Abstract
Carotid bodies and neonatal adrenal medullary chromaffin cells (AMC) rapidly respond to acute hypoxia within seconds before compromising cellular functions. Responses to acute hypoxia are dynamically altered by chronic perturbations in arterial blood O2 levels resulting from breathing disorders. Sleep disordered breathing with recurrent apneas cause periodic decreases in arterial blood O2 or intermittent hypoxia (IH). Recent studies suggest that reactive oxygen species (ROS) mediate cellular adaptations to prolonged hypoxia. In this article we discuss the evidence for ROS in mediating exaggerated carotid body and AMC responses to acute hypoxia by IH and the underlying cellular and molecular mechanisms. IH increases ROS levels, and anti-oxidants prevent IH-induced augmented responses of the carotid body and AMC to hypoxia. The enhanced hypoxic sensitivity by IH involves ROS-dependent recruitment of transmitters/modulators in the carotid body and Ca2+ signaling mechanisms in AMC. Mechanisms by which IH elevates ROS include activation of NADPH oxidases, inhibition of mitochondrial complex I activity and down regulation of anti-oxidant enzymes. Transcriptional regulation of pro-and anti-oxidant enzymes by hypoxia-inducible factors 1 and 2 appears to be a major molecular mechanism regulating ROS generation by IH.
Keywords: NADPH oxidases, mitochondrial electron transport chain, S-glutathionylation, T-type Ca2+ channels, Ryanodine receptors, endothelin, 5-HT
1. Introduction
O2 derived from breathing is primarily utilized for generating energy and a small portion of it is enzymatically converted to reactive oxygen species (ROS) by oxidases and also by electron leak from mitochondrion (Halliwell & Gutteridge, 1990). It is increasingly becoming evident that ROS mediate a variety of physiological processes including cellular adaptations to prolonged hypoxia (Desireddy et al. 2010; Guzy et al. 2007, Waypa and Schumacher, 2008, Waypa et al. 2010).
Arterial chemoreceptors, especially the carotid bodies respond to hypoxia within seconds after its onset and the resulting autonomic reflexes ensure adequate O2 delivery to tissues (see Prabhakar, 2000 for references). In neonates, carotid bodies are immature and respond poorly to acute hypoxia (see Donnelly, 2000 for references). However, neonatal adrenal medullary chromaffin cells (AMC) respond to acute hypoxia with catecholamine secretion (Bournaud et al, 2007, Mojet et al, 1997, Rico et al., 2005, Seidler and Slotkin, 1985, Souvannakitti et al, 2009, Thomson et al., 1997). The resulting increase in circulating catecholamines is beneficial to neonates for withstanding stress imposed by hypoxia. Several studies assessed the role of ROS in the acute hypoxic sensing by the carotid body and AMC (Agapito et al. 2009; Dinger et al., 2007, Gonzalez et al., 2007). These studies, however, do not provide compelling evidence for ROS mediating the responses to acute hypoxia.
Physiological responses to acute hypoxia are dynamically altered by chronic perturbations in systemic O2 resulting from breathing disorders. Sleep disordered breathing with recurrent apneas represent one such breathing problem in adult humans (Nieto et al., 2000) and in preterm infants (Poets, 1994). Recurrent apneas are characterized by brief, repetitive cessations of breathing (each episode lasting no more than 10 to 30 seconds) resulting in periodic decreases in arterial blood O2 or intermittent hypoxia (IH). Recent studies suggest that IH profoundly impacts carotid body and AMC responses to acute hypoxia. In this review we present evidence for ROS signaling in mediating the carotid body and AMC responses to acute hypoxia under IH and highlight the underlying cellular and molecular mechanisms.
