysis revealed that our MAG cDNA was derived from the same mRNA as clone p1B236, a randomly selected, brain-specific, partial cDNA isolated by Sutcliffe et al. [Sutcliffe, J. G., Milner, R. J., Shinnick, T. M. & Bloom, F. E. (1983) Cell 33, 671-682]. Analysis of the predicted protein sequence suggests that MAG has a long extracellular domain (499 amino acids), followed by a short transmembrane segment (20 amino acids) and an intracellular carboxyl-terminal domain (90 amino acids). The molecule has several glycosylation sites, three internal repeats homologous to a repeat in the neural cell adhesion molecule (N-CAM), and sites for phosphorylation near the carboxyl terminus. The primary structure reported here provides a molecular framework for further investigations into the function of the MAG molecule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P. E., Frail D. E., Latov N. Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy. J Neurochem. 1982 Nov;39(5):1261–1265. doi: 10.1111/j.1471-4159.1982.tb12563.x. [DOI] [PubMed] [Google Scholar]
- Dautigny A., Mattei M. G., Morello D., Alliel P. M., Pham-Dinh D., Amar L., Arnaud D., Simon D., Mattei J. F., Guenet J. L. The structural gene coding for myelin-associated proteolipid protein is mutated in jimpy mice. 1986 Jun 26-Jul 2Nature. 321(6073):867–869. doi: 10.1038/321867a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Frail D. E., Braun P. E. Abnormal expression of the myelin-associated glycoprotein in the central nervous system of dysmyelinating mutant mice. J Neurochem. 1985 Oct;45(4):1071–1075. doi: 10.1111/j.1471-4159.1985.tb05525.x. [DOI] [PubMed] [Google Scholar]
- Frail D. E., Braun P. E. Two developmentally regulated messenger RNAs differing in their coding region may exist for the myelin-associated glycoprotein. J Biol Chem. 1984 Dec 10;259(23):14857–14862. [PubMed] [Google Scholar]
- Hemperly J. J., Murray B. A., Edelman G. M., Cunningham B. A. Sequence of a cDNA clone encoding the polysialic acid-rich and cytoplasmic domains of the neural cell adhesion molecule N-CAM. Proc Natl Acad Sci U S A. 1986 May;83(9):3037–3041. doi: 10.1073/pnas.83.9.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Krangel M. S., Strominger J. L. Cysteines in the transmembrane region of major histocompatibility complex antigens are fatty acylated via thioester bonds. J Biol Chem. 1984 Jun 10;259(11):7230–7238. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Bakhit C., Bloom F. E., Sutcliffe J. G., Milner R. J. Brain-specific polypeptide 1B236 exists in multiple molecular forms. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2009–2013. doi: 10.1073/pnas.82.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nobile-Orazio E., Hays A. P., Latov N., Perman G., Golier J., Shy M. E., Freddo L. Specificity of mouse and human monoclonal antibodies to myelin-associated glycoprotein. Neurology. 1984 Oct;34(10):1336–1342. doi: 10.1212/wnl.34.10.1336. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R. H., Barbarash G. R., Figlewicz D. A., McIntyre L. J. Purification and partial characterization of the myelin-associated glycoprotein from adult rat brain. Biochim Biophys Acta. 1983 May 4;757(1):140–143. doi: 10.1016/0304-4165(83)90162-9. [DOI] [PubMed] [Google Scholar]
- Quarles R. H. Myelin-associated glycoprotein in development and disease. Dev Neurosci. 1983;6(6):285–303. doi: 10.1159/000112356. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Pasnak C. F. A rapid procedure for selectively isolating the major glycoprotein from purified rat brain myelin. Biochem J. 1977 Jun 1;163(3):635–637. doi: 10.1042/bj1630635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riopelle R. J., McGarry R. C., Roder J. C. Adhesion properties of a neuronal epitope recognized by the monoclonal antibody HNK-1. Brain Res. 1986 Mar 5;367(1-2):20–25. doi: 10.1016/0006-8993(86)91573-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Quarles R. H., Itoyama Y., Webster H. D. Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1510–1514. doi: 10.1073/pnas.76.3.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Shinnick T. M., Bloom F. E. Identifying the protein products of brain-specific genes with antibodies to chemically synthesized peptides. Cell. 1983 Jul;33(3):671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- Webster H. D., Palkovits C. G., Stoner G. L., Favilla J. T., Frail D. E., Braun P. E. Myelin-associated glycoprotein: electron microscopic immunocytochemical localization in compact developing and adult central nervous system myelin. J Neurochem. 1983 Nov;41(5):1469–1479. doi: 10.1111/j.1471-4159.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]