Abstract
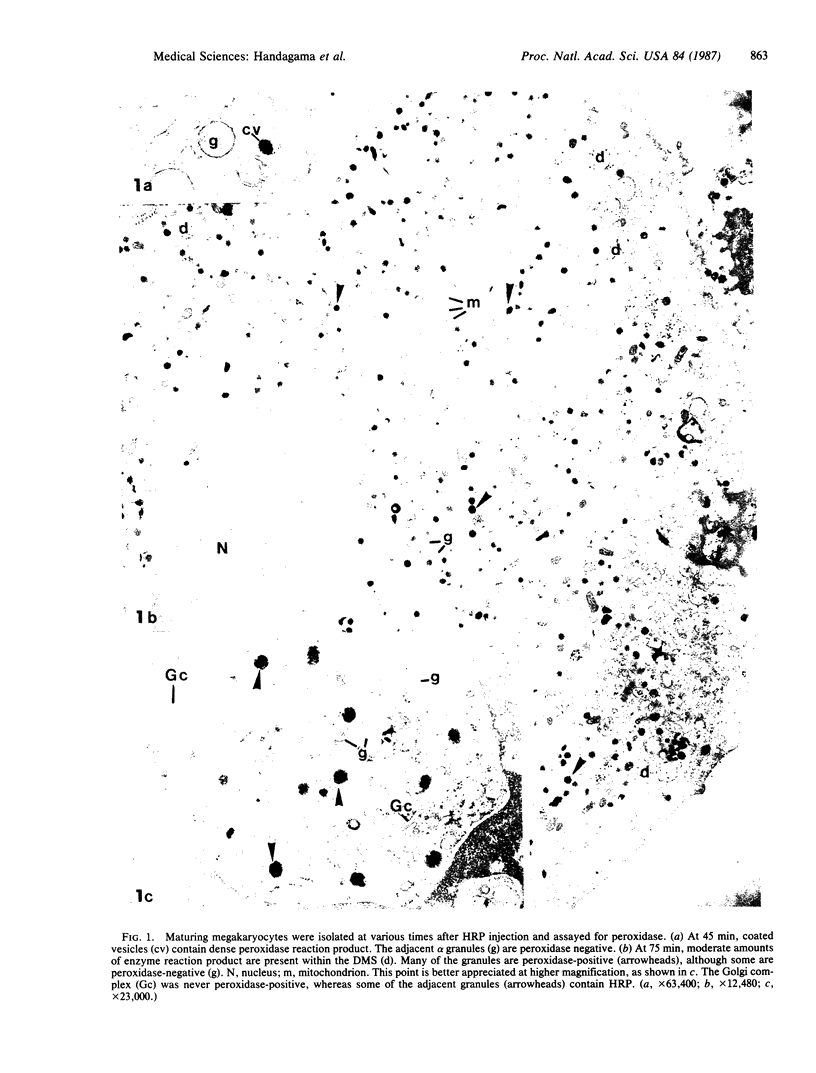
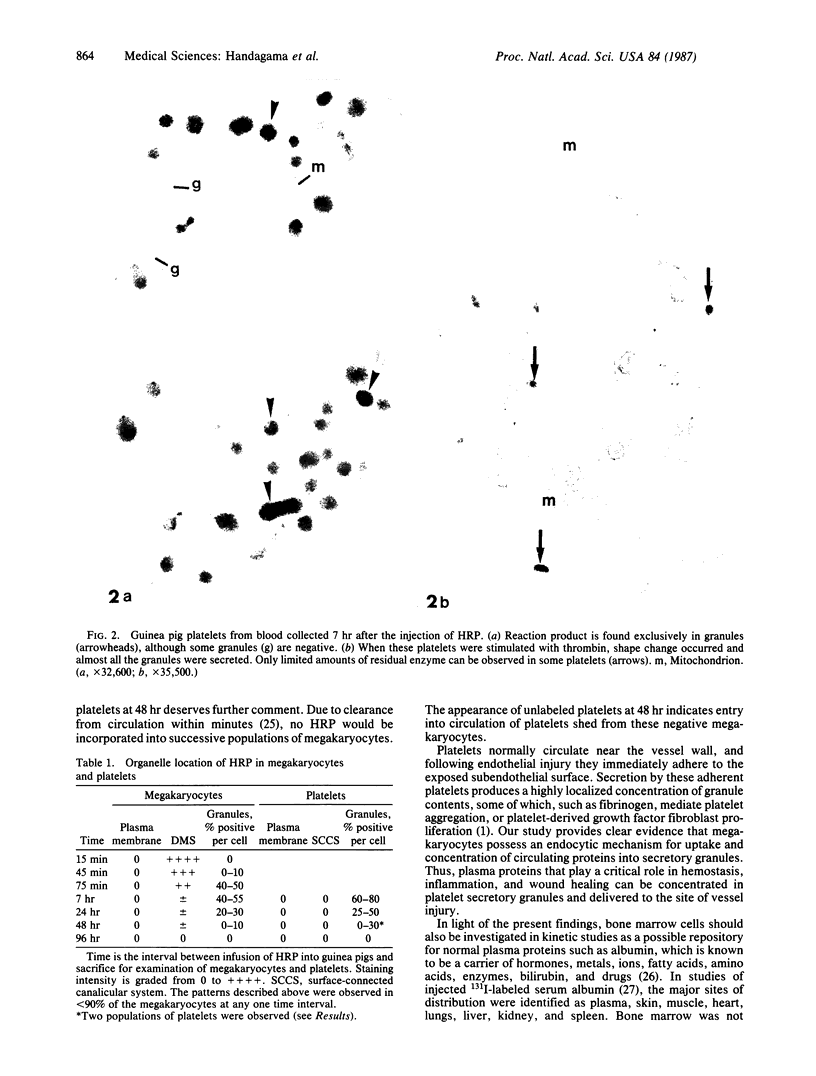
To determine whether or not proteins circulating in plasma can be incorporated into megakaryocytes and platelets, horseradish peroxidase (HRP) was injected intravenously into guinea pigs and these cells were examined for its uptake by electron microscopy and cytochemistry. Enriched samples of megakaryocytes enabled ultrastructural analysis of large numbers of these rare cells. In megakaryocytes, 50% of alpha granules contained HRP between 75 min and 7 hr after injection. At 24 hr, 25% of the megakaryocyte granules were peroxidase-positive, less were positive by 48 hr, and there were none at 4 days. Thus, the findings demonstrate that a circulating protein can be endocytosed by megakaryocytes and rapidly packaged into alpha granules. Platelet granules also contain HRP by 7 hr after injection, and they can secrete it in response to thrombin. Unfortunately, our present studies do not allow us to distinguish between direct endocytosis by the platelet and/or shedding of new platelets from recently labeled megakaryocytes. It is concluded that while some alpha granule proteins are synthesized by megakaryocytes, others may be acquired from plasma by endocytosis. In addition to providing evidence that some of the proteins of alpha granules may be of exogenous origin, this study has allowed the definition of a pathway whereby plasma proteins may be temporarily sequestered in megakaryocytes before reentering the circulation in platelets.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J Ultrastruct Res. 1968 Sep;24(5):412–433. doi: 10.1016/s0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- Belloc F., Hourdille P., Fialon P., Boisseau M. R., Soria J. Fibrinogen synthesis by megakaryocyte rich human marrow cell concentrates. Thromb Res. 1985 May 15;38(4):341–351. doi: 10.1016/0049-3848(85)90133-1. [DOI] [PubMed] [Google Scholar]
- Bentfeld-Barker M. E., Bainton D. F. Identification of primary lysosomes in human megakaryocytes and platelets. Blood. 1982 Mar;59(3):472–481. [PubMed] [Google Scholar]