2. Effects of IH on acute responses to hypoxia
Carotid Body
Carotid body responses to acute hypoxia are exaggerated in IH treated adult rats (Peng and Prabhakar, 2004), mice (Peng et al. 2006b) and cats (Rey et al. 2004), whereas response to hypercapnia (high CO2) were unaffected (Peng and Prabhakar, 2004). Repetitive hypoxia elicits long-lasting increase in baseline carotid body activity in IH treated rodents, a phenomenon referred to as sensory long-term facilitation or sLTF (Peng et al., 2003; Peng et al., 2006b). However, sLTF was not expressed by carotid bodies from control rats or mice. The effects of IH on carotid body function were: a) time-dependent; b) reversible after re-exposure to normoxia and c) associated with no apparent alterations in carotid body morphology. Comparable cumulative duration of continuous hypoxia neither sensitized the hypoxic response nor elicited sLTF, suggesting that the effects were unique to IH.
Although neonatal carotid bodies respond poorly to acute hypoxia, IH markedly augments sensory response to low O2 (Peng et al, 2004). Unlike adults, IH treated neonatal carotid bodies display hyperplasia of glomus cells, do not exhibit sLTF and the hypersensitivity to hypoxia persists into juvenile life (Pawar et al. 2008, 2009).
Adrenal medullary chromaffin Cells (AMC)
IH treated adult (Kumar et al., 2006) and neonatal AMC (Souvannakitti et al., 2009) respond to acute hypoxia with enhanced catecholamine secretion. The effects were unique to IH because continuous hypoxia does not facilitate low-O2 evoked catecholamine secretion either in the adult (Kumar et al., 2006) or from neonatal AMC (Souvannakitti et al., 2009). On the other hand, IH decreases neurogenic catecholamine secretion in adult rat adrenal medulla as evidenced by marked suppression of nicotine or 2-deoxyglucose-evoked catecholamine efflux (Kumar et al., 2006). The decreased neurogenic response is in part due to down-regulation of nicotinic cholinergic receptor expression in AMC (Souvannakitti et al., 2010a).
3. Role of ROS in altered responses to acute hypoxia by IH
The above outlined studies suggest that IH selectively augments carotid body and AMC responses to acute hypoxia in adult and neonatal rodents. It has been proposed that ROS signaling mediates IH-induced augmented carotid body responses to acute hypoxia (Prabhakar, 2001). Supporting this possibility are the studies showing elevated ROS levels in IH treated carotid bodies from adult (Peng et al., 2003) and neonatal (Pawar et al., 2009) rats as evidenced by decreased aconitase activity and elevated MDA levels, which are established biochemical markers of ROS (Gardner, 2002; Ramanathan et al., 2005). Similar elevations in ROS were also seen in adrenal medullae from IH treated adult rats (Kumar et al 2006), mice (Kuri et al., 2007) and neonatal rats (Souvannakitti et al. 2009).
Functional significance of increased ROS levels was ascertained by anti-oxidant treatment strategy. Rats were treated with a stable superoxide dismutase mimetic [manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP), 5 mg/kg, i.p.], a potent scavenger of ROS every day prior to subjecting them to 8h regimen of IH for 10days. MnTMPyP prevented IH-induced exaggerated carotid body response to hypoxia in adult and neonatal rats (Peng et al. 2003, 2004; Pawar et al. 2009). However, acute application of a single dose of MnTMPyP on the 10th day of IH exposure, however, was ineffective in preventing the sensitization of the hypoxic response (Peng et al., 2003), implying that ROS-mediated signaling cascade rather than acute generation ROS is necessary for evoking augmented hypoxic sensitivity following IH. Ascorbic acid, another anti-oxidant is also effective in preventing IH-evoked sensitization of the carotid body response to acute hypoxia (DelRio et al., 2010).
Anti-oxidants also abolish the enhanced catecholamine secretion from IH treated AMC from neonatal (Souvannakitti et al. 2009) and adult rats (Kumar et al. 2006) and mice (Kuri et al. 2007). These studies suggest that ROS is critical for evoking the exaggerated hypoxic responses of the carotid body and AMC in IH treated rodents. However, further studies are needed to delineate relative contributions of O2 ˙−, H2O2 or OH˙− to IH-induced exaggerated sensitivity of the carotid body and AMC to acute hypoxia.
