Abstract
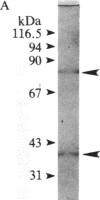
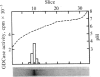
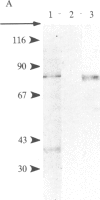
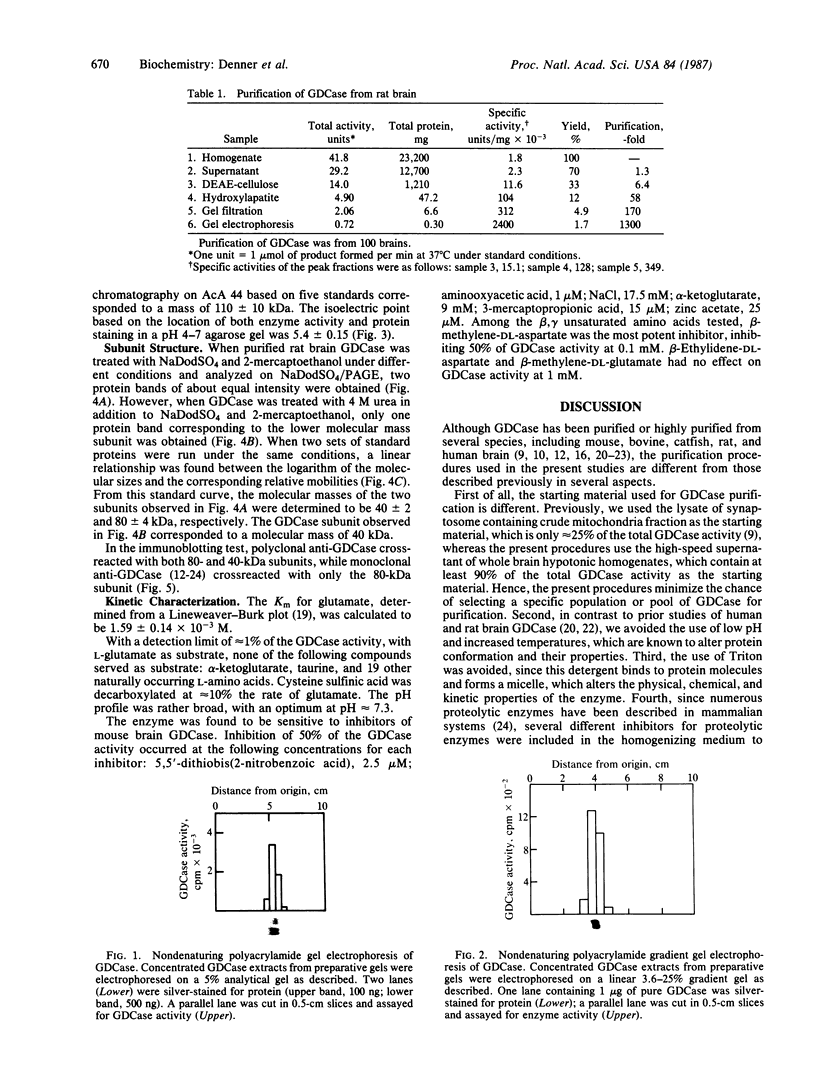
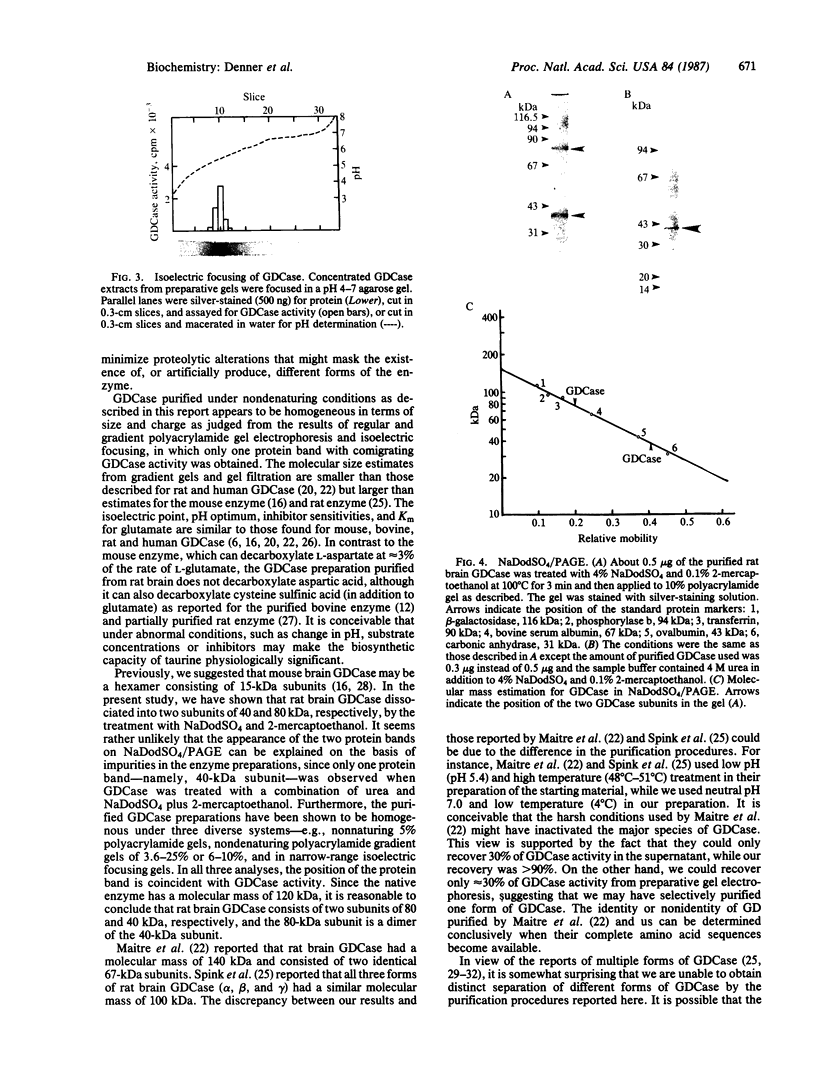
Glutamate decarboxylase (GDCase; L-glutamate-1-carboxy-lyase, EC 4.1.1.15) was purified from whole rat brain approximately equal to 1300-fold to apparent homogeneity with a specific activity of 2.4 units per mg of protein by a combination of column chromatographies on DEAE-cellulose, hydroxylapatite, and gel filtration, and preparative nondenaturing polyacrylamide gel electrophoresis. The purified preparation contained a single protein band that comigrated with GDCase activity in three diverse analyses: nondenaturing regular (5%) and gradient (3.6-25%) polyacrylamide gel electrophoresis and isoelectric focusing at pH 4-7. The native molecular mass was calculated to be 120 +/- 10 kDa from gradient polyacrylamide gel electrophoresis and 110 +/- 10 kDa from gel filtration. Under the treatment with NaDodSO4 and 2-mercaptoethanol, GDCase dissociated into two subunits of 40 +/- 2 and 80 +/- 4 kDa, as estimated from NaDodSO4 gel electrophoresis. However, only a 40-kDa subunit was detected when GDCase was treated with 4 M urea plus NaDodSO4 and 2-mercaptoethanol, suggesting that the 80-kDa subunit is the dimer of the 40-kDa subunit. In immunoblotting, polyclonal antibodies against GDCase reacted with both 40- and 80-kDa subunits, while monoclonal antibody reacted with only 80-kDa subunits. The isoelectric point of the native enzyme was 5.4. The Km for glutamate was 1.59 X 10(-3) M. In addition to L-glutamate, cysteine sulfinic acid was also decarboxylated at approximately equal to 10% of the rate of glutamate. The pH optimum was fairly broad, with a maximum at approximately equal to 7.3. The enzyme was strongly inhibited by carbonyl-trapping agents, sulfhydryl reagents, thiol compounds, and beta-methylene-DL-aspartate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blindermann J. M., Maitre M., Ossola L., Mandel P. Purification and some properties of L-glutamate decarboxylase from human brain. Eur J Biochem. 