- Chiu H. C., Schick P. K., Colman R. W. Biosynthesis of factor V in isolated guinea pig megakaryocytes. J Clin Invest. 1985 Feb;75(2):339–346. doi: 10.1172/JCI111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Karnovsky M. J. Uptake of horseradish peroxidase by guinea pig basophilic leukocytes. Lab Invest. 1972 Jan;26(1):27–39. [PubMed] [Google Scholar]
- Dvorak A. M., Klebanoff S. J., Henderson W. R., Monahan R. A., Pyne K., Galli S. J. Vesicular uptake of eosinophil peroxidase by guinea pig basophils and by cloned mouse mast cells and granule-containing lymphoid cells. Am J Pathol. 1985 Mar;118(3):425–438. [PMC free article] [PubMed] [Google Scholar]
- EBBE S., BALDINI M., DONOVAN J. COMPARATIVE STUDIES OF PLATELET SURVIVAL BY DIFFERENT METHODS IN THE RABBIT. Blood. 1965 Apr;25:548–566. [PubMed] [Google Scholar]
- Fedorko M. E. The functional capacity of guinea pig megakaryocytes. II. The uptake of particles and macromolecules and the effect of rabbit antiguinea pig platelet antiserum. Lab Invest. 1977 Mar;36(3):321–328. [PubMed] [Google Scholar]
- George J. N., Nurden A. T., Phillips D. R. Molecular defects in interactions of platelets with the vessel wall. N Engl J Med. 1984 Oct 25;311(17):1084–1098. doi: 10.1056/NEJM198410253111705. [DOI] [PubMed] [Google Scholar]
- George J. N., Saucerman S., Levine S. P., Knieriem L. K., Bainton D. F. Immunoglobulin G is a platelet alpha granule-secreted protein. J Clin Invest. 1985 Nov;76(5):2020–2025. doi: 10.1172/JCI112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz A. M., Keefer M., Doshi K., Annamalai A. E., Chiu H. C., Colman R. W. Biology of human megakaryocyte factor V. Blood. 1986 Jun;67(6):1639–1648. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Holmsen H. Platelet metabolism and activation. Semin Hematol. 1985 Jul;22(3):219–240. [PubMed] [Google Scholar]
- Leven R. M., Schick P. K., Budzynski A. Z. Fibrinogen biosynthesis in isolated guinea pig megakaryocytes. Blood. 1985 Feb;65(2):501–504. [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSCHILD M. A., BAUMAN A., YALOW R. S., BERSON S. A. Tissue distribution of I131 labeled human serum albumin following intravenous administration. J Clin Invest. 