4. Cellular targets of ROS
Carotid body
Neurotransmitters/modultators are essential for evoking sensory excitation of the carotid body by acute hypoxia (Prabhakar, 2000). Recent studies suggest that ROS-dependent recruitment of endothelin-1 (ET-1) and 5-hydroxytryptamine (5-HT) mediate sensitization and sLTF of the IH treated carotid body, respectively.
ET-1 is normally expressed at low levels in the carotid body. Exogenous application of ET-1 by itself has very little effect on the sensory activity, but markedly augments sensory response to acute hypoxia (Chen et al., 2002, 2007). Recent studies showed that IH: a) up-regulates ET-1 expression in glomus cells of the carotid body (Rey et al 2006; Pawar et al., 2009); b) facilitates ET-1 release, and c) up-regulates ETA but not ETB receptor mRNAs (Pawar et al., 2009). Anti-oxidant treatment prevents the effects of IH on ET-1 expression in the carotid body (Pawar et al., 2009). More importantly, ETA but not ETB receptor antagonist abolishes IH-evoked sensitization of the hypoxic response of the carotid body (Rey et al 2006; Pawar et al 2009) but not the sLTF in adult carotid body (Peng and Prabhakar, 2010, unpublished observations).
5-HT mediates long-lasting neuronal activation in the vertebrate nervous system (Machacek et al. 2001). Carotid bodies express 5-HT (Gronblad et al. 1983; Zhang et al. 2003; Jacono et al. 2005) and exogenous application of 5-HT augments the carotid body activity (Kirby & McQueen, 1984; Jacono et al. 2005). Although the role of 5-HT in evoking sensory excitation by acute hypoxia remains uncertain, several lines of evidence suggest that 5-HT mediates IH-evoked sLTF of the carotid bodies. These include: a) repetitive hypoxia releases 5-HT from IH treated but not from control carotid bodies; b) 5-HT activates NADPH oxidase (Nox) via 5-HT2 receptors; c) IH-induced sLTF was absent in mice deficient in 5-HT (Peng et al. 2009) and d) exogenous spaced application of 5-HT induces sLTF in control carotid bodies via Nox activation (Peng et al., 2006a).
The mechanisms by which ROS up-regulates ET-1 and ETA receptors, and how IH facilitates the release of ET-1 and 5-HT, which are critical steps in evoking sensitization of the hypoxic response and sLTF of the carotid body remain to be elucidated.
AMC
It is well established that Ca2+ signaling is essential for catecholamine release from AMC. Acute hypoxia-evoked increases in [Ca2+]i were markedly enhanced in IH treated AMC, which was due to enhanced Ca2+ influx as well as mobilization of intracellular Ca2+ stores (Souvannakitti et al., 2009). A recent study reported that IH leads to ROS-dependent transcriptional up-regulation of T-type Ca2+ channels (Cav3.1 and Cav3.2) and rynaodine receptors (RyRs) in AMC, which mediate the enhanced Ca2+ influx and mobilization of intracellular Ca2+ stores, respectively (Souvannakitti et al., 2010b). In IH treated AMC basal [Ca2+]i was elevated, which was due to activation of RyRs by ROS-dependent S-glutathionylation of RyRs (Souvannakitti et al., 2010b).
4. Sources of ROS generation by IH
Cellular levels of ROS depend on the balance between pro-and anti-oxidant enzyme activities. The following sections summarize the effects of IH on pro-and anti-oxidants.
4.1 IH and Pro-oxidants
4.1.1. NADPH oxidases (Nox)
The family of NADPH oxidases (Nox) constitutes one of the major sources of ROS (see Bedard and Krause, 2004). Peng et al (2009) reported that IH up-regulates Nox2 mRNA and increases Nox enzyme activity by ~12-fold in carotid bodies. More importantly, IH treated Nox2 knock-out mice do not exhibit sLTF and show reduced hypoxic response of the carotid body (Peng et al 2009). IH also up-regulates Nox2 mRNA, increases Nox activity in adrenal medullae from neonatal rats and Nox inhibitors prevent the exaggerated catecholamine secretion as well as [Ca2+]i responses to hypoxia in AMC from IH treated neonatal rats (Souvannakitti et al. 2010b).
