Abstract
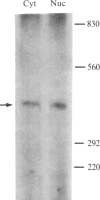
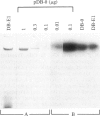
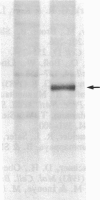
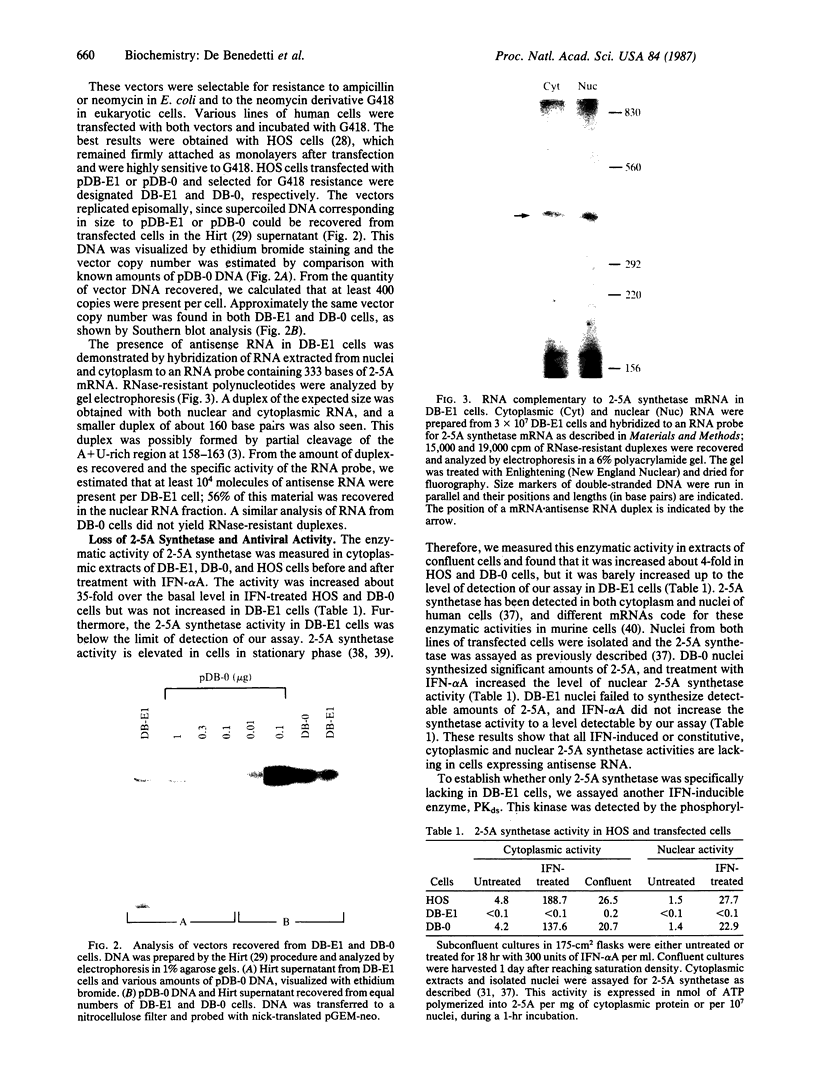
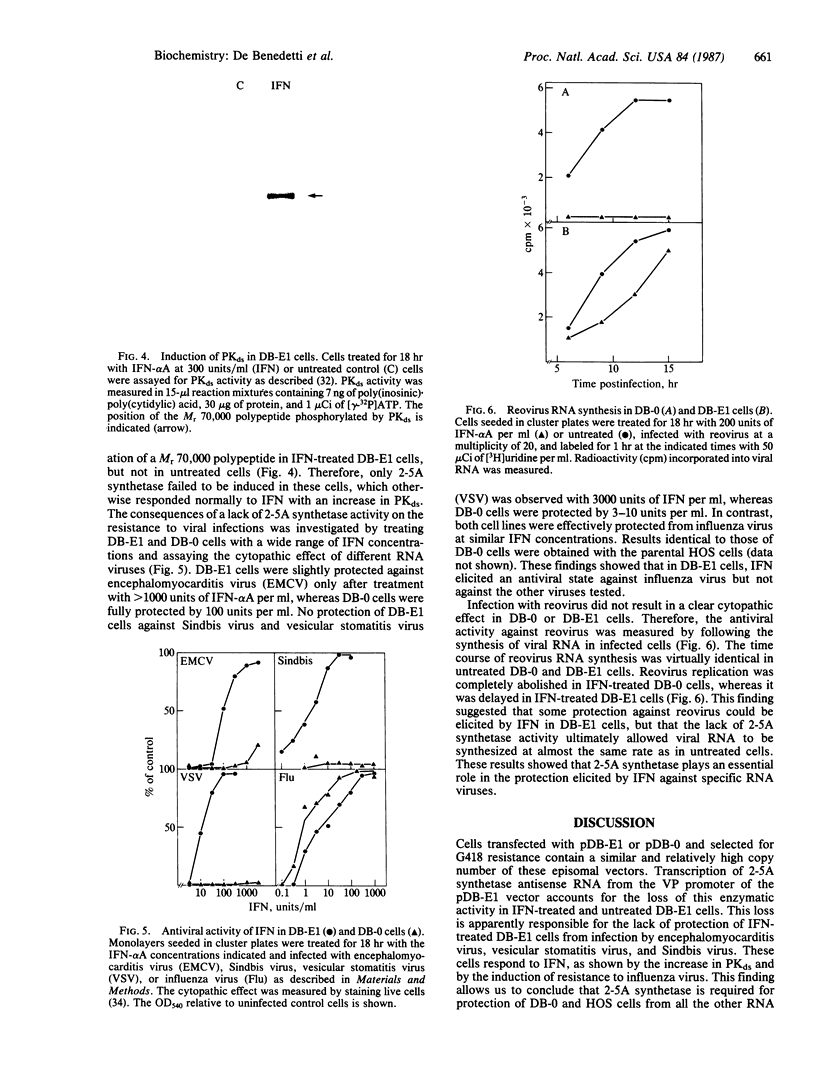
An expression vector was constructed that carries part of the human BK papovavirus with 0.5 kilobases of (2'-5')oligoadenylate (2-5A) synthetase cDNA inserted in inverted orientation downstream from the virion proteins (VP) promoter and the neomycin-resistance gene neo under the control of a simian virus 40 promoter. Cells transfected with this vector and selected for resistance to the neomycin derivative G418 synthesized RNA complementary to 2-5A synthetase mRNA. These cells lacked 2-5A synthetase activity, and the enzyme was not inducible by interferon. In contrast, 2-5A synthetase was induced in cells transfected with a control vector without the cDNA insert. Such cells were protected by interferon from RNA viruses, whereas cells lacking 2-5A synthetase were not protected from encephalomyocarditis virus, vesicular stomatitis virus, and Sindbis virus but were fully protected from influenza virus. These findings show that a high level of 2-5A synthetase is required for interferon-induced protection from the cytoplasmic RNA viruses tested.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Benech P., Merlin G., Revel M., Chebath J. 3' end structure of the human (2'-5') oligo A synthetase gene: prediction of two distinct proteins with cell type-specific expression. Nucleic Acids Res. 1985 Feb 25;13(4):1267–1281. doi: 10.1093/nar/13.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden E. C., Leonhardt P. H. Aquanititave semimicro, semiautomated colorimetric assay for interferon. J Lab Clin Med. 1977 May;89(5):1036–1042. [PubMed] [Google Scholar]
- Chebath J., Merlin G., Metz R., Benech P., Revel M. Interferon-induced 56,000 Mr protein and its mRNA in human cells: molecular cloning and partial sequence of the cDNA. Nucleic Acids Res. 1983 Mar 11;11(5):1213–1226. doi: 10.1093/nar/11.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Green P. J., Inouye M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell. 1984 Jun;37(2):429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Williams G. J., Comeau L., Baglioni C. Inhibition of viral mRNA translation in interferon-treated L cells infected with reovirus. J Virol. 