1955 Sep;34(9):1354–1358. doi: 10.1172/JCI103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E. M., Nachman R. L., Williams N., Winchester R. J., Ross G. D. Human megakaryocytes. I. Characterization of the membrane and cytoplasmic components of isolated marrow megakaryocytes. J Exp Med. 1979 Jun 1;149(6):1273–1287. doi: 10.1084/jem.149.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman J. S., Schlesinger P., Stahl P. Rat plasma clearance of horseradish peroxidase and yeast invertase is mediated by specific recognition. FEBS Lett. 1978 Jan 15;85(2):345–348. doi: 10.1016/0014-5793(78)80488-8. [DOI] [PubMed] [Google Scholar]
- Rudnick G., Humphreys C. J., Dean G. E. Serotonin transport by platelet plasma and granule membranes. Ann N Y Acad Sci. 1985;456:277–278. doi: 10.1111/j.1749-6632.1985.tb14876.x. [DOI] [PubMed] [Google Scholar]
- Ryo R., Nakeff A., Huang S. S., Ginsberg M., Deuel T. F. New synthesis of a platelet-specific protein: platelet factor 4 synthesis in a megakaryocyte-enriched rabbit bone marrow culture system. J Cell Biol. 1983 Feb;96(2):515–520. doi: 10.1083/jcb.96.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranzer J. P., da Prada M., Pletscher A. Storage of 5-hydroxytryptamine in megakaryocytes. J Cell Biol. 1972 Jan;52(1):191–197. doi: 10.1083/jcb.52.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. The transfer of thorium particles from plasma to platelets and platelet granules. Am J Pathol. 1968 Oct;53(4):567–575. [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. Endocytosis by human platelets: metabolic and freeze-fracture studies. J Cell Biol. 1981 Dec;91(3 Pt 1):706–715. doi: 10.1083/jcb.91.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Petursson S. Thrombocytopoiesis--analysis by membrane tracer and freeze-fracture studies on fresh human and cultured mouse megakaryocytes. J Cell Biol. 1984 Aug;99(2):390–402. doi: 10.1083/jcb.99.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. The submembranous fibrils of human blood platelets. J Cell Biol. 1970 Oct;47(1):293–299. doi: 10.1083/jcb.47.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]