IH in addition to Nox2, also up-regulates Nox4 in carotid bodies (Peng et al., 2009) and in neonatal AMC (Souvannakitti et al., 2010b). Immunocytochemical analysis revealed localization of Nox2 to cytosol and Nox4 to the nucleus of glomus cells (Peng et al., 2009) and neonatal AMC (Souvannakitti et al., 2010b). Functional significance of Nox4 up-regulation by IH in the carotid body and AMC remains to be examined.
4.1.2. Mitochondrial Electron Transport Chain (ETC)
In addition to oxidases, mitochondrial electron transport chain (ETC) constitutes another major source of ROS (Ambrosio et al.., 1993). IH inhibits mitochondrial complex I but not the complex III activity in carotid bodies (Peng et al., 2003). Similar inhibition of the complex I activity was also reported in IH treated rat pheochromocytoma -12 (PC12) cells, which are of adrenal medullary origin (Yuan et al. 2004; Khan et al 2010). The decreased complex I activity was associated with increased mitochondrial ROS in IH treated PC12 cells. Thus, in addition to Nox activation, inhibition of mitochondrial ETC at the complex I also contributes to ROS generation by IH.
4.1.3. Mechanism(s) of mitochondrial complex I inhibition by IH
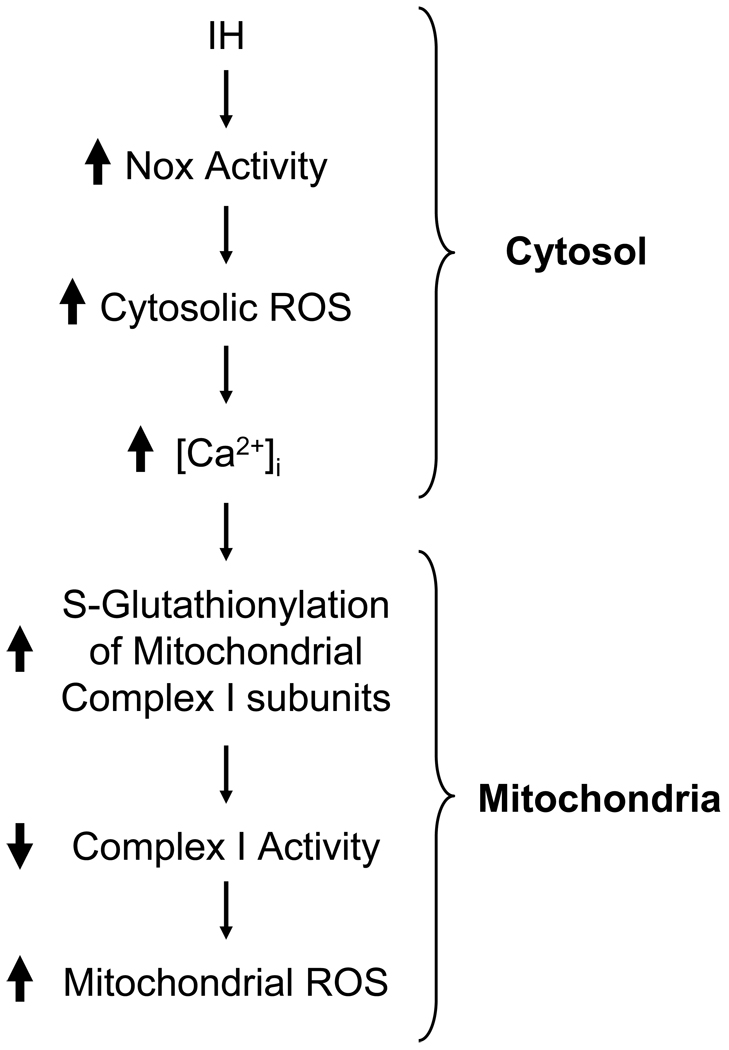
Khan et al (2010) examined the mechanisms underlying the complex I inhibition by IH. These investigators found that Nox inhibitors (i.e., apocynin or AEBSF) as well as silencing Nox2 by siRNA prevent complex I inhibition in IH treated cells. Furthermore, complex I inhibition was absent in IH treated Nox2 knockout mice. In addition, they also found that ROS generated by Nox facilitate Ca2+ flux into mitochondria and cause S-glutathionylation of 75-and 50-kDa subunits of complex I resulting in inhibition of the complex I activity. Together, these findings provide evidence for positive feed-forward interactions between Nox2 and mitochondrial complex I resulting in ROS-induced ROS for sustained oxidative stress under IH condition as schematically illustrated in Fig.1.