1985 Sep;55(3):588–593. doi: 10.1128/jvi.55.3.588-593.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Anti-mitogenic function of interferon-induced (2'-5')oligo(adenylate) and growth-related variations in enzymes that synthesize and degrade this oligonucleotide. Eur J Biochem. 1981;114(1):5–10. doi: 10.1111/j.1432-1033.1981.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Krishnan I., Baglioni C. Elevated levels of (2'-5')oligoadenylic acid polymerase activity in growth-arrested human lymphoblastoid Namalva cells. Mol Cell Biol. 1981 Oct;1(10):932–938. doi: 10.1128/mcb.1.10.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Shaw M., Broni B., Shapiro G., Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985 Oct;56(1):201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., Jonak G., Cheng Y. S., Korant B., Knight E., Darnell J. E., Jr Transcriptional induction of two genes in human cells by beta interferon. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6733–6737. doi: 10.1073/pnas.81.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- McMahon M., Stark G. R., Kerr I. M. Interferon-induced gene expression in wild-type and interferon-resistant human lymphoblastoid (Daudi) cells. J Virol. 1986 Jan;57(1):362–366. doi: 10.1128/jvi.57.1.362-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin G., Chebath J., Benech P., Metz R., Revel M. Molecular cloning and sequence of partial cDNA for interferon-induced (2'-5')oligo(A) synthetase mRNA from human cells. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4904–4908. doi: 10.1073/pnas.80.16.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi G., Barbanti-Brodano G., Negrini M., Lee D., Corallini A., Caputo A., Grossi M. P., Ricciardi R. P. BK virus-plasmid expression vector that persists episomally in human cells and shuttles into Escherichia coli. Mol Cell Biol. 1984 Aug;4(8):1551–1560. doi: 10.1128/mcb.4.8.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Synthesis of 2'5'-oligo(A) in extracts of interferon-treated HeLa cells. J Biol Chem. 1979 Jun 25;254(12):5058–5064. [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J Biol Chem. 1982 Dec 25;257(24):14593–14596. [PubMed] [Google Scholar]
