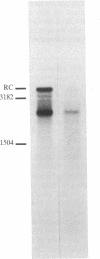
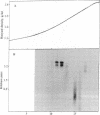
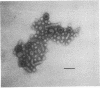
Abstract
The hepatoblastoma cell line Hep G2 was transfected with a plasmid carrying the gene that confers resistance to G418 and four 5'-3' tandem copies of the hepatitis B virus (HBV) genome positioned such that two dimers of the genomic DNA are 3'-3' with respect to one another. Cells of one clone that grew in the presence of G418 produce high levels of hepatitis B e antigen and of hepatitis B surface antigen. HBV DNA is carried by these cells as chromosomally integrated sequences and episomally as relaxed circular, covalently closed, and incomplete copies of the HBV genome. Viral DNA was detected also in conditioned growth medium at the buoyant densities characteristic for infectious Dane and immature core particles. Finally, HBV-specific components morphologically identical to the 22-nm spherical and filamentous hepatitis B surface antigen particles as well as 42-nm Dane particles were visualized by immunoelectron microscopic analysis. Therefore, we have demonstrated that the Hep G2 cell line can support the assembly and secretion not only of several of the replicative intermediates of HBV DNA but also of Dane-like particles. This in vitro system can now be used to study the life cycle of HBV and the reaction of immunocompetent cells with cells carrying HBV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum H. E., Haase A. T., Harris J. D., Walker D., Vyas G. N. Asymmetric replication of hepatitis B virus DNA in human liver: demonstration of cytoplasmic minus-strand DNA by blot analyses and in situ hybridization. Virology. 1984 Nov;139(1):87–96. doi: 10.1016/0042-6822(84)90332-5. [DOI] [PubMed] [Google Scholar]
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Gerber M., Price P. M., Flordellis C., Edelman J., Acs G. Amplification of expression of hepatitis B surface antigen in 3T3 cells cotransfected with a dominant-acting gene and cloned viral DNA. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1815–1819. doi: 10.1073/pnas.79.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Murray K. Expression of the hepatitis B virus surface, core and E antigen genes by stable rat and mouse cell lines. J Mol Biol. 1982 Nov 25;162(1):43–67. doi: 10.1016/0022-2836(82)90161-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Robinson W. S. The proteins of hepatitis B Dane particle cores. J Med Virol. 1977;1(2):119–131. doi: 10.1002/jmv.1890010205. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Landers T. A., Greenberg H. B., Robinson W. S. Structure of hepatitis B Dane particle DNA and nature of the endogenous DNA polymerase reaction. J Virol. 1977 Aug;23(2):368–376. doi: 10.1128/jvi.23.2.368-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Hepatitis B virus DNA forms in nuclear and cytoplasmic fractions of infected human liver. Virology. 1984 Sep;137(2):390–399. doi: 10.1016/0042-6822(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Morgan T. L., Maher V. M., McCormick J. J. Optimal parameters for the polybrene-induced DNA transfection of diploid human fibroblasts. In Vitro Cell Dev Biol. 1986 Jun;22(6):317–319. doi: 10.1007/BF02623404. [DOI] [PubMed] [Google Scholar]
- Moriarty A. M., Hoyer B. H., Shih J. W., Gerin J. L., Hamer D. H. Expression of the hepatitis B virus surface antigen gene in cell culture by using a simian virus 40 vector. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2606–2610. doi: 10.1073/pnas.78.4.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möröy T., Etiemble J., Trépo C., Tiollais P., Buendia M. A. Transcription of woodchuck hepatitis virus in the chronically infected liver. EMBO J. 1985 Jun;4(6):1507–1514. doi: 10.1002/j.1460-2075.1985.tb03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P., Ostrove S., Flordellis C., Sells M. A., Thung S., Gerber M., Christman J., Acs G. Characterization of RNA transcripts and virally coded proteins synthesized in mouse fibroblasts transfected with hepatitis B DNA: HBeAg synthesis in HBcAg-negative cells with active core-antigen genes. Biosci Rep. 1983 Nov;3(11):1017–1026. doi: 10.1007/BF01121028. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler F., Robinson W. S. Hepatitis B viral DNA molecules have cohesive ends. J Virol. 1979 Oct;32(1):226–233. doi: 10.1128/jvi.32.1.226-233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A. Expression of hepatitis B virus surface antigen gene in cultured cells by using recombinant plasmid vectors. Mol Cell Biol. 1983 Jan;3(1):143–146. doi: 10.1128/mcb.3.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Stratowa C., Schaefer-Ridder M., Doehmer J., Hofschneider P. H. Enhanced production of hepatitis B surface antigen in NIH 3T3 mouse fibroblasts by using extrachromosomally replicating bovine papillomavirus vector. Mol Cell Biol. 1983 Jun;3(6):1032–1039. doi: 10.1128/mcb.3.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser B., Ganem D., Seeger C., Varmus H. E. Closed circular viral DNA and asymmetrical heterogeneous forms in livers from animals infected with ground squirrel hepatitis virus. J Virol. 1983 Oct;48(1):1–9. doi: 10.1128/jvi.48.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Cattaneo R., Koch H. G., Darai G., Schaller H., Schellekens H., van Eerd P. M., Deinhardt F. Cloned HBV DNA causes hepatitis in chimpanzees. Nature. 1982 Oct 21;299(5885):740–742. doi: 10.1038/299740a0. [DOI] [PubMed] [Google Scholar]
- Will H., Cattaneo R., Pfaff E., Kuhn C., Roggendorf M., Schaller H. Expression of hepatitis B antigens with a simian virus 40 vector. J Virol. 1984 May;50(2):335–342. doi: 10.1128/jvi.50.2.335-342.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H., Korba B. E., Lechner J. F., Tokiwa T., Gazdar A. F., Seeley T., Siegel M., Leeman L., Autrup H., Harris C. C. High-frequency transfection and cytopathology of the hepatitis B virus core antigen gene in human cells. Science. 1983 Oct 28;222(4622):385–389. doi: 10.1126/science.6194563. [DOI] [PubMed] [Google Scholar]