Figure 1.
Schematic representation of the mechanisms associated with inhibition of Complex I activity by intermittent hypoxia (IH). Nox= NADPH oxidase.
4.2. IH and Anti-oxidants
In addition to activating pro-oxidants, there is some evidence showing that IH down-regulates anti-oxidant enzymes. Recently, Nanduri et al. (2009) reported down-regulation of superoxide dismutase-2 (Sod-2) mRNA as well as the Sod-2 enzyme activity in IH treated rat adrenal medullae. Likewise, 60–70% down-regulation of Sod-2 mRNA was also seen in IH treated rat carotid bodies (Nanduri et al., 2010, unpublished observations). In rat PC12 cell cultures IH decreased glutathione peroxidase-1 (Gpx-1) activity, an enzyme that catalyzes degradation of H2O2 (Khan et al., 2010, unpublished observations). These observations suggest that IH-evoked increase in ROS levels involves not only up-regulation of pro-oxidants but also down-regulation of anti-oxidants.
5. Molecular mechanisms of ROS generation by IH
IH affects a variety of transcription factors, including the hypoxia-inducible factors (HIF-1 and HIF-2s), Activator Protein-1 (AP-1), nuclear factor of activated T-cells (NFAT), and nuclear factor κB (NF-κB; see Nanduri et al. 2008 for references). The following section summarizes emerging evidence for regulation of genes encoding pro-and anti-oxidant enzymes by the HIF family of transcriptional activators during IH.
5.1. IH causes differential regulation of HIF-1 and HIF-2
HIF-1 is the prototypical member of the HIF family of transcriptional activators and comprises an O2-regulated α subunit and a constitutive β subunit (Wang et al. 1995). HIF-1 transcriptional activity is induced under the conditions of continuous hypoxia as a consequence of HIF-1α protein accumulation resulting from decreased O2-dependent proline hydroxylation, ubiquitination, and proteasomal degradation (Coleman and Ratcliffe, 2007). HIF-2α (also known as endothelial PAS domain protein-1, EPAS-1) is another member of the HIF family, which shares 80% sequence homology to HIF-1α and also interacts with HIF-1β (Tian et al., 1997).
Although continuous hypoxia leads to accumulation of both HIF-1α and HIF-2α (Holmquist-Mengelbier et al., 2006), IH up-regulates HIF-1α and down-regulates HIF-2α protein in cell cultures and in rodents (Nanduri et al. 2009). Furthermore, the effects of IH on HIF-1α and HIF-2α persist for several hours after termination of IH stimulus as opposed to return to basal levels within minutes after terminating continuous hypoxia (Yuan et al., 2008, Nanduri et al., 2009), indicating that continuous and intermittent hypoxia exert strikingly different effects on HIF-1 and HIF-2.
5.2. Significance of differential regulation of HIF-1 and HIF-2 by IH
Complete HIF-1α deficiency results in embryonic lethality at mid-gestation, whereas Hif1α+/− heterozygous (HET) mice, which are partially deficient in HIF-1α expression, develop normally and are indistinguishable from wild type (WT) littermates under normoxic conditions (Yu et al., 1999). IH treated WT mice exhibit enhanced carotid body responses to hypoxia, sLTF and elevated ROS levels, and remarkably these responses are absent in IH treated HET mice (Peng et al., 2006b). Interestingly, IH-induced up-regulation of Nox2 mRNA in the carotid body is absent in Hif-1a+/− mice (Yuan et al. 2010; unpublished observations), raising intriguing possibility that HIF-1 regulates Nox2 and the ensuing mitochondrial complex I inhibition during IH. However, detailed studies are needed to ascertain this possibility.