1978 May;86(1):143–152. doi: 10.1111/j.1432-1033.1978.tb12293.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Tapia R. Brain glutamate decarboxylase: properties of its calcium-dependent binding to liposomes and kinetics of the bound and the free enzyme. J Neurochem. 1980 Jun;34(6):1682–1688. doi: 10.1111/j.1471-4159.1980.tb11261.x. [DOI] [PubMed] [Google Scholar]
- Hadjian R. A., Stewart J. A. Immunological quantitation of glutamic acid decarboxylase in developing mouse brain. J Neurochem. 1977 Jun;28(6):1249–1257. doi: 10.1111/j.1471-4159.1977.tb12318.x. [DOI] [PubMed] [Google Scholar]
- Maitre M., Blindermann J. M., Ossola L., Mandel P. Comparison of the structures of L-glutamate decarboxylases from human and rat brains. Biochem Biophys Res Commun. 1978 Dec 14;85(3):885–890. doi: 10.1016/0006-291x(78)90626-5. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Wu J. Y., Roberts E. Electrophoresis of glutamic acid decarboxylase (EC 4.1.1.15) from mouse brain in sodium dodecyl sulphate polyacrylamide gels. J Neurochem. 1973 Jul;21(1):167–172. doi: 10.1111/j.1471-4159.1973.tb04236.x. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Tappaz M. L., Kopin I. J. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981;6(12):2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Weise V. K., Ransom D. H., Tappaz M. L., Krutzsch H. C., Kopin I. J. Comparison of cysteine sulphinic acid decarboxylase isoenzymes and glutamic acid decarboxylase in rat liver and brain. Neuroscience. 1981;6(12):2701–2714. doi: 10.1016/0306-4522(81)90114-7. [DOI] [PubMed] [Google Scholar]
- Pérez de la Mora M., Feria-Velasco A., Tapia R. Pyridoxal phosphate and glutamate decarboxylase in subcellular particles of mouse brain and their relationship to convulsions. J Neurochem. 1973 Jun;20(6):1575–1587. doi: 10.1111/j.1471-4159.1973.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Roberts E., Kuriyama K. Biochemical-physiological correlations in studies of the gamma-aminobutyric acid system. Brain Res. 1968 Apr;8(1):1–35. doi: 10.1016/0006-8993(68)90170-4. [DOI] [PubMed] [Google Scholar]