- Nilsen T. W., Wood D. L., Baglioni C. Presence of 2',5'-oligo(A) and of enzymes that synthesize, bind, and degrade 2',5'-oligo(A) in HeLa cell nuclei. J Biol Chem. 1982 Feb 25;257(4):1602–1605. [PubMed] [Google Scholar]
- Nilsen T. W., Wood D. L., Baglioni C. Virus-specific effects of interferon in embryonal carcinoma cells. Nature. 1980 Jul 10;286(5769):178–180. doi: 10.1038/286178a0. [DOI] [PubMed] [Google Scholar]
- O'Malley R. P., Mariano T. M., Siekierka J., Mathews M. B. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986 Feb 14;44(3):391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Preiss A., Seifert E., Jäckle H., Knipple D. C. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985 Feb 21;313(6004):703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Wreschner D. H., Oberman F., Panet A., Bakhanashvili M. Isolation and characterization of an interferon-resistant cell line deficient in the induction of (2'-5')oligoadenylate synthetase activity. Mol Cell Biol. 1983 Oct;3(10):1759–1765. doi: 10.1128/mcb.3.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- Samuel C. E., Knutson G. S. Mechanism of interferon action: human leukocyte and immune interferons regulate the expression of different genes and induce different antiviral states in human amnion U cells. Virology. 1983 Oct 30;130(2):474–484. doi: 10.1016/0042-6822(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- St Laurent G., Yoshie O., Floyd-Smith G., Samanta H., Sehgal P. B., Lengyel P. Interferon action: two (2'-5')(A)n synthetases specified by distinct mRNAs in Ehrlich ascites tumor cells treated with interferon. Cell. 1983 May;33(1):95–102. doi: 10.1016/0092-8674(83)90338-0. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Simili M., Baglioni C. Binding of viral and cellular messenger RNAs to ribosomes in eukaryotic cell extracts. Methods Enzymol. 1979;60:351–360. doi: 10.1016/s0076-6879(79)60033-2. [DOI] [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- West D. K., Baglioni C. Induction of interferon in HeLa cells of a protein kinase activated by double-stranded RNA. Eur J Biochem. 1979 Nov;101(2):461–468. doi: 10.1111/j.1432-1033.1979.tb19740.x. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Hovanessian A. G. Interferon enhances 2-5A synthetase in embryonal carcinoma cells. Nature. 1979 Nov 1;282(5734):74–76. doi: 10.1038/282074a0. [DOI] [PubMed] [Google Scholar]