Scortegagna et al (2003) reported that HIF-2 regulates transcription of several antioxidant enzymes, including SOD-2. IH-induced down-regulation of Sod-2 mRNA and Sod-2 activity in PC12 cell cultures are prevented by overexpression of transcriptionally active—but not inactive—HIF-2α plasmid (Nanduri et al. 2009). In intact rats, systemic administration of ALLM, a potent inhibitor of calpains, rescues IH-induced HIF-2α degradation in the carotid body and adrenal medulla, restores Sod2 activity and prevents elevation of ROS (Nanduri et al. 2009). Thus, down regulation of HIF-2 contributes to IH-induced increase in ROS via insufficient transcription of anti-oxidative enzymes, such as Sod-2.
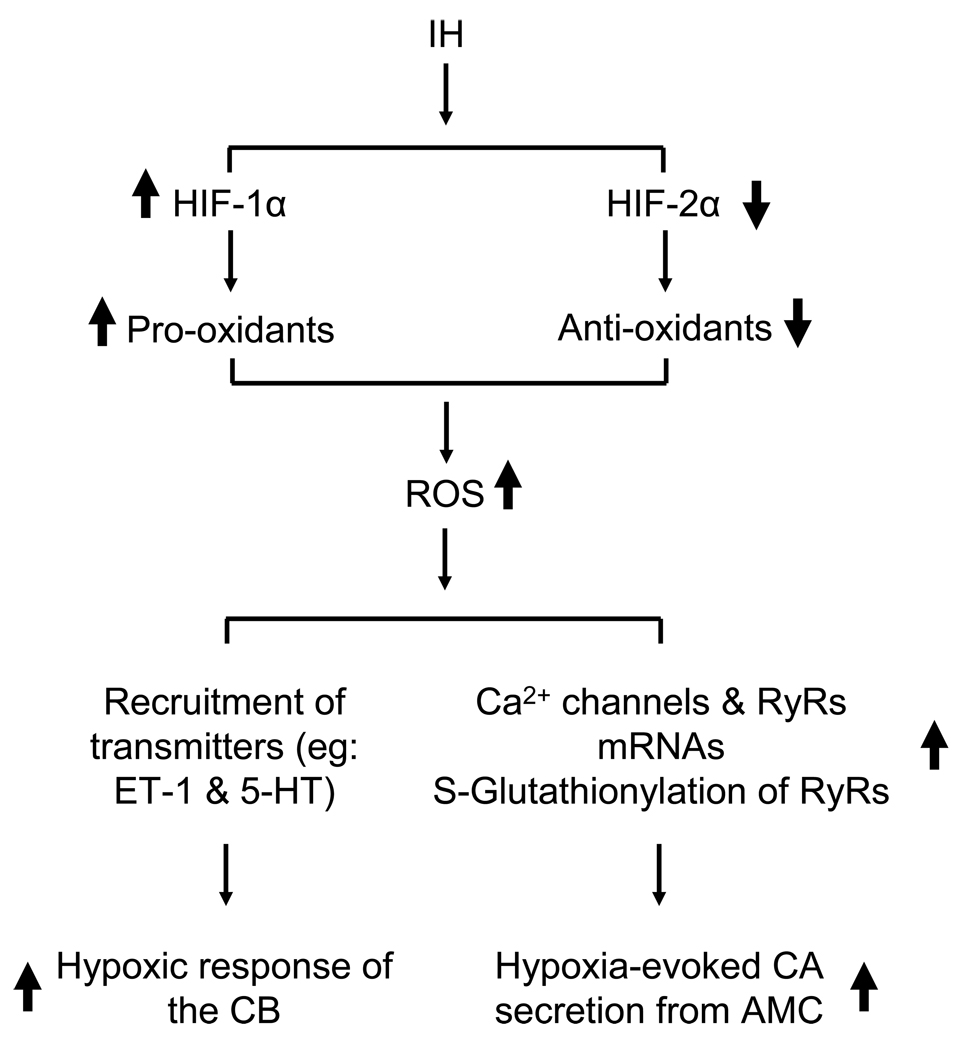
The above outlined observations indicate that up-regulation of pro-oxidants via HIF-1 and down-regulation of anti-oxidants by HIF-2 contribute in part to the elevated ROS levels under IH (Fig. 2). However, it should be noted that HIF-1 and HIF-2 regulate several other genes related to maintenance of homeostasis during hypoxia including those encoding erythropoietin, vascular endothelial growth factor, enzymes associated with glucose metabolism (see Semenza and Prabhakar, 2007 for references). The effects of IH on the expression of these genes have not been yet established. Furthermore, as stated at the beginning of this section, IH also activates other transcriptional activators including AP-1, NFAT and NF-κB (Nanduri et al., 2008). Very little is known on the role(s) of transcriptional activators other than HIFs to the augmented hypoxic sensitivity of the carotid body and AMC by IH.