- Slater G. G. Stable pattern formation and determination of molecular size by pore-limit electrophoresis. Anal Chem. 1969 Jul;41(8):1039–1041. doi: 10.1021/ac60277a003. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Wu S. J., Martin D. L. Multiple forms of glutamate decarboxylase in porcine brain. J Neurochem. 1983 Apr;40(4):1113–1119. doi: 10.1111/j.1471-4159.1983.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Su Y. Y., Wu J. Y., Lam D. M. Purification of L-glutamic acid decarboxylase from catfish brain. J Neurochem. 1979 Jul;33(1):169–179. doi: 10.1111/j.1471-4159.1979.tb11719.x. [DOI] [PubMed] [Google Scholar]
- Tapia R., Meza-Ruíz G. Differences in some properties of newborn and adult brain glutamate decarboxylase. J Neurobiol. 1975 Mar;6(2):171–181. doi: 10.1002/neu.480060205. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turský T. Inhibition of brain glutamate decarboxylase by adenosine triphosphate. Eur J Biochem. 1970 Feb;12(3):544–549. doi: 10.1111/j.1432-1033.1970.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Denner L. A., Wei S. C., Lin C. T., Song G. X., Xu Y. F., Liu J. W., Lin H. S. Production and characterization of polyclonal and monoclonal antibodies to rat brain L-glutamate decarboxylase. Brain Res. 1986 May 14;373(1-2):1–14. doi: 10.1016/0006-8993(86)90309-4. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Matsuda T., Roberts E. Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem. 1973 May 10;248(9):3029–3034. [PubMed] [Google Scholar]
- Wu J. Y. Purification and characterization of cysteic acid and cysteine sulfinic acid decarboxylase and L-glutamate decarboxylase from bovine brain. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4270–4274. doi: 10.1073/pnas.79.14.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Roberts E. Properties of brain L-glutamate decarboxylase: inhibition studies. J Neurochem. 1974 Oct;23(4):759–767. doi: 10.1111/j.1471-4159.1974.tb04401.x. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Wong E., Saito K., Roberts E., Schousboe A. Properties of L-glutamate decarboxylase from brains of adult and newborn mice. J Neurochem. 1976 Sep;27(3):653–659. doi: 10.1111/j.1471-4159.1976.tb10390.x. [DOI] [PubMed] [Google Scholar]