Figure 2.
Schematic presentation of molecular and cellular mechanisms associated with intermittent hypoxia (IH)-induced enhanced hypoxic response of the carotid body (CB) and adrenal medullary chromaffin cells (AMC). HIF-1 and HIF-2= hypoxia-inducible factors 1 and 2; ET-1= Endothelin 1; 5-HT= 5-hydroxytryptamine; RyR= ryanodine receptor.
6. Summary and perspective
In this review, we attempted to summarize recent studies showing the effects of IH on acute hypoxic sensing by the carotid body and AMC in experimental models. Available evidence indicates that IH augments carotid body and AMC responses to acute hypoxia and this effect involves ROS signaling. The IH evoked exaggerated hypoxic sensitivity might explain persistent sympathetic excitation and elevated plasma catecholamines seen in recurrent apnea patients. However, one must be cautious in extrapolating the results from experimental models of IH to morbidities in recurrent apnea patients, because the latter in addition to periodic hypoxia is also associated with hypercapnia as well. In addition, feed back from pulmonary afferents is also altered during apneic episodes in these patients, which might also contribute to autonomic changes. None-the-less, studies on experimental models as delineated in this article provide much needed conceptual frame work on cellular and molecular mechanisms that are unique to IH, which can be further validated in patients with sleep disordered breathing.
Acknowledgement
We thank Drs. Y-J. Peng, G-Yuan, S.A. Khan, A.P. Fox, and D. Souvannakitti for their contribution to various experiments reported in this article. This work is supported by grants from National Institutes of Health, Heart, Lung and Blood Institute HL-90554, HL-76537, HL-86493, HL-089616.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agapito MT, Sanz-Alfayate G, Gomez-Niño A, Gonzalez C, Obeso A. General redox environment and carotid body chemoreceptor function. Am J Physiol Cell Physiol. 2009;296:C620–C631. doi: 10.1152/ajpcell.00542.2008. [DOI] [PubMed] [Google Scholar]
- Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty JT. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bournaud RJ, Hidalgo H, Yu J. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxia sensitivity. Pflugers Arch. 2007;454:83–92. doi: 10.1007/s00424-006-0185-z. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1314–L1323. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1257–L1262. doi: 10.1152/ajplung.00419.2006. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. In: Peers C, editor. Oxygen Sensing and Hypoxia-Induced Responses. London: Portland Press; 2007. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Desireddi JR, Farrow KN, Marks JD, Waypa GB, Schumacker PT. Hypoxia increases ROS signaling and cytosolic Ca(2+) in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal. 2010;12:595–602. doi: 10.1089/ars.2009.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J. 2010;36:143–150. doi: 10.1183/09031936.00158109. [DOI] [PubMed] [Google Scholar]
- Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007;157:45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF. Developmental aspects of oxygen sensing by the carotid body. J. Appl. Physiol. 2000;88:2296–2301. doi: 10.1152/jappl.2000.88.6.2296. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Agapito MT, Rocher A, Gonzalez-Martin MC, Vega-Agapito V, Gomez-Niño A, Rigual R, Castañeda J, Obeso A. Chemoreception in the context of the general biology of ROS. Respir Physiol Neurobiol. 2007;157:30–44. doi: 10.1016/j.resp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Gronblad M, Liesi P, Rechardt L. Serotonin-like immunoreactivity in rat carotid body. Brain Res. 1983;276:348–350. doi: 10.1016/0006-8993(83)90745-x. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Mack MM, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal. 2007;9:1317–1328. doi: 10.1089/ars.2007.1708. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Påhlman S. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Jacono FJ, Peng YJ, Kumar GK, Prabhakar NR. Modulation of the hypoxic sensory response of the carotid body by 5-hydroxytryptamine: role of the 5-HT2 receptor. Respir Physiol Neurobiol. 2005;145:135–142. doi: 10.1016/j.resp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kirby GC, McQueen DS. Effects of the antagonists MDL 72222 and ketanserin on responses of cat carotid body chemoreceptors to 5-hydroxytryptamine. Br J Pharmacol. 1984;83:259–269. doi: 10.1111/j.1476-5381.1984.tb10142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. Nox2 mediates intermittent hypoxia-induced mitochondrial complex i inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal. 2010 Jul 10; doi: 10.1089/ars.2010.3213. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri BA, Khan SA, Chan SA, Prabhakar NR, Smith CB. Increased secretory capacity of mouse adrenal chromaffin cells by chronic intermittent hypoxia: involvement of protein kinase C. J Physiol. 2007;584:313–319. doi: 10.1113/jphysiol.2007.140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek DW, Garraway SM, Shay BL, Hochman S. Serotonin 5-HT2 receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J Physiol. 2001;537:201–207. doi: 10.1111/j.1469-7793.2001.0201k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojet MH, Mills E, Duchen MR. Hypoxia-induced catecholamine secretion in isolated newborn rat adrenal chromaffin cells is mimicked by inhibition of mitochondrial respiration. J Physiol. 1997;504:175–189. doi: 10.1111/j.1469-7793.1997.175bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study, Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R735–R742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J. Appl. Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J. Appl. Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006a;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J. Physiol. 2006b;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J. Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol. Neonate. 1994;65:211–219. doi: 10.1159/000244055. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J. Appl. Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J. Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Rio RD, Iturriaga R. Contribution of endothelin-1 to the enhanced CB chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006;1086:152–159. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- Rico AJ, Prieto-Lloret J, Gonzalez C, Rigual R. Hypoxia and acidosis increase the secretion of catecholamines in the neonatal rat adrenal medulla: an in vitro study. Am J Physiol Cell Physiol. 2005;289:C1417–C1425. doi: 10.1152/ajpcell.00023.2005. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol. 1985;358:1–16. doi: 10.1113/jphysiol.1985.sp015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Kuri B, Yuan G, Pawar A, Kumar GK, Smith C, Fox AP, Prabhakar NR. Neonatal intermittent hypoxia impairs neuronal nicotinic receptor expression and function in adrenal chromaffin cells. Am J Physiol Cell Physiol. 2010a May 12; doi: 10.1152/ajpcell.00530.2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and Ryanodine Receptors mediate the Augmented Exocytosis of Catecholamines from Intermittent Hypoxia treated Neonatal Rat Chromaffin Cells. J.Neurosci. 2010b doi: 10.1523/JNEUROSCI.2307-10.2010. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol. 1997;498:503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa GB, Schumacker PT. Oxygen sensing in hypoxic pulmonary vasoconstriction: using new tools to answer an age-old question. Exp Physiol. 2008;93:133–138. doi: 10.1113/expphysiol.2007.041236. [DOI] [PubMed] [Google Scholar]
- Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J. Clin. Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fearon IM, Zhong H, Nurse CA. Presynaptic modulation of rat arterial chemoreceptor function by 5-HT: role of K+ channel inhibition via protein kinase C. J Physiol. 2003;551:825–842. doi: 10.1113/jphysiol.2002.038489. [DOI] [PMC free article] [PubMed] [